Volume 11, Issue 2 (June 2024)
J. Food Qual. Hazards Control 2024, 11(2): 127-134 |
Back to browse issues page
Ethics code: 0000
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Narwati N, Setiawan S. Reduction of the Cyanide from Cassava Leaves Using NaHCO3. J. Food Qual. Hazards Control 2024; 11 (2) :127-134
URL: http://jfqhc.ssu.ac.ir/article-1-1136-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1136-en.html
Department of Environmental Health, Poltekkes Kemenkes Surabaya, Jawa Timur, Indonesia , narwati@poltekkesdepkes-sby.ac.id
Full-Text [PDF 381 kb]
(2781 Downloads)
| Abstract (HTML) (1569 Views)
N. Narwati *, S. Setiawan
Department of Environmental Health, Poltekkes Kemenkes Surabaya, Jawa Timur, Indonesia
HIGHLIGHTS:
To cite: Narwati N., Setiawan S. (2024). Reduction of the cyanide from cassava leaves using NaHCO3. Journal of Food Quality and Hazards Control. 11: 127-134.
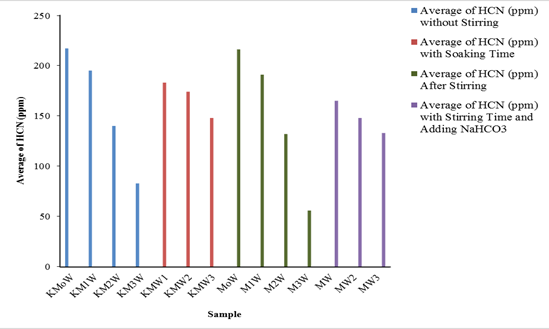
Figure 1: Average of Hydrogen Cyanide (HCN) levels in cassava leaves without stirring, with soaking time, after stirring, and adding average Sodium Bicarbonate (NaHCO3) 20 g
(K=Control group; Mo=0 g NaHCO3; M1=10 g NaHCO3; M2=20 g NaHCO3; M3=30 g NaHCO3; W1=15 min; W2=30 min; W3=45 min; W=Average of stirring time (22.5 min))
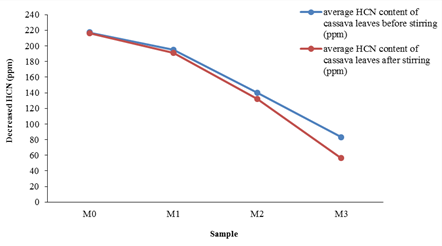
Figure 2: Decreased Hydrogen Cyanide (HCN) levels of cassava leaves before and after stirring with mass Sodium Bicarbonate (NaHCO3) variations (M0=0 g NaHCO3; M1=10 g NaHCO3; M2=20 g NaHCO3; M3=30 g NaHCO3)
Figure 3: Decrease in Hydrogen Cyanide (HCN) content of cassava leaves before-after adding Sodium Bicarbonate (NaHCO3) with stirring time variations (M=Average of mass NaHCO3 (20 g); W1=15 min; W2=30 min; W3=45 min)
Table 1: Comparison of Hydrogen Cyanide (HCN) levels based on Sodium Bicarbonate (NaHCO3) mass treatment and stirring time using post-hoc test
* Data Analyze
Full-Text: (41 Views)
Reduction of the Cyanide from Cassava Leaves Using NaHCO3
N. Narwati *, S. Setiawan
Department of Environmental Health, Poltekkes Kemenkes Surabaya, Jawa Timur, Indonesia
- Cassava leaves naturally contain cyanide acid.
- Hydrogen Cyanide in cassava leaves is in high concentrations exceeding maximum permissible limits.
- Sodium Bicarbonate and stirring synergize in reducing Hydrogen Cyanide levels.
| Article type Original article |
ABSTRACT Background: Cassava leaves are one part of the cassava plant, which can be consumed. Cassava leaves used as vegetable ingredients are weak as the presence of toxic cyanogenic glycosides. The pre-processing method of cassava leaves is performed to reduce the cyanide content. The method was modified using Sodium Bicarbonate (NaHCO3) substance, capable of binding cyanide acid, with stirring intervention from the stirrer chamber. This method is believed to contribute in lowering Hydrogen Cyanide (HCN) levels in a shorter period of time. The objective of this study was to assess the effectiveness of using NaHCO3 and a stirring chamber in decreasing the cyanide content of cassava leaves. Methods: A total of 48 samples were examined, all collected in June 2023. The assessment was based on comparing the NaHCO3 mass and the stirring time. The NaHCO3 mass was adjusted to 0, 10, 20, or 30 g with stirring times of 0, 15, 30, or 45 min. Cassava leaves are separated from the stalk and thoroughly washed then sliced 2 mm thick. Identification of cassava leaf HCN levels was measured using a spectrophotometer, wavelength 500 nm. The statistical variance analysis technique (ANOVA) was employed to detect the disparity in cyanide content among the cassava leaves of the various experimental groups. Results: The results indicated the highest cassava leaf HCN levels in the 0 g NaHCO3 group and the length of stirring time was 0 min, which was 217 ppm. The lowest cassava leaf HCN content in the group with the addition of 30 g NaHCO3 treatment with 150 rpm stirring is 56 ppm, with a percentage decrease of 32.5%. Conclusion: The finding of adding NaHCO3 and stirring cassava leaves in water is regarded as an effort to reduce HCN levels to prevent the risk of food poisoning. © 2024, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Hydrogen Cyanide Cyanogenic Glycosides Manihot Sodium Bicarbonate |
||
| Article history Received: 31 Oct 2023 Revised: 5 Feb 2024 Accept: 15 May 2024 |
||
| Acronyms and abbreviations HCN=Hydrogen Cyanide NaHCO3=Sodium Bicarbonate |
Introduction
Cassava with the scientific name Manihot esculenta Crantz is a plant that easily grows in the tropical regions therefore, it is commonly observed in Indonesia. This tuber plant is extremely popular in Indonesia. Cassava serves as a source of carbohydrates. Additionally, cassava is a cultivated plant that is widely planted in several regions in Indonesia. Cassava leaves, a component of the cassava plant, are valuable for consumption, particularly as a vegetable base. Based on the easy availility and nutritional content, cassava leaves are one of the basic vegetable ingredients that Indonesian individuals depend on. Cassava leaves possess higher protein content, contain vitamin C and A, and provide several dietary fiber (Ndubuisi and Chidiebere, 2018). The nutritional content in cassava leaves consists of protein of 19.73 to 29.47%, carbohydrates between 66.1 to 72.1%, and total energy of 385.9 to 397.9 kcal/100 g (Modesto Junior et al., 2019). Conversely, cassava leaves have a weakness due to the toxic content of cyanogenic glycosides (Hawashi et al., 2019). Result of enzymatic hydrolysis by beta-glucosidase following the maceration of plant tissues as they are consumed, or by the gut microflora, cyanogenic glycosides are broken down to release Hydrogen Cyanide (HCN) which is toxic to both animals and humans (Kwok, 2008). This led to complications of cyanide toxicity such as decreased consciousness, headache, confusion, nausea, and feebleness (Mosayyebi et al., 2020).
Nyirenda (2021) clarified that each cassava variety contains cyanogenic glycosides, although in varying concentrations. The difference in cyanogenic glycoside content is indicated by the presence of HCN in cassava plant parts. The normal cyanogenic compounds were higher within the cortex (804 ppm) and cleared out (655 ppm) than in the root parenchymal (305 ppm) (Ospina et al., 2024). Hawashi et al. (2019) explained that cassava leaves contain 5 to 20 times more cyanogenic than the root and are considered highly toxic if consumed.
