Volume 11, Issue 4 (December 2024)
J. Food Qual. Hazards Control 2024, 11(4): 291-296 |
Back to browse issues page
Ethics code: IR.TUMS.REC.1399.321
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Heidari T, Jahed Khaniki G, Sadighara P, Shariatifar N. Assessment of Toxic Histamine Contents in Processed Cheeses and the Effect of Salt and pH Levels on the Amount of Histamine. J. Food Qual. Hazards Control 2024; 11 (4) :291-296
URL: http://jfqhc.ssu.ac.ir/article-1-1137-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1137-en.html
Food Safety and Hygiene Division, Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran , ghjahed@sina.tums.ac.ir
Full-Text [PDF 511 kb]
(1770 Downloads)
| Abstract (HTML) (1224 Views)
Full-Text: (969 Views)
Assessment of Toxic Histamine Contents in Processed Cheeses and the Effect of Salt and pH Levels on the Amount of Histamine
T. Heidari, G. Jahed Khaniki [*]* , P. Sadighara, N. Shariatifar
Food Safety and Hygiene Division, Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
HIGHLIGHTS
To cite: Heidari T., Jahed Khanik G., Sadighara P., Shariatifar N. (2024). Assessment of toxic histamine contents in processed cheeses and the effect of salt and pH levels on the amount of histamine. Journal of Food Quality and Hazards Control. 11: 291-296.
Introduction
T. Heidari, G. Jahed Khaniki [*]*
Food Safety and Hygiene Division, Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- All 68 cheese samples contained histamine.
- The highest concentration of histamine was in Parmesan cheese.
- The concentration of histamine in the sample was affected by the pH value.
- Among different cheese groups, hard-ripened cheeses had more histamine.
| Article type Original article |
ABSTRACT Background: Histamine is a chemical released by the body's immune system in response to allergens, which causes messages to be sent between different cells. There is a significant amount of histamine in certain foods, such as fermented foods and alcoholic beverages. Food allergy reactions occur after consuming contaminated foods with high amounts of histamine. Due to increased consumption of processed cheeses over the past decade, this study aims to measure histamine in cheeses processed by some dairy factories in Tehran. Methods: Sixty-eight samples of four types of processed cheese (Mozzarella, Gouda, Cheddar, and Parmesan) from seven brands were randomly collected in Tehran city and transferred to the laboratory (January to March 2021). Histamine concentration was measured with a UV-Visible spectrophotometer at 600 nm. The pH and salt of the samples were also measured. Statistical analysis was done with SPSS software version 24. Results: The lowest concentration of histamine was found in Gouda soft cheese and the highest concentration was in Parmesan cheese. A significant difference was observed in the histamine concentration of different cheese groups. Histamine concentration was higher in Parmesan cheese samples, which may be due to its longer ripening period than other cheese groups. A significant relationship (p-value<0.05) was observed in the effect of pH and salt on the increase in histamine concentration. Conclusion: The average histamine in all cheese groups was higher than the limit of histamine concentration in fermented foods in the European :union: (200 mg/kg). Considering the high concentration of histamine in cheeses, it is suggested to investigate its health and safety aspects for the health of cheese consumers. © 2024, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Biogenic Amines Histamine Cheese Spectrophotometry |
||
| Article history Received: 28 Dec 2023 Revised: 03 Apr 2024 Accept: 21 Oct 2024 |
||
| Abbreviations BA=Biogenic Amine HPLC=High-Performance Liquid Chromatography |
Introduction
Biogenic Amines (BAs) are biologically active substances produced in the metabolism of animals, plants, and microorganisms, and are involved in physiological functions such as neurotransmission, blood pressure control, cell growth regulation, and allergic response (Erdag et al., 2018). These are non-volatile and heat-resistant bases with biological activity and aliphatic, aromatic, or heterocyclic structures (Tapingkae et al., 2010). These amines include: tyramine, putrescine, tryptamine, cadaverine, spermidine, 2-phenylethylamine, and histamine, which are present in various foods (Önal, 2007). Histamine and tyramine are the most toxic BAs known (Selim et al., 2023). Histamine is a heterocyclic BAs formed through oxidative decarboxylation in the presence of a catalyst (Moniente et al., 2022).
The immune response to histamine depends on the receptor and the cell type (Thangam et al., 2018). The most common symptoms of poisoning with different receptors (gastric, respiratory, etc.) include diarrhea and vomiting, heart palpitations, low blood pressure, headache, respiratory, and asthma attacks and skin rashes(Annunziata et al., 2022).