The acute dose of cyanide that can result in death ranges from 0.5-3.5 mg/kg body weight, whereas the chronic cyanide dose for daily consumption is 0.02 mg cyanide/kg body weight (Harenčár et al., 2021). An individual weighing 60 kg has the potential to die with a dose of 30 mg HCN. Consumption of cassava containing cyanide in an amount exceeding the body's tolerance limit will cause cyanide disorders such as cyanide poisoning, Tropical Ataxic Neuropathy (TAN), paralysis, growth retardation, and goiter/hyperthyroidism. Moreover, cyanide poisoning has symptoms of headache, nausea, shortness of breath, diarrhea, convulsions, fast pulse, and potentially lead to fatality (Nyamekye, 2021). An individual can consume cassava leaves in greater quantities in case the HCN levels in cassava leaves are minimized, initiating with the pre-processing process. This is so as cassava leaves are further processed, HCN levels can be tolerated by the body. Research has been conducted to decrease HCN levels, composing of drying, heating, and fermentation. (Ndubuisi and Chidiebere, 2018)
The process of reducing the cyanide acid in cassava leaves involved washing the pounded cassava leaves, soaking the cassava leaves, and utilizing the kinetics method during the washing process of mashed cassava leaves. Although processing methods can minimize linamarin and cyanide in food, improperly processed cassava products would contain some amount of residual linamarin and hydrogen cyanide. (Ndubuisi and Chidiebere, 2018) Previous research indicated that residual HCN levels in cassava leaves decreased as the duration of washing and the water volume ratio increased (Hawashi et al., 2019). It was reiterated by (Ojiambo et al., 2017) that the decrease in HCN levels as a result of soaking cassava was influenced by soaking time and variations of soaking. Even though the HCN content decreased by 88.45%, the issue of a lengthy washing time remains , therefore it is considered less efficient as applied to pre-processing among the community particularly housewives. Modification of the method using certain materials with stirring treatment followed by the heat method is believed to lead to a reduction of HCN levels in a relatively short time. Modification of the stirring method in soaking and heating cassava is thought to result in a diffusion process that causes HCN to dissolve in the water. Several proven and effective chemical, physical, and biological treatment processes have been developed for the removal and recovery of cyanide (Maciel et al., 2023). The rotation of the stirrer provides a frictional force that expands the surface of Sodium Bicarbonate (NaHCO3) allowing the cyanide content to dissolve more quickly in water. Meanwhile, the stirrer rotates and generates heat which is transferred through the soaking water media to accelerate the cyanide reduction process. The addition of NaHCO3 as an ingredient in the immersion water was applied in this study since it has the ability to bind the formed cyanide. The physical method in the form of stirring using a stirrer chamber has been proven by Narwati and Suryono (2019) in minimizing heavy metals, free fatty acids, and peroxide numbers. The results demonstrated that there was a reduction in the heavy metal Cadmium (Cd) with the intervention of stirring through the stirrer chamber (Narwati and Suryono, 2019) as well as a decrease in free fatty acids and peroxide numbers (Narwati et al., 2021). These findings suggest that the stirring process enhances the ability of the adsorbent to bind the adsorbate. Novita et al. (2021) explained that increased stirring improves adsorption efficiency, although time affects the process velocity. This research is based on a theoretical principle and aims to decrease cyanide acid in cassava leaves by utilizing a modified physical method. Materials and methods
Collection of samples
Cassava leaf samples were collected from traders and purchased from farms in Bogor-Indonesia in May 2023. The type of cassava utilized is kuru cassava.
Sample preparation
Cassava leaves were collected as much as 960 g, 24 treatments with two observations. Cassava leaves are separated from the stems, washed clean, and cut into 1 mm thick pieces. Twenty g of cassava leaves were weighed for each treatment of 15, 30, and 45 min of stirring and the addition of 10, 20, and 30 g of NaHCO3 (Pudak, Indonesia). Cassava leaves are put into soaking water with a volume-to-mass ratio of 20 g/100 ml of cassava leaves in a beaker. For each immersion ratio variable, stirring and adding mass of NaHCO3 were performed for 15, 30, 45 min, and 10, 20, and 30 g, respectively with a stirring speed of 150 rpm. Next, the sample is drained and placed in a sample plastic. This process was repeated 2 times for each treatment.
Sample analysis
The total cyanide content in cassava leaves and fractions was analyzed using the picrate paper kit method (Ayele et al., 2022). Picrate paper was prepared by dipping 0.3 mm thick filter paper into a 2.5% (w/v) picrate solution (Sigma-Aldrich, St. Louis, MO, USA) followed by drying in a fume hood. The dried papers were sliced into a 3×1 cm rectangle and affixed to the plastic strip measuring 5×1 cm, with a thickness of 1 mm. Linamarase was obtained by isolating enzymes through extraction followed by subsequent purification using gel filtration chromatography (Perkin Elmer 2,400 CHN/O Analyzer, USA). A sample of 0.05 g, 1 ml of 0.1 M Na-phosphate buffer (Merck, Germany), and 100 µl linamarase were combined in a vile and the strip carrying a picrate paper was inserted the vial, which was closed immediately with a screw cap. The sample and solutions in the vial were gently blended and left at 30 ◦C for 24 h. Next, the picrate paper was removed and soaked in 5 mL distilled water for 30 min. A picrate paper suspended in a vial without a sample was exploited as a blank. The standard curve for cyanide content was prepared from a series of linamarin (Sigma-Aldrich, USA) concentrations (0.2-2.4 µM). The picrate papers from the blank and the standard were processed in the identical way as the picrate papers of the samples. The absorbance of the solutions was measured at 510 nm. Phytate was examined in the samples. The extraction process consisted of adding 0.5 g of the dried sample to a 10 ml solution of 3.5% Hydrochloric Acid (HCl; Pubchem, USA). The solution was stirred for 1 h and centrifuged for 10 min at 3,000×g. The aliquot was removed from the supernatant, filled into a 2 ml tube, and centrifuged again at 10,000×g for 10 min. The preparation of Wade reagent involved combining 30 mg of Iron (III) Chloride Hexahydrate (FeC13·6H2O; Merckmillipore, Germany) and 300 mg of sulfosalicylic acid (Merck, Germany) in 100 ml of distilled water. Standard phytate solution was prepared by dissolving 2,632 mg sodium phytate (Sigma-P8810, Merck KGaA, Darmstadt, Germany) in 1 ml of distilled water (2,632 mg/ml). Distilled water (9 ml) was introduced into the solution (dilution of 1:10). A standard curve was prepared with a range of 0.0-1.0 ml and absorbance levels were measured at 500 nm using a UV-spectrophotometer (model 1800 PC, Japan).
Nyirenda (2021) clarified that each cassava variety contains cyanogenic glycosides, although in varying concentrations. The difference in cyanogenic glycoside content is indicated by the presence of HCN in cassava plant parts. The normal cyanogenic compounds were higher within the cortex (804 ppm) and cleared out (655 ppm) than in the root parenchymal (305 ppm) (Ospina et al., 2024). Hawashi et al. (2019) explained that cassava leaves contain 5 to 20 times more cyanogenic than the root and are considered highly toxic if consumed.
The acute dose of cyanide that can result in death ranges from 0.5-3.5 mg/kg body weight, whereas the chronic cyanide dose for daily consumption is 0.02 mg cyanide/kg body weight (Harenčár et al., 2021). An individual weighing 60 kg has the potential to die with a dose of 30 mg HCN. Consumption of cassava containing cyanide in an amount exceeding the body's tolerance limit will cause cyanide disorders such as cyanide poisoning, Tropical Ataxic Neuropathy (TAN), paralysis, growth retardation, and goiter/hyperthyroidism. Moreover, cyanide poisoning has symptoms of headache, nausea, shortness of breath, diarrhea, convulsions, fast pulse, and potentially lead to fatality (Nyamekye, 2021). An individual can consume cassava leaves in greater quantities in case the HCN levels in cassava leaves are minimized, initiating with the pre-processing process. This is so as cassava leaves are further processed, HCN levels can be tolerated by the body. Research has been conducted to decrease HCN levels, composing of drying, heating, and fermentation. (Ndubuisi and Chidiebere, 2018)
The process of reducing the cyanide acid in cassava leaves involved washing the pounded cassava leaves, soaking the cassava leaves, and utilizing the kinetics method during the washing process of mashed cassava leaves. Although processing methods can minimize linamarin and cyanide in food, improperly processed cassava products would contain some amount of residual linamarin and hydrogen cyanide. (Ndubuisi and Chidiebere, 2018) Previous research indicated that residual HCN levels in cassava leaves decreased as the duration of washing and the water volume ratio increased (Hawashi et al., 2019). It was reiterated by (Ojiambo et al., 2017) that the decrease in HCN levels as a result of soaking cassava was influenced by soaking time and variations of soaking. Even though the HCN content decreased by 88.45%, the issue of a lengthy washing time remains , therefore it is considered less efficient as applied to pre-processing among the community particularly housewives. Modification of the method using certain materials with stirring treatment followed by the heat method is believed to lead to a reduction of HCN levels in a relatively short time. Modification of the stirring method in soaking and heating cassava is thought to result in a diffusion process that causes HCN to dissolve in the water. Several proven and effective chemical, physical, and biological treatment processes have been developed for the removal and recovery of cyanide (Maciel et al., 2023). The rotation of the stirrer provides a frictional force that expands the surface of Sodium Bicarbonate (NaHCO3) allowing the cyanide content to dissolve more quickly in water. Meanwhile, the stirrer rotates and generates heat which is transferred through the soaking water media to accelerate the cyanide reduction process. The addition of NaHCO3 as an ingredient in the immersion water was applied in this study since it has the ability to bind the formed cyanide. The physical method in the form of stirring using a stirrer chamber has been proven by Narwati and Suryono (2019) in minimizing heavy metals, free fatty acids, and peroxide numbers. The results demonstrated that there was a reduction in the heavy metal Cadmium (Cd) with the intervention of stirring through the stirrer chamber (Narwati and Suryono, 2019) as well as a decrease in free fatty acids and peroxide numbers (Narwati et al., 2021). These findings suggest that the stirring process enhances the ability of the adsorbent to bind the adsorbate. Novita et al. (2021) explained that increased stirring improves adsorption efficiency, although time affects the process velocity. This research is based on a theoretical principle and aims to decrease cyanide acid in cassava leaves by utilizing a modified physical method. Materials and methods
Collection of samples
Cassava leaf samples were collected from traders and purchased from farms in Bogor-Indonesia in May 2023. The type of cassava utilized is kuru cassava.
Sample preparation
Cassava leaves were collected as much as 960 g, 24 treatments with two observations. Cassava leaves are separated from the stems, washed clean, and cut into 1 mm thick pieces. Twenty g of cassava leaves were weighed for each treatment of 15, 30, and 45 min of stirring and the addition of 10, 20, and 30 g of NaHCO3 (Pudak, Indonesia). Cassava leaves are put into soaking water with a volume-to-mass ratio of 20 g/100 ml of cassava leaves in a beaker. For each immersion ratio variable, stirring and adding mass of NaHCO3 were performed for 15, 30, 45 min, and 10, 20, and 30 g, respectively with a stirring speed of 150 rpm. Next, the sample is drained and placed in a sample plastic. This process was repeated 2 times for each treatment.
Sample analysis
The total cyanide content in cassava leaves and fractions was analyzed using the picrate paper kit method (Ayele et al., 2022). Picrate paper was prepared by dipping 0.3 mm thick filter paper into a 2.5% (w/v) picrate solution (Sigma-Aldrich, St. Louis, MO, USA) followed by drying in a fume hood. The dried papers were sliced into a 3×1 cm rectangle and affixed to the plastic strip measuring 5×1 cm, with a thickness of 1 mm. Linamarase was obtained by isolating enzymes through extraction followed by subsequent purification using gel filtration chromatography (Perkin Elmer 2,400 CHN/O Analyzer, USA). A sample of 0.05 g, 1 ml of 0.1 M Na-phosphate buffer (Merck, Germany), and 100 µl linamarase were combined in a vile and the strip carrying a picrate paper was inserted the vial, which was closed immediately with a screw cap. The sample and solutions in the vial were gently blended and left at 30 ◦C for 24 h. Next, the picrate paper was removed and soaked in 5 mL distilled water for 30 min. A picrate paper suspended in a vial without a sample was exploited as a blank. The standard curve for cyanide content was prepared from a series of linamarin (Sigma-Aldrich, USA) concentrations (0.2-2.4 µM). The picrate papers from the blank and the standard were processed in the identical way as the picrate papers of the samples. The absorbance of the solutions was measured at 510 nm. Phytate was examined in the samples. The extraction process consisted of adding 0.5 g of the dried sample to a 10 ml solution of 3.5% Hydrochloric Acid (HCl; Pubchem, USA). The solution was stirred for 1 h and centrifuged for 10 min at 3,000×g. The aliquot was removed from the supernatant, filled into a 2 ml tube, and centrifuged again at 10,000×g for 10 min. The preparation of Wade reagent involved combining 30 mg of Iron (III) Chloride Hexahydrate (FeC13·6H2O; Merckmillipore, Germany) and 300 mg of sulfosalicylic acid (Merck, Germany) in 100 ml of distilled water. Standard phytate solution was prepared by dissolving 2,632 mg sodium phytate (Sigma-P8810, Merck KGaA, Darmstadt, Germany) in 1 ml of distilled water (2,632 mg/ml). Distilled water (9 ml) was introduced into the solution (dilution of 1:10). A standard curve was prepared with a range of 0.0-1.0 ml and absorbance levels were measured at 500 nm using a UV-spectrophotometer (model 1800 PC, Japan).
Data analysis
Statistical analysis involved conducting analysis of variance (ANOVA) with Tukey's test to assess variations between the two groups. The value of p<0.05 is considered significant. Two-way analysis of variance was conducted which followed by the post-hoc test. Statistical analysis was performed using the SPSS application version 20.
Results
Statistical analysis involved conducting analysis of variance (ANOVA) with Tukey's test to assess variations between the two groups. The value of p<0.05 is considered significant. Two-way analysis of variance was conducted which followed by the post-hoc test. Statistical analysis was performed using the SPSS application version 20.
Results
HCN in cassava leaves in the control group
The following table presents the average HCN levels in cassava leaves without stirring, with various variations of NaHCO3 (control groups). Code K represents a control group, Mo defines a sample without NaHCO3, M1 identifies a sample to which 10 g of NaHCO3 is added, M2 represents a sample to which 20 g of NaHCO3 is added, and M3 is for a sample with 30 g of NaHCO3. The KW code indicates a control sample that was not subjected to stirring (Figure 1). The HCN levels of the control group without the addition of NaHCO3 and stirring. The sample was exclusively immersed in water with time variations of 15 (W1), 30 (W2), and 45 min (W3). The experimental results obtained the highest HCN levels in the sample group soaked in water for 15 min (KMW1), which was 183 ppm. The lowest HCN level in the 45 min immersed sample (KMW3) was 148 ppm, although the decline in the HCN rate remains higher than the specified rate.
The following table presents the average HCN levels in cassava leaves without stirring, with various variations of NaHCO3 (control groups). Code K represents a control group, Mo defines a sample without NaHCO3, M1 identifies a sample to which 10 g of NaHCO3 is added, M2 represents a sample to which 20 g of NaHCO3 is added, and M3 is for a sample with 30 g of NaHCO3. The KW code indicates a control sample that was not subjected to stirring (Figure 1). The HCN levels of the control group without the addition of NaHCO3 and stirring. The sample was exclusively immersed in water with time variations of 15 (W1), 30 (W2), and 45 min (W3). The experimental results obtained the highest HCN levels in the sample group soaked in water for 15 min (KMW1), which was 183 ppm. The lowest HCN level in the 45 min immersed sample (KMW3) was 148 ppm, although the decline in the HCN rate remains higher than the specified rate.

Figure 1: Average of Hydrogen Cyanide (HCN) levels in cassava leaves without stirring, with soaking time, after stirring, and adding average Sodium Bicarbonate (NaHCO3) 20 g
(K=Control group; Mo=0 g NaHCO3; M1=10 g NaHCO3; M2=20 g NaHCO3; M3=30 g NaHCO3; W1=15 min; W2=30 min; W3=45 min; W=Average of stirring time (22.5 min))
HCN in cassava leaves intervention group
The average HCN following treatment can be displayed in Figure 1. The highest average HCN levels in the group without NaHCO3 and stirred in water (M0W), is 216 ppm HCN. The lowest average HCN content in the group to which NaHCO3 was added was as much as 30 g stirred in water with a stirring speed of 150 rpm, which is 56 ppm HCN. These results indicate that increasing the dose of NaHCO3 added to the water-stirred sample results in decreased levels of HCN. The highest average HCN levels in the treatment group stirred in 15 min (MW1) of water, which is 165 ppm HCN. The lowest HCN level in the treatment group was stirred for 45 min (MW3), which was 133 ppm HCN. Both treatments were conducted with a speed of 150 rpm and included the addition of 20 g NaHCO3. Stirring time contributed to a decrease in HCN in each treatment group to which NaHCO3 had been added to the sample. The longer the stirring time against the sample soaked with the addition of NaHCO3 leads to the lower level of HCN.
Analyzing variations in HCN levels in cassava leaves
Decreased HCN levels of cassava leaves before and after stirring with mass NaHCO3 has shown in Figure 2. The highest percentage of reduction in HCN levels in the M3 code was 32.5% in the sample group given the additional treatment of 30 g NaHCO3. These results uncover that the addition of 30 g NaHCO3 has the potential to reduce. Decrease in HCN content of cassava leaves before-after adding NaHCO3 with stirring time variations has shown in Figure 3.