Histamine poisoning occurs when consuming foods with high histamine content, such as fish and fermented cheeses. Recently, the presence and high concentration of BAs in foods have been one of the foremost issues in food safety (Omer et al., 2021). Many Gram-positive and Gram-negative bacteria are capable of producing histamine, some of which are common food contaminants (EFSA Panel on Biological Hazards, 2011). The growth and activity of BAs-producing microorganisms in milk and cheese are affected by factors such as water activity, salt concentration, pH, and redox potential (Schirone et al., 2022). Ripe cheese is one of the most studied fermented foods, as this product is commonly associated with histamine poisoning (Muthukumar et al., 2020). Cheese is the second most consumed dairy product in Europe, North America, and the Pacific, and its per capita consumption is increasing (OECD/FAO, 2019). Unfortunately, accurate and clear information about the permissible limit of histamine or BAs concentration in cheese is not available in any domestic or international standard. Considering the high consumption of processed cheeses over the past decade, no research has been done in this field in Iran. Therefore, this research was conducted to measure the amount of histamine in processed cheeses produced and supplied by some dairy factories in Tehran.
Materials and methods
Materials
The histamine standard was purchased from Sigma (Science Park Drive, Singapore). Copper (II) sulfate hexahydrate (CuSO4(6H2O)), potassium dichromate (K2Cr2O7), silver nitrate (AgNO3), phosphate buffered saline pH 7 (PBS), and alizarin red S were purchased from Sigma-Aldrich, US. Methanol was of pure analytical grade. Whatman filter paper 52 from Merck, Darmstadt, Germany was used.
Instrumentation
A UV-visible spectrophotometer (HACH DR 5,000, Germany) was used to measure the absorption of the formed complexes (Miftakhul et al., 2019). A pH meter (7,020, Richmond, Surrey) was used to measure the sample's pH (ISIRI, 2022).
Sampling
Four types of processed cheese (n=68) were purchased from January to March 2021 (from different production dates) from supermarkets located in Tehran. The number of each type of cheese was 25 Mozzarellas, 15 Goudas, 15 Cheddar, and 13 Parmesans. All samples were transported to the laboratory under standard conditions and kept in the freezer (at -21 °C) until the test.
Reagent preparation
The reagents were prepared as follows: 0.3929 g of CuSO4.6H2O and 0.1 g of alizarin red S were dissolved in distilled water. Each reagent reached a volume of 100 ml in a volumetric flask.
Preparation of mixed solution of alizarin red S- (Cu+2)
Alizarin red S solution (0.25 ml), one ml of sulfate solution (Cu+2), and one ml of phosphate buffer solution (pH 7) were added to a 10 ml volumetric flask. Afterward, methanol was added over 15 min at room temperature up to the mark.
Preparation of mixed solution of histamine alizarin red S-(Cu+2)
Alizarin red S solution (0.25 ml) was slowly transferred to a 10 ml volumetric flask, and then (Cu+2) sulfate (one ml), histamine (1.25 ml), and phosphate buffer (one ml) solutions were added. Methanol was added over 15 min at room temperature up to the mark.
Calibration curve
Histamine (0.1 g) was weighed, and dissolved in methanol. The solution in a volumetric flask with methanol reached a volume of 100 ml. Concentrations of 100, 250, 500, 750, and 1,000 mg/kg were prepared to draw the calibration curve. The concentration of histamine in the samples was measured 10 min after preparation and complex formation with a UV-vis spectrophotometer at a wavelength of 600 nm.
Samples preparation and extraction
All test steps were performed using Jannatin et al.'s method with slight modifications based on other articles (Jannatin et al., 2017; Karami et al., 2020; Miftakhul et al., 2019). Five g of each cheese sample was weighed, finely chopped, and transferred to a 50 ml centrifuge tube, then 20 ml of methanol is added to it. Next, it was rapidly mixed for five min and then homogenized for 10 min using an ultrasonic bath (TAT, China). Then the sample (Universal 320, PIT Company, Iran) was centrifuged at 4,500 rpm for 15 min. One and twenty-five hundredths ml of the supernatant was transferred to a beaker, then one ml of CuSO4.6H2O, one ml of buffer solution, and 0.25 ml of alizarin red S were added. The spectrophotometer was zeroed each time before loading the samples with a blank (methanol) and then the absorption wavelength was read. The optimal response time was seen 10 min after preparing the solution. The absorption of the complex was measured by UV-vis spectrophotometer at 600 nm wavelength.
Measuring the amount of salt
The measurement of salt in the samples was done by Mohr's method (titration with silver nitrate).
pH measurement
According to the national standard of Iran (ISIRI, 2022), the pH value of the cheeses was determined with an electric pH meter.