The average HCN following treatment can be displayed in Figure 1. The highest average HCN levels in the group without NaHCO3 and stirred in water (M0W), is 216 ppm HCN. The lowest average HCN content in the group to which NaHCO3 was added was as much as 30 g stirred in water with a stirring speed of 150 rpm, which is 56 ppm HCN. These results indicate that increasing the dose of NaHCO3 added to the water-stirred sample results in decreased levels of HCN. The highest average HCN levels in the treatment group stirred in 15 min (MW1) of water, which is 165 ppm HCN. The lowest HCN level in the treatment group was stirred for 45 min (MW3), which was 133 ppm HCN. Both treatments were conducted with a speed of 150 rpm and included the addition of 20 g NaHCO3. Stirring time contributed to a decrease in HCN in each treatment group to which NaHCO3 had been added to the sample. The longer the stirring time against the sample soaked with the addition of NaHCO3 leads to the lower level of HCN.
Analyzing variations in HCN levels in cassava leaves
Decreased HCN levels of cassava leaves before and after stirring with mass NaHCO3 has shown in Figure 2. The highest percentage of reduction in HCN levels in the M3 code was 32.5% in the sample group given the additional treatment of 30 g NaHCO3. These results uncover that the addition of 30 g NaHCO3 has the potential to reduce. Decrease in HCN content of cassava leaves before-after adding NaHCO3 with stirring time variations has shown in Figure 3.

Figure 2: Decreased Hydrogen Cyanide (HCN) levels of cassava leaves before and after stirring with mass Sodium Bicarbonate (NaHCO3) variations (M0=0 g NaHCO3; M1=10 g NaHCO3; M2=20 g NaHCO3; M3=30 g NaHCO3)
Figure 3: Decrease in Hydrogen Cyanide (HCN) content of cassava leaves before-after adding Sodium Bicarbonate (NaHCO3) with stirring time variations (M=Average of mass NaHCO3 (20 g); W1=15 min; W2=30 min; W3=45 min)
According to the graph, it appears that stirring time could lower HCN concentrations. Samples stirred for 30 min (MW2) displayed the most significant reduction in HCN levels, with a percentage decrease of 14.9%.
The results of the Tukey test in Table 1 analyzed the correlation between HCN content in cassava leaves and the mass of NaHCO3. The post-hoc test determined that the addition of 30 g NaHCO3 led to a significant decrease (p<0.05) in HCN. The data indicated that the HCN content diminished by 14.9% with the addition of 30 g of NaHCO3.
This research intends to examine the variations in sample stirring time and their impact on the HCN level data. The data demonstrated that the sample stirring time of 30 min was not significantly different from the stirring time of 15 min with a signification value of 0.060 Addition of NaHCO3 had a high influence on reducing HCN in cassava leaves. Increased stirring time fails to guarantee a high reduction in HCN (Table 1).
The results of the Tukey test in Table 1 analyzed the correlation between HCN content in cassava leaves and the mass of NaHCO3. The post-hoc test determined that the addition of 30 g NaHCO3 led to a significant decrease (p<0.05) in HCN. The data indicated that the HCN content diminished by 14.9% with the addition of 30 g of NaHCO3.
This research intends to examine the variations in sample stirring time and their impact on the HCN level data. The data demonstrated that the sample stirring time of 30 min was not significantly different from the stirring time of 15 min with a signification value of 0.060 Addition of NaHCO3 had a high influence on reducing HCN in cassava leaves. Increased stirring time fails to guarantee a high reduction in HCN (Table 1).
Table 1: Comparison of Hydrogen Cyanide (HCN) levels based on Sodium Bicarbonate (NaHCO3) mass treatment and stirring time using post-hoc test
| Post-hoc test | Mean difference | Sig. | |
| Control (0 g NaHCO3) | 10 g NaHCO3 | 0.2533* | 0.021 |
| 20 g NaHCO3 | 0.8450* | <0.001 | |
| 30 g NaHCO3 | 1.6000* | <0.001 | |
| Mixing time (15 min) | Mixing time (30 min) | 0.1663 | 0.060 |
Discussion
Cassava leaves in this study still contain HCN in case consumed consistently for an extended period of time. The utilized cassava leaves sourced from agricultural land in the Bogor Region containing 219.5 ppm of cyanide. The HCN content of cassava leaves originating from Bogor agricultural land was lower in this research compared to the levels found in cassava leaves from Salvaterra-Brazil as reported by Modesto Junior et al. (2019). The researchers investigated nine varieties of cassava leaves with codes M1 to M9. Three varieties, namely M1, M2, and M8, included the highest HCN as compared to the other 6 varieties, namely 425, 561, and 230 mg/kg, respectively (Modesto Junior et al., 2019). This indicates that the cassava leaves are a type of cassava that has a high content of cyanogenic glycosides. The typical level of cyanogenic glycoside content in cassava ranges from 1 to 1,300 mg per kg dry weight. In a review of other reports by him revealed that the total cyanogenic content of the roots had no correlation with the cyanogenic content in the leaves and stems of the same cassava plant (Modesto Junior et al., 2019).
Levels of cyanogenic glycoside in leaves and stems were higher than in roots. It has been reported that cassava leaves contain 53-1,300 mg per kg dry matter, while cassava roots include 10-500 mg of cyanide per kg dry matter (Amelework and Bairu, 2022). The plant harbors cyanogenic glycoside compounds. Cyanide acid is a toxic compound resulting from the hydrolysis process of cyanogenic glycoside compounds (Pratiwi et al., 2023). The hydrolysis of cyanogenic glycosides is performed by β-glucosidase to produce the appropriate cyanohydrin, which subsequently decomposes and releases HCN and aldehydes or ketones. It was further explained that the cyanogenic glycosides linamarin (α-hydroxybutyronitrile-β-d-glucopyranoside) and lotaustralin (ethyl linamarin) are distributed in cassava cell vacuoles, whereas the enzyme linamarase is present in the cell walls ( Nyirenda, 2021). Linamarin and lotaustralin found in cassava leaves are among the cyanogenic glycosides which release HCN through enzymatic hydrolysis (Modesto Junior et al., 2019). Nyamekye (2021) modified that cyanide affects individuals who frequently consume cassava over a prolonged period of time. Cyanide is readily absorbed in the body. The acute lethal dose of HCN for humans is reported to be 0.5 to 3.5 mg per kg of body weight (Agustiar et al., 2018).
The presence of cyanide in the body affects the body's cells to utilize oxygen. Cyanide will bind to key iron-containing enzymes and halt cellular respiration. Exposure to low doses of cyanide can lead to heart failure (Nyamekye, 2021). Consumption of cassava and its products high in cyanogens may cause cyanide poisoning with symptoms of vomiting, nausea, dizziness, stomach pains, weakness, headache, exacerbated goiter (Maciel et al., 2023), and diarrhea and occasionally death (Ndubuisi and Chidiebere, 2018)
Cyanide poisoning is a serious risk to human health, particularly in the tropical regions. It was further explained that cyanide inhibits oxygen utilization and enhances anaerobic metabolism which results in excess lactic acid and metabolic acidosis and ultimately causes cell death due to lack of energy (Panter, 2018). Castada et al. (2020) elaborated that non-lethal doses can lead to headaches, hyperventilation, vomiting, weakness, stomach cramps, and partial failure of the circulatory and respiratory systems. It was reiterated by Harenčár et al. (2021) if daily consumption of 20 µg/kg of cyanide can cause acute symptoms and chronic symptoms of 80 µg/kg cyanide.
Cyanide in cassava leaves is both natural and toxic which can affect human health since cyanide is extremely lethal. As cyanide poison is ingested and initiates to reach the stomach, the poison can penetrate the intestinal wall, and enter the blood vessels. Cyanide can diffuse quickly in tissues and has the potential to bind to target organs within seconds. Cyanide compounds have the ability to inactivate enzymes and destroy the structural integrity (Maciel et al., 2023).
The pre-processing of cassava leaves in this study consisted of slicing the cassava leaves to one mm in order to reduce the cyanide levels. Apart from slicing or chopping the cassava leaves, is the process continued with washing and soaking the cassava leaves in a NaHCO3 solution with stirring. In the study by Ayele et al. (2022), cassava pre-processing was performed not only to reduce HCN but also to minimize the loss of nutrients from cassava leaves. The reduction of HCN was 57% using the enumeration technique mixed at 25 ºC (Ayele et al., 2022). This indicates that the method of chopping and stirring cassava leaves in pre-processing can decrease HCN levels and minimize the loss of nutrients from cassava leaves. Andama and Oloya (2017) explained in their research that a combined method of cassava pre-processing is required to detoxify HCN before consumption.
Soaking time plays an important role in reducing HCN. As a result of this study, soaking with water without stirring results in the elevated levels of HCN, which is 148 ppm. This is presumably due to the lack of soaking time in the stirring process and without changing the soaking water. Cassava leaves which are sliced into small pieces will provide surface expansion for glucoside and glucosidase interactions to interact in liquid media. Ukonu and Lasisi (2022) described in their research that immersion in liquid media permits the extraction of cyanides that are larger to dissolve in the soaking water. Soaking for 4 h can remove 20% of cyanide. In this study, the reduction of HCN has not provided a safe limit for the consumption of cassava leaves. The duration required is typically between 15 to 45 min, resulting in a significant decrease in HCN levels, specifically above 40 ppm. Ukonu and Lasisi (2022) further explained that the decrease in cyanide was more significant if the immersion water was changed regularly within 3-5 days.
The use of NaHCO3 is a modified method to diminish HCN. An increase in the mass of NaHCO3 results in a greater reduction in HCN. Furthermore, the decrease in HCN with the addition of NaHCO3 was proven in a research by Sari et al. (2022). Cassava leaves soaked in a solution of NaHCO3 for 1 h, obtained a decrease in HCN of 22.05% with an HCN level of 41.2656 ppm. The addition of NaHCO3 causes softening of the cassava leaf tissue, making it easier for the linamarin contained in cassava leaves to decompose. It was further explained that the process of decomposing linamarin was due to the hydrolysis reaction of the linamarase enzyme. The process of soaking cassava leaves at room temperature is able to break down plant cell dissolve organic solvents, which can be adjusted for the soaking time (Sari et al., 2022). Increasing the duration of soaking required for improved results is 6-24 h (Nebiyu and Getachew, 2011). Based on this information, the suboptimal decrease in HCN in this study was suspected due to the lack of soaking time and without changing the soaking water. Therefore, it fails to give the effect of hydrolyzing linamarin in cassava leaves. The effect of immersion time in NaHCO3 solution on the reduction of HCN has been conducted by Triana and Kamila (2018). The results proved that the cyanide content decreased by 84.22% after soaking for 12 h with 20% NaHCO3 mass (Triana and Kamila, 2018). The limitation of this research remains in indicating that NaHCO3 with modified stirring does not provide a high reduction in cyanide levels. Based on the results of this research, further research is required to enhance the effectiveness of NaHCO3 by providing heating temperature interventions at certain stirring speeds. Study Modesto Junior et al. 2019 explained that cooking (70-100 °C) is an efficient process in the removal of total HCN from cassava leaf.
Levels of cyanogenic glycoside in leaves and stems were higher than in roots. It has been reported that cassava leaves contain 53-1,300 mg per kg dry matter, while cassava roots include 10-500 mg of cyanide per kg dry matter (Amelework and Bairu, 2022). The plant harbors cyanogenic glycoside compounds. Cyanide acid is a toxic compound resulting from the hydrolysis process of cyanogenic glycoside compounds (Pratiwi et al., 2023). The hydrolysis of cyanogenic glycosides is performed by β-glucosidase to produce the appropriate cyanohydrin, which subsequently decomposes and releases HCN and aldehydes or ketones. It was further explained that the cyanogenic glycosides linamarin (α-hydroxybutyronitrile-β-d-glucopyranoside) and lotaustralin (ethyl linamarin) are distributed in cassava cell vacuoles, whereas the enzyme linamarase is present in the cell walls ( Nyirenda, 2021). Linamarin and lotaustralin found in cassava leaves are among the cyanogenic glycosides which release HCN through enzymatic hydrolysis (Modesto Junior et al., 2019). Nyamekye (2021) modified that cyanide affects individuals who frequently consume cassava over a prolonged period of time. Cyanide is readily absorbed in the body. The acute lethal dose of HCN for humans is reported to be 0.5 to 3.5 mg per kg of body weight (Agustiar et al., 2018).
The presence of cyanide in the body affects the body's cells to utilize oxygen. Cyanide will bind to key iron-containing enzymes and halt cellular respiration. Exposure to low doses of cyanide can lead to heart failure (Nyamekye, 2021). Consumption of cassava and its products high in cyanogens may cause cyanide poisoning with symptoms of vomiting, nausea, dizziness, stomach pains, weakness, headache, exacerbated goiter (Maciel et al., 2023), and diarrhea and occasionally death (Ndubuisi and Chidiebere, 2018)
Cyanide poisoning is a serious risk to human health, particularly in the tropical regions. It was further explained that cyanide inhibits oxygen utilization and enhances anaerobic metabolism which results in excess lactic acid and metabolic acidosis and ultimately causes cell death due to lack of energy (Panter, 2018). Castada et al. (2020) elaborated that non-lethal doses can lead to headaches, hyperventilation, vomiting, weakness, stomach cramps, and partial failure of the circulatory and respiratory systems. It was reiterated by Harenčár et al. (2021) if daily consumption of 20 µg/kg of cyanide can cause acute symptoms and chronic symptoms of 80 µg/kg cyanide.
Cyanide in cassava leaves is both natural and toxic which can affect human health since cyanide is extremely lethal. As cyanide poison is ingested and initiates to reach the stomach, the poison can penetrate the intestinal wall, and enter the blood vessels. Cyanide can diffuse quickly in tissues and has the potential to bind to target organs within seconds. Cyanide compounds have the ability to inactivate enzymes and destroy the structural integrity (Maciel et al., 2023).
The pre-processing of cassava leaves in this study consisted of slicing the cassava leaves to one mm in order to reduce the cyanide levels. Apart from slicing or chopping the cassava leaves, is the process continued with washing and soaking the cassava leaves in a NaHCO3 solution with stirring. In the study by Ayele et al. (2022), cassava pre-processing was performed not only to reduce HCN but also to minimize the loss of nutrients from cassava leaves. The reduction of HCN was 57% using the enumeration technique mixed at 25 ºC (Ayele et al., 2022). This indicates that the method of chopping and stirring cassava leaves in pre-processing can decrease HCN levels and minimize the loss of nutrients from cassava leaves. Andama and Oloya (2017) explained in their research that a combined method of cassava pre-processing is required to detoxify HCN before consumption.
Soaking time plays an important role in reducing HCN. As a result of this study, soaking with water without stirring results in the elevated levels of HCN, which is 148 ppm. This is presumably due to the lack of soaking time in the stirring process and without changing the soaking water. Cassava leaves which are sliced into small pieces will provide surface expansion for glucoside and glucosidase interactions to interact in liquid media. Ukonu and Lasisi (2022) described in their research that immersion in liquid media permits the extraction of cyanides that are larger to dissolve in the soaking water. Soaking for 4 h can remove 20% of cyanide. In this study, the reduction of HCN has not provided a safe limit for the consumption of cassava leaves. The duration required is typically between 15 to 45 min, resulting in a significant decrease in HCN levels, specifically above 40 ppm. Ukonu and Lasisi (2022) further explained that the decrease in cyanide was more significant if the immersion water was changed regularly within 3-5 days.
The use of NaHCO3 is a modified method to diminish HCN. An increase in the mass of NaHCO3 results in a greater reduction in HCN. Furthermore, the decrease in HCN with the addition of NaHCO3 was proven in a research by Sari et al. (2022). Cassava leaves soaked in a solution of NaHCO3 for 1 h, obtained a decrease in HCN of 22.05% with an HCN level of 41.2656 ppm. The addition of NaHCO3 causes softening of the cassava leaf tissue, making it easier for the linamarin contained in cassava leaves to decompose. It was further explained that the process of decomposing linamarin was due to the hydrolysis reaction of the linamarase enzyme. The process of soaking cassava leaves at room temperature is able to break down plant cell dissolve organic solvents, which can be adjusted for the soaking time (Sari et al., 2022). Increasing the duration of soaking required for improved results is 6-24 h (Nebiyu and Getachew, 2011). Based on this information, the suboptimal decrease in HCN in this study was suspected due to the lack of soaking time and without changing the soaking water. Therefore, it fails to give the effect of hydrolyzing linamarin in cassava leaves. The effect of immersion time in NaHCO3 solution on the reduction of HCN has been conducted by Triana and Kamila (2018). The results proved that the cyanide content decreased by 84.22% after soaking for 12 h with 20% NaHCO3 mass (Triana and Kamila, 2018). The limitation of this research remains in indicating that NaHCO3 with modified stirring does not provide a high reduction in cyanide levels. Based on the results of this research, further research is required to enhance the effectiveness of NaHCO3 by providing heating temperature interventions at certain stirring speeds. Study Modesto Junior et al. 2019 explained that cooking (70-100 °C) is an efficient process in the removal of total HCN from cassava leaf.