Statistical analysis
In statistical analysis, the normality of data distribution was checked by the Kolmogorov-Smirnov test. Using a one-way ANOVA test, the difference in histamine concentration between different groups of cheese was examined. The Pearson correlation coefficient test was used to investigate the effect of salt and pH variables on the increase of histamine concentration in the samples. All analyses were performed in SPSS software version 24.
Results and Discussion
Cheese provides a favorable environment for the production of BAs. Histamine can produce during the production, ripening, and storage of cheese. Histamine production is mainly due to the microbial decarboxylation of the amino acid histidine or the catalysis of the decarboxylation reaction caused by decarboxylase enzymes (Fernandes et al., 2001; Halasz et al., 1994). Linear regression analysis was analyzed according to Figure 1; the linear regression equation was y=0. 0012 x+0.6291, and R2=0.9918. The recovery percentage was obtained about 96.25% for the Cu+2 complex is shown in Table 1. Histamine concentration in 68 processed cheese samples was investigated. The range of histamine levels was between 143.3-979.9 mg/kg. The highest and lowest amount of histamine was found in parmesan cheese (646.07±219.52 mg/kg) and mozzarella cheese (359.78±174.84 mg/kg), respectively. The description of primary characteristics such as the total amount, mean, and standard deviation of histamine are described separately in different types of cheese according to Table 2. The highest amount of histamine was observed in parmesan cheese (Figure 2). A supplementary one-way ANOVA test was performed to determine the significant difference between the groups. The p-value of Parmesan cheese with Mozzarella, Gouda, and Cheddar were 0, 0.014, and 0.018, respectively. No significant difference was observed between the groups except for Parmesan cheese and other cheeses. The p-value of Parmesan cheese with Mozzarella was zero and p<0.05 (Table 3).
Table 1: The recovery of method
Table 2: Histamine concentration in various type of cheese (mg/kg)
* p<0.05
Table 3: Amount of Salt and pH in various type of cheese
* p<0.05
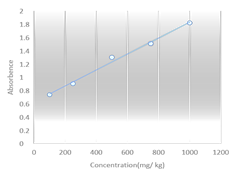
Figure 1: Calibration curve for the spectrophotometric determination of copper (II) (Cu+2)
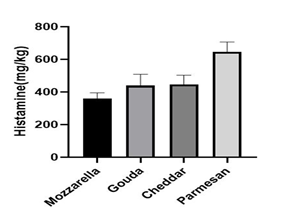
Figure 2: The amount of histamine in various type of cheese
In the present study, histamine was detected in Parmesan, Gouda, Cheddar, and Mozzarella cheeses. The average histamine level in all cheese groups was higher than the limit of histamine concentration in fermented foods in the European :union: (200 mg/kg). The European Food Safety Authority (EFSA) has declared the maximum histamine amount in fresh and hard cheeses to be about 119 and 1,240 mg/kg, respectively (EFSA Panel on Biological Hazards, 2011). In a research, common BAs in ripened cheeses were determined in Slovakia using the High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) method. Results showed that histamine was detected in 54.5% of the cheeses, but its concentration was not more than 68.7 mg/kg (in the sample of hard cheese ripened for 18 months). The lowest and highest concentrations of histamine were 9.60 and 2.1 mg/kg, respectively (Jakabová et al., 2023). These findings were in contrary to our study, where histamine was found in all samples and its amount was high in most cheeses. In our study, the highest concentration of histamine was found in Parmesan cheese (hard-ripened) which was consistent with the mentioned study.
In another study, the BAs of ripened cheeses produced in different seasons from pasteurized sheep's milk were measured by the Ultra-HPLC method. The average histamine was 204.9 mg/kg in hard cheeses and 38.3 mg/kg in semi-hard cheeses (Renes et al., 2021). In our research, the mean level of histamine was high in different groups of cheese. Histamine levels in Parmesan cheeses (as hard cheese) were higher than Cheddar and Gouda cheeses (as semi-hard). The higher concentration of histamine in semi-hard and hard cheeses is justified due to the longer ripening period.
Mayer and Fiechter (2018) measured BAs in 151 commercial cheese samples using the UHPLC. Histamine was present in 79% of the samples. Its maximum concentration was up to 116 mg/100 g in hard cheeses, but only 5% of samples had histamine levels higher than 17 mg/100 g (Mayer and Fiechter, 2018). The maximum concentration of histamine in the sample from this study was higher than in our samples, but our study had a greater number of samples with high histamine concentration.