Conclusions
The addition of NaHCO3 had a high influence on the reduction of HCN in cassava leaves. The longer stirring time does not guarantee a high reduction of HCN. The research concluded that the addition of 30 g NaHCO3 with a modified stirring time could reduce cyanide levels in cassava leaves. This research recommends the use of a pre-processing technique for cassava leaves by adding NaHCO3 with stirring to produce cassava leaves with low HCN.
The addition of NaHCO3 had a high influence on the reduction of HCN in cassava leaves. The longer stirring time does not guarantee a high reduction of HCN. The research concluded that the addition of 30 g NaHCO3 with a modified stirring time could reduce cyanide levels in cassava leaves. This research recommends the use of a pre-processing technique for cassava leaves by adding NaHCO3 with stirring to produce cassava leaves with low HCN.
Author's contributions
N.N. and S.S. designed the study, conducted the experimental work, analyzed the data, wrote the manuscript; reviewed the manuscript. Both authors read and approved the final manuscript.
Conflicts of interest
All authors declare that there was no conflict of interest regarding the publication of this article.
Acknowledgements
We express our gratitude to the ethics commission of the Health Polytechnic of the Ministry of Health in Surabaya for allowing the research to be carried out by issuing an ethical clearance certificate. We would also like to thank the Laboratory team for the Department of Environmental Health, and the Indonesian Sukodono-Sidoarjo Environmental Laboratory for supporting and facilitating the implementation of this research.
Funding
This research received internal funding from the Health Polytechnic of the Ministry of Health, Surabaya, Indonesia.
Acknowledgements
We express our gratitude to the ethics commission of the Health Polytechnic of the Ministry of Health in Surabaya for allowing the research to be carried out by issuing an ethical clearance certificate. We would also like to thank the Laboratory team for the Department of Environmental Health, and the Indonesian Sukodono-Sidoarjo Environmental Laboratory for supporting and facilitating the implementation of this research.
Funding
This research received internal funding from the Health Polytechnic of the Ministry of Health, Surabaya, Indonesia.
Ethical considerations
All necessary ethical considerations were met, and a research ethics certificate was issued by the Research Ethics Commission, Health Polytechnic, Ministry of Health, Surabaya, Indonesia. Certificate Identification Number: EA/1664/KEPK-Poltekkes_Sby/V/2023Ethical consideration No.EA/1664/KEPK-Poltekkes_Sby/V/2023.
References
Agustiar H.Y., Pato U., Ali A. (2018). Variation of water temperature in the agitation on the cyianide content and quality of rubber seed oil (Havea brasiliensis). JOMFaperta. 5: 1-14.
Amelework A.B., Bairu M.W. (2022). Advances in genetic analysis and breeding of cassava (Manihot esculenta Crantz): a review. Plants. 11: 1617. [DOI: 10.3390/plants11121617]
Andama M., Oloya B. (2017). Effectiveness of traditional processing techniques in reducing cyanide levels in selected cassava varieties in zombo district. International Journal of Food Science and Biotechnology. 2: 121-125. [DOI: 10.11648/j.ijfsb.20170204.14]
Ayele H.H., Latif S., Müller J. (2022). Influence of temperature and screw pressing on the quality of cassava leaf fractions. Agriculture. 12: 42. [DOI: 10.3390/agriculture12010042]
Castada H.Z., Liu J., Barringer S.A., Huang X. (2020). Cyanogenesis in macadamia and direct analysis of hydrogen cyanide in macadamia flowers, leaves, husks, and nuts using selected ion flow tube–mass spectrometry. Foods. 9: 174. [DOI: 10.3390/foods9020174]
Harenčár Ľ., Ražná K., Nôžková J. (2021). Cyanogenic glycosides - their role and potential in plant food resources. Journal of Microbiology, Biotechnology and Food Sciences. 11: e4771. [DOI: 10.15414/JMBFS.4771]
Hawashi M., Sitania C., Caesy C., Aparamarta H.W., Widjaja T., Gunawan S. (2019). Kinetic data of extraction of cyanide during the soaking process of cassava leaves. Data in Brief. 25: 104279. [DOI: 10.1016/j.dib.2019.104279]
Kwok J. (2008). Cyanide poisoning and cassava. Centre for Food Safety. URL: https://www.cfs.gov.hk/english/ multimedia/ multimedia_pub/multimedia_pub_fsf_29_02.html.
Maciel A.C., Da Silva Pena R., Do Nascimento L.D., De Oliveira T.A., Chagas-Junior G.C.A., Lopes A.S. (2023). Health exposure risks and bioremediation of cyanide in cassava processing effluents: an overview. Journal of Water Process Engineering. 55: 104079. [DOI: 10.1016/j.jwpe.2023.104079]
Modesto Junior E.N., Chisté R.C., Pena R.D.S. (2019). Oven drying and hot water cooking processes decrease HCN contents of cassava leaves. Food Research International. 119: 517-523. [DOI: 10.1016/j.foodres.2019.01.029]
Mosayyebi B., Imani M., Mohammadi L., Akbarzadeh A., Zarghami N., Edalati M., Alizadeh E., Rahmati M. (2020). An update on the toxicity of cyanogenic glycosides bioactive compounds: possible clinical application in targeted cancer therapy. Materials Chemistry and Physics. 246: 122841. [DOI: 10.1016/j. matchemphys. 2020.122841]
Narwati, Suryono H. (2019). Stirring chamber design development to increase the potention of chicken egg shells to decrease cadmium (Cd) level in blood cockle (Anadara granosa). Medico-Legal Update. 19: 269-274. [DOI: 10.5958/0974-1283.2019.00054.9]
Narwati N., Suryono H., Setiawan S. (2021). Potential development of chicken egg shell in recycling of waste cooking oil innovation through the stirer chamber device. Open Access Macedonian Journal of Medical Sciences. 9: 1256-1260. [DOI: 10.3889/oamjms.2021.7189]
Ndubuisi N.D., Chidiebere A.C.U. (2018). Cyanide in cassava: a review. International Journal of Genomics and Data Mining. 02: 118. [DOI: 10.29011/2577-0616.000118]
Nebiyu A., Getachew E. (2011). Soaking and drying of cassava roots reduced cyanogenic potential of three cassava varieties at Jimma, Southwest Ethiopia. African Journal of Biotechnology. 10: 13465-13469. [DOI: 10.5897/AJB10.2636]
Novita E., Nur Aeni S., Pradana H.A. (2021). Time and speed of stirring treatment in adsorptionefficiencyof coffee processing wastewater. Jurnal Keteknikan Pertanian, 9: 41-48. [DOI: 10.19028/JTEP.09.2.41-48]. [Indonesian with English abstract]
Nyamekye C.A. (2021). Health issues related to the production and consumption of cassava as a staple food. Master's thesis, Norwegian University of Life Sciences, As. URL: https://nmbu.brage.unit.no/nmbu-xmlui/bitstream/handle/ 11250/2771129/ NYAMEKYE2021. pdf?sequence=1.
Nyirenda K.K. (2021). Toxicity potential of cyanogenic glycosides in edible plants. In: Erkekoglu P., Ogawa T. (Editors). Medical toxicology. IntechOpen, London, United Kingdom. [DOI: 10.5772/intechopen.91408]
Ojiambo O.C., Nawiri M.P., Masika E. (2017). Reduction of cyanide levels in sweet cassava leaves grown in Busia county, Kenya based on different processing methods. Food Research. 1: 97-102. [DOI: 10.26656/fr.2017.3.024]
Ospina M.A., Tran T., Pizarro M., Luna J., Salazar S., Londoño L., Ceballos H., Becerra Lopez-Lavalle L.A., Dufour D. (2024). Content and distribution of cyanogenic compounds in cassava roots and leaves in association with physiological age. Journal of the Science of Food and Agriculture. 104: 4851-4859. [DOI: 10.1002/jsfa.13123]
Panter K.E. (2018). Cyanogenic glycoside-containing plants. In: Gupta R.C. (Editor). Veterinary toxicology: basic and clinical principles. 3rd edition. Academic Press, United States. pp: 935-940. [DOI: 10.1016/B978-0-12-811410-0.00064-7]
Pratiwi D., Masyrofah D., Martia E., Putri G.K., Putri T.R. (2023). Article review: analysis of cyanogenic compounds in plants. Jurnal Farmasetis. 12: 9-14. [Indonesian with English abstract]
Sari E.M., Nurfajriah S., Ramadhyan D. (2022). Comparison of cyanide compounds in cassava leaves by soaking in NaHCO3 and Ca(OH)2. Journal of Research and Education Chemistry. 4: 9-28. [DOI: 10.25299/jrec.2022.vol4(1).9332]. [Indonesian with English abstract]
Triana L., Kamila L. (2018). Analysis of cyanide acid levels in cassava soaked in 20% NaHCO3 solution with time variations. Jurnal Laboratorium Khatulistiwa. 2: 130-136. [DOI: 10.30602/jlk.v1i2.150]. [Indonesian with English abstract]
Ukonu C., Lasisi O. (2022). Health risk associated with cassava and it’s cyanogenic properties : a review of the literature. American Journal of Multidisciplinary Research in Africa. 2: 1-11. [DOI: 10.13140/RG.2.2.16068.30084]
Agustiar H.Y., Pato U., Ali A. (2018). Variation of water temperature in the agitation on the cyianide content and quality of rubber seed oil (Havea brasiliensis). JOMFaperta. 5: 1-14.
Amelework A.B., Bairu M.W. (2022). Advances in genetic analysis and breeding of cassava (Manihot esculenta Crantz): a review. Plants. 11: 1617. [DOI: 10.3390/plants11121617]
Andama M., Oloya B. (2017). Effectiveness of traditional processing techniques in reducing cyanide levels in selected cassava varieties in zombo district. International Journal of Food Science and Biotechnology. 2: 121-125. [DOI: 10.11648/j.ijfsb.20170204.14]
Ayele H.H., Latif S., Müller J. (2022). Influence of temperature and screw pressing on the quality of cassava leaf fractions. Agriculture. 12: 42. [DOI: 10.3390/agriculture12010042]
Castada H.Z., Liu J., Barringer S.A., Huang X. (2020). Cyanogenesis in macadamia and direct analysis of hydrogen cyanide in macadamia flowers, leaves, husks, and nuts using selected ion flow tube–mass spectrometry. Foods. 9: 174. [DOI: 10.3390/foods9020174]
Harenčár Ľ., Ražná K., Nôžková J. (2021). Cyanogenic glycosides - their role and potential in plant food resources. Journal of Microbiology, Biotechnology and Food Sciences. 11: e4771. [DOI: 10.15414/JMBFS.4771]
Hawashi M., Sitania C., Caesy C., Aparamarta H.W., Widjaja T., Gunawan S. (2019). Kinetic data of extraction of cyanide during the soaking process of cassava leaves. Data in Brief. 25: 104279. [DOI: 10.1016/j.dib.2019.104279]
Kwok J. (2008). Cyanide poisoning and cassava. Centre for Food Safety. URL: https://www.cfs.gov.hk/english/ multimedia/ multimedia_pub/multimedia_pub_fsf_29_02.html.
Maciel A.C., Da Silva Pena R., Do Nascimento L.D., De Oliveira T.A., Chagas-Junior G.C.A., Lopes A.S. (2023). Health exposure risks and bioremediation of cyanide in cassava processing effluents: an overview. Journal of Water Process Engineering. 55: 104079. [DOI: 10.1016/j.jwpe.2023.104079]
Modesto Junior E.N., Chisté R.C., Pena R.D.S. (2019). Oven drying and hot water cooking processes decrease HCN contents of cassava leaves. Food Research International. 119: 517-523. [DOI: 10.1016/j.foodres.2019.01.029]
Mosayyebi B., Imani M., Mohammadi L., Akbarzadeh A., Zarghami N., Edalati M., Alizadeh E., Rahmati M. (2020). An update on the toxicity of cyanogenic glycosides bioactive compounds: possible clinical application in targeted cancer therapy. Materials Chemistry and Physics. 246: 122841. [DOI: 10.1016/j. matchemphys. 2020.122841]
Narwati, Suryono H. (2019). Stirring chamber design development to increase the potention of chicken egg shells to decrease cadmium (Cd) level in blood cockle (Anadara granosa). Medico-Legal Update. 19: 269-274. [DOI: 10.5958/0974-1283.2019.00054.9]
Narwati N., Suryono H., Setiawan S. (2021). Potential development of chicken egg shell in recycling of waste cooking oil innovation through the stirer chamber device. Open Access Macedonian Journal of Medical Sciences. 9: 1256-1260. [DOI: 10.3889/oamjms.2021.7189]
Ndubuisi N.D., Chidiebere A.C.U. (2018). Cyanide in cassava: a review. International Journal of Genomics and Data Mining. 02: 118. [DOI: 10.29011/2577-0616.000118]
Nebiyu A., Getachew E. (2011). Soaking and drying of cassava roots reduced cyanogenic potential of three cassava varieties at Jimma, Southwest Ethiopia. African Journal of Biotechnology. 10: 13465-13469. [DOI: 10.5897/AJB10.2636]
Novita E., Nur Aeni S., Pradana H.A. (2021). Time and speed of stirring treatment in adsorptionefficiencyof coffee processing wastewater. Jurnal Keteknikan Pertanian, 9: 41-48. [DOI: 10.19028/JTEP.09.2.41-48]. [Indonesian with English abstract]
Nyamekye C.A. (2021). Health issues related to the production and consumption of cassava as a staple food. Master's thesis, Norwegian University of Life Sciences, As. URL: https://nmbu.brage.unit.no/nmbu-xmlui/bitstream/handle/ 11250/2771129/ NYAMEKYE2021. pdf?sequence=1.
Nyirenda K.K. (2021). Toxicity potential of cyanogenic glycosides in edible plants. In: Erkekoglu P., Ogawa T. (Editors). Medical toxicology. IntechOpen, London, United Kingdom. [DOI: 10.5772/intechopen.91408]
Ojiambo O.C., Nawiri M.P., Masika E. (2017). Reduction of cyanide levels in sweet cassava leaves grown in Busia county, Kenya based on different processing methods. Food Research. 1: 97-102. [DOI: 10.26656/fr.2017.3.024]
Ospina M.A., Tran T., Pizarro M., Luna J., Salazar S., Londoño L., Ceballos H., Becerra Lopez-Lavalle L.A., Dufour D. (2024). Content and distribution of cyanogenic compounds in cassava roots and leaves in association with physiological age. Journal of the Science of Food and Agriculture. 104: 4851-4859. [DOI: 10.1002/jsfa.13123]
Panter K.E. (2018). Cyanogenic glycoside-containing plants. In: Gupta R.C. (Editor). Veterinary toxicology: basic and clinical principles. 3rd edition. Academic Press, United States. pp: 935-940. [DOI: 10.1016/B978-0-12-811410-0.00064-7]
Pratiwi D., Masyrofah D., Martia E., Putri G.K., Putri T.R. (2023). Article review: analysis of cyanogenic compounds in plants. Jurnal Farmasetis. 12: 9-14. [Indonesian with English abstract]
Sari E.M., Nurfajriah S., Ramadhyan D. (2022). Comparison of cyanide compounds in cassava leaves by soaking in NaHCO3 and Ca(OH)2. Journal of Research and Education Chemistry. 4: 9-28. [DOI: 10.25299/jrec.2022.vol4(1).9332]. [Indonesian with English abstract]
Triana L., Kamila L. (2018). Analysis of cyanide acid levels in cassava soaked in 20% NaHCO3 solution with time variations. Jurnal Laboratorium Khatulistiwa. 2: 130-136. [DOI: 10.30602/jlk.v1i2.150]. [Indonesian with English abstract]
Ukonu C., Lasisi O. (2022). Health risk associated with cassava and it’s cyanogenic properties : a review of the literature. American Journal of Multidisciplinary Research in Africa. 2: 1-11. [DOI: 10.13140/RG.2.2.16068.30084]
* Corresponding author (N. Narwati)
* E-mail: narwati@poltekkesdepkes-sby.ac.id
ORCID ID: https://orcid.org/0000-0003-1445-3142
* E-mail: narwati@poltekkesdepkes-sby.ac.id
ORCID ID: https://orcid.org/0000-0003-1445-3142
Type of Study: Original article |
Subject:
Special
Received: 23/10/31 | Accepted: 24/05/15 | Published: 24/06/30
Received: 23/10/31 | Accepted: 24/05/15 | Published: 24/06/30
References
1. Agustiar H.Y., Pato U., Ali A. (2018). Variation of water temperature in the agitation on the cyianide content and quality of rubber seed oil (Havea brasiliensis). JOMFaperta. 5: 1-14.