A study was conducted in Basrah city in 2018 on nine groups of traditional and imported cheeses using the Enzyme-Linked Immunosorbent Assay (ELISA) method. The results showed that most traditional cheeses have small amounts of histamine. The average concentration of histamine in local cheese samples was 0.2725 and 23.5025 mg/kg in Arabic cheese and Dhafayer cheese, respectively. The average in six imported cheese samples from low to high was 0.6875, 1.9250, 2.5775, 4.4100, 4.7600, 6.8275, 10.5325 mg/kg, which is the highest amount related to Goody cheese imported from Saudi Arabia (Issa et al., 2018). Poveda et al. (2016) measured amino acid concentration and BAs content in goat milk cheeses using HPLC. Low concentrations of BAs were observed, the highest of which were tyramine and histamine with values between 4.2 and 50.7 and 10.2 and 60.5 mg/kg, which are considered less than dangerous for humans. Low histamine concentrations in cheeses were in contrast to the observations of our present study.
A study in Poland on samples of moldy cheese and hard cheeses was investigated using RP-HPLC. The highest concentration in Gorgonzola Piccante cheese (moldy cheese ripened for 42 days) was 730.47 mg/kg. The highest average concentration of histamine in cheeses ripened at 4 °C was 405.21 mg/kg in Camembert cheese. The concentration of histamine in some types of cheeses was higher than the toxic threshold dose (Madejska et al., 2018). The high concentration of histamine in ripened cheeses confirms the results of our study.
Razavi Rohani et al. (2013) conducted research on the presence of BA in western Iranian cheese. Eighty-five cheese samples were evaluated using a modified technique of HPLC. In Koopeh cheese, the average concentrations of putrescine, cadaverine, histamine, and tyramine were 156.09, 282.34, 70.80, and 8.48 ppm, respectively. For Liqvan cheese, these values were 53.277, 342.74, 37.58, and 351.12 ppm, and for Salmas red cheese, they were 438.03, 701.05, 105.21, and 182.62 ppm, respectively (Razavi Rohani et al., 2013). Our study aligns with other research in highlighting the potential health risks associated with high histamine levels in certain cheese samples (Madejeska et al., 2018; Razavi Rohani et al., 2013).
Based on statistical analysis, there was a significant relationship between pH and high concentration of histamine in the samples (p<0.05). Correlation was also observed between salt amounts and histamine concentration (p<0.05). The details of the measured amount of salt and pH for each sample group are described in Table 3. Histamine accumulation in cheese is influenced by the type of milk, heat treatment, ripening time, and storage conditions (Møller et al., 2020). The use of salt in cheese with high humidity content (4.7-5.7%) and ripening at low temperature can be associated with suppressing the growth of undesirable microorganisms and thus it reducing the risk of histamine formation (Guinee and Sutherland, 2011). As a result, the high concentration of histamine is found in low-salt cheeses (Møller et al., 2020). The salt level is directly related to increasing pH and can lead to a decrease in the activity of microorganisms, especially reducing lactic acid production in high salt concentration (Madadlou et al., 2007; Pastorino et al., 2003). While in the findings of the present research, the relationship between the increase of salt and the high concentration of histamine in the samples was significant.
Zhang et al. (2010) reported that the amount of BAs decreases with increasing salt concentration and it can be due to the reduction of microorganisms in fresh cheese samples. While in ripened cheese samples, increasing the brine concentration has a positive effect on BAs accumulation and it possibly leads to progressively greater enzymatic decarboxylation. Considering that our study was carried out on ripened cheeses, the positive correlation of high salt and increased histamine concentration confirms the results of Zhang et al. (2010) study. In another study, it was also noted that there is higher accumulation of histamine in saltier, wetter, and less oxidized parts of cheese, which is consistent with our findings in cheeses with higher salt contents (Moniente et al., 2022). The spectrophotometric method used in this study can be used quickly, cheaply, and effectively for monitoring in a short time, although it has lower accuracy compared to expensive and time-consuming methods such as HPLC.
Conclusion
The average histamine in all cheese groups was found higher than the limit of histamine concentration in fermented foods according to the European :union: limit. Considering the high concentration of histamine in cheeses, it is suggested to monitor histamine in cheeses and conduct further research on its health and safety implications for consumers. Additionally, there is a need for broader studies on other BAs and factors such as temperature, starter bacteria, ripening duration, etc. to address the safety concerns associated with histamine in cheese.
Author contributions
T.H., G.J.K., P.S., and N.S. designed and edited the study; T.H. conducted the experimental work, analyzed the data, and wrote the manuscript; all authors read and approved the final manuscript.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study is a part of a master's thesis in the field of food safety and hygiene at the School of Public Health with financial support from Tehran University of Medical Sciences, Tehran, Iran (Project no.1400-1-99-52389).