2. Amelework A.B., Bairu M.W. (2022). Advances in genetic analysis and breeding of cassava (Manihot esculenta Crantz): a review. Plants. 11: 1617. [DOI: 10.3390/plants11121617] [DOI:10.3390/plants11121617] [PMID] [PMCID]
3. Andama M., Oloya B. (2017). Effectiveness of traditional processing techniques in reducing cyanide levels in selected cassava varieties in zombo district. International Journal of Food Science and Biotechnology. 2: 121-125. [DOI: 10.11648/j.ijfsb.20170204.14]
4. Ayele H.H., Latif S., Müller J. (2022). Influence of temperature and screw pressing on the quality of cassava leaf fractions. Agriculture. 12: 42. [DOI: 10.3390/agriculture12010042] [DOI:10.3390/agriculture12010042]
5. Castada H.Z., Liu J., Barringer S.A., Huang X. (2020). Cyanogenesis in macadamia and direct analysis of hydrogen cyanide in macadamia flowers, leaves, husks, and nuts using selected ion flow tube-mass spectrometry. Foods. 9: 174. [DOI: 10.3390/foods9020174] [DOI:10.3390/foods9020174] [PMID] [PMCID]
6. Harenčár Ľ., Ražná K., Nôžková J. (2021). Cyanogenic glycosides - their role and potential in plant food resources. Journal of Microbiology, Biotechnology and Food Sciences. 11: e4771. [DOI: 10.15414/JMBFS.4771] [DOI:10.15414/jmbfs.4771]
7. Hawashi M., Sitania C., Caesy C., Aparamarta H.W., Widjaja T., Gunawan S. (2019). Kinetic data of extraction of cyanide during the soaking process of cassava leaves. Data in Brief. 25: 104279. [DOI: 10.1016/j.dib.2019.104279] [DOI:10.1016/j.dib.2019.104279] [PMID] [PMCID]
8. Kwok J. (2008). Cyanide poisoning and cassava. Centre for Food Safety. URL: https://www.cfs.gov.hk/english/ multimedia/ multimedia_pub/multimedia_pub_fsf_29_02.html.
9. Maciel A.C., Da Silva Pena R., Do Nascimento L.D., De Oliveira T.A., Chagas-Junior G.C.A., Lopes A.S. (2023). Health exposure risks and bioremediation of cyanide in cassava processing effluents: an overview. Journal of Water Process Engineering. 55: 104079. [DOI: 10.1016/j.jwpe.2023.104079] [DOI:10.1016/j.jwpe.2023.104079]
10. Modesto Junior E.N., Chisté R.C., Pena R.D.S. (2019). Oven drying and hot water cooking processes decrease HCN contents of cassava leaves. Food Research International. 119: 517-523. [DOI: 10.1016/j.foodres.2019.01.029] [DOI:10.1016/j.foodres.2019.01.029] [PMID]
11. Mosayyebi B., Imani M., Mohammadi L., Akbarzadeh A., Zarghami N., Edalati M., Alizadeh E., Rahmati M. (2020). An update on the toxicity of cyanogenic glycosides bioactive compounds: possible clinical application in targeted cancer therapy. Materials Chemistry and Physics. 246: 122841. [DOI: 10.1016/j. matchemphys. 2020.122841] [DOI:10.1016/j.matchemphys.2020.122841]
12. Narwati, Suryono H. (2019). Stirring chamber design development to increase the potention of chicken egg shells to decrease cadmium (Cd) level in blood cockle (Anadara granosa). Medico-Legal Update. 19: 269-274. [DOI: 10.5958/0974-1283.2019.00054.9] [DOI:10.5958/0974-1283.2019.00054.9]
13. Narwati N., Suryono H., Setiawan S. (2021). Potential development of chicken egg shell in recycling of waste cooking oil innovation through the stirer chamber device. Open Access Macedonian Journal of Medical Sciences. 9: 1256-1260. [DOI: 10.3889/oamjms.2021.7189] [DOI:10.3889/oamjms.2021.7189]
14. Ndubuisi N.D., Chidiebere A.C.U. (2018). Cyanide in cassava: a review. International Journal of Genomics and Data Mining. 02: 118. [DOI: 10.29011/2577-0616.000118] [DOI:10.29011/2577-0616.000118]
15. Nebiyu A., Getachew E. (2011). Soaking and drying of cassava roots reduced cyanogenic potential of three cassava varieties at Jimma, Southwest Ethiopia. African Journal of Biotechnology. 10: 13465-13469. [DOI: 10.5897/AJB10.2636] [DOI:10.5897/AJB10.2636]
16. Novita E., Nur Aeni S., Pradana H.A. (2021). Time and speed of stirring treatment in adsorptionefficiencyof coffee processing wastewater. Jurnal Keteknikan Pertanian, 9: 41-48. [DOI: 10.19028/JTEP.09.2.41-48]. [Indonesian with English abstract] [DOI:10.19028/jtep.09.2.41-48]
17. Nyamekye C.A. (2021). Health issues related to the production and consumption of cassava as a staple food. Master's thesis, Norwegian University of Life Sciences, As. URL: https://nmbu.brage.unit.no/nmbu-xmlui/bitstream/handle/ 11250/2771129/ NYAMEKYE2021. pdf?sequence=1.
18. Nyirenda K.K. (2021). Toxicity potential of cyanogenic glycosides in edible plants. In: Erkekoglu P., Ogawa T. (Editors). Medical toxicology. IntechOpen, London, United Kingdom. [DOI: 10.5772/intechopen.91408] [DOI:10.5772/intechopen.91408]
19. Ojiambo O.C., Nawiri M.P., Masika E. (2017). Reduction of cyanide levels in sweet cassava leaves grown in Busia county, Kenya based on different processing methods. Food Research. 1: 97-102. [DOI: 10.26656/fr.2017.3.024] [DOI:10.26656/fr.2017.3.024]
20. Ospina M.A., Tran T., Pizarro M., Luna J., Salazar S., Londoño L., Ceballos H., Becerra Lopez-Lavalle L.A., Dufour D. (2024). Content and distribution of cyanogenic compounds in cassava roots and leaves in association with physiological age. Journal of the Science of Food and Agriculture. 104: 4851-4859. [DOI: 10.1002/jsfa.13123] [DOI:10.1002/jsfa.13123] [PMID]
21. Panter K.E. (2018). Cyanogenic glycoside-containing plants. In: Gupta R.C. (Editor). Veterinary toxicology: basic and clinical principles. 3rd edition. Academic Press, United States. pp: 935-940. [DOI: 10.1016/B978-0-12-811410-0.00064-7] [DOI:10.1016/B978-0-12-811410-0.00064-7]
22. Pratiwi D., Masyrofah D., Martia E., Putri G.K., Putri T.R. (2023). Article review: analysis of cyanogenic compounds in plants. Jurnal Farmasetis. 12: 9-14. [Indonesian with English abstract]
23. Sari E.M., Nurfajriah S., Ramadhyan D. (2022). Comparison of cyanide compounds in cassava leaves by soaking in NaHCO3 and Ca(OH)2. Journal of Research and Education Chemistry. 4: 9-28. [DOI: 10.25299/jrec.2022.vol4(1).9332]. [Indonesian with English abstract] [DOI:10.25299/jrec.2022.vol4(1).9332]
24. Triana L., Kamila L. (2018). Analysis of cyanide acid levels in cassava soaked in 20% NaHCO3 solution with time variations. Jurnal Laboratorium Khatulistiwa. 2: 130-136. [DOI: 10.30602/jlk.v1i2.150]. [Indonesian with English abstract] [DOI:10.30602/jlk.v1i2.150]
25. Ukonu C., Lasisi O. (2022). Health risk associated with cassava and it's cyanogenic properties : a review of the literature. American Journal of Multidisciplinary Research in Africa. 2: 1-11. [DOI: 10.13140/RG.2.2.16068.30084]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