Funding
This study was funded by Tehran University of Medical Sciences, Tehran, Iran.
Ethical consideration
Ethical considerations were taken into account at all stages of the research. Certificate Identification Number: IR.TUMS.REC.1399.321.
References
Annunziata L., Schirone M., Campana G., De Massis M.R., Scortichini G., Visciano P. (2022). Histamine in fish and fish products: an 8-year survey. Follow up and official control activities in the Abruzzo region (Central Italy). Food Control. 133: 108651. [DOI: 10.1016/j.foodcont.2021.108651]
EFSA Panel on Biological Hazards (BIOHAZ). (2011). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA Journal. 9: 2393. [DOI: 10.2903/j.efsa.2011.2393]
Erdag D., Merhan O., Yildiz B. (2018). Biochemical and pharmacological properties of biogenic amines. Biogenic Amines. 8: 1-14. [DOI: 10.5772/intechopen.81569]
Fernandes J.O., Judas I.C., Oliveira M.B., Ferreira I.M.P.L.V.O., Ferreira M.A. (2001). A GC–MS method for quantitation of histamine and other biogenic amines in beer. Chromatography. 53: S-327–S-331.
Guinee T.P., Sutherland B.J. (2011). Cheese | salting of cheese. In: Fuquay J.W. (editor). Encyclopedia of dairy sciences. 2nd Edition. Academic Press, San Diego. pp: 595-606.
Halasz A., Barath A., Simon-sarkadi L., Holzapfel W. (1994). Biogenic amines and their production by microorganisms in food. Trends of Food Science and Technology. 5: 42-49. [DOI: 10.1016/0924-2244(94)90070-1]
Institute of Standards and Industrial Research of Iran (ISIRI). (2022). Milk and milk products- determination of titrable acidity and pH - test method. National Standard No. 2852. URL: https://standard.inso.gov.ir/StandardView.aspx?Id=57037. Accessed 8 September 2024.
Issa A.H., Saewan S.A., Abdulrahman B.A. (2018). Studying of histamine concentration and quality parameters in some local soft cheese and imported processed cheese types in basrah markets. Journal of Zankoy Sulaimani, 2nd International Conference of Agricultural Sciences. 555-560.
Jakabová S., Árvay J., Benešová L., Zajác P., Čapla J., Čurlej J., Golian J. (2023). Evaluation of biogenic amines in goat and sheep cheeses of Slovak origin. Journal of Microbiology, Biotechnology and Food Sciences. 13: 1-5. [DOI: 10.55251/jmbfs.10000]
Jannatin M., Supriyanto G., Pudjiastuti P. (2017). A novel spectrophotometric method for determination of histamine based on its complex reaction with ni (ii) and alizarin red s. Indonesian Journal of Chemistry. 17: 139-143. [DOI: 10.22146/ijc.23621]
Karami M., Alizadeh-Sani M., Sadighara P., Tajdar-Oranj B., Shariatifar N., Molaee-Aghaee E., Peivasteh-Roudsari L. (2020). Rapid determination of histamine levels in canned tuna by a novel spectrophotometric method. Journal of Food Safety and Hygiene. 6: 198-204.
Madadlou A., Khosrowshahi A., Mousavi M.E., Farmani J. (2007). The influence of brine concentration on chemical composition and texture of Iranian white cheese. Journal of Food Engineering. 81: 330-335. [DOI: 10.1016/j.jfoodeng. 2006.11.010]
Madejska A., Michalski M., Pawul-Gruba M., Osek J. (2018). Histamine content in rennet ripening cheeses during storage at different temperatures and times. Journal of Veterinary Research. 62: 65-69. [DOI: 10.1515/jvetres-2018-0009]
Mayer H. K., Fiechter G. (2018). UHPLC analysis of biogenic amines in different cheese varieties. Food Control. 93: 9-16. [DOI: 10.1016/j.foodcont.2018.05.040]
Miftakhul J., Ayu Nabila I.L., Sri W., Superiyanto G., Ibrahim W.A.W. (2019). Rapid spectrophotometric method for histamine determination in fish using alizarin red s and metal. Malaysian Journal of Analytical Sciences. 23: 505-515. [DOI: 10.17576/mjas-2019-2303-15]
Møller C.D.A., Ücok E., Rattray F. (2020). Histamine forming behaviour of bacterial isolates from aged cheese. Food Research International. 128: 108719. [DOI: 10.1016/j.foodres.2019. 108719]
Moniente M., García-Gonzalo D., Llamas-Arriba M.G., Garate J., Ontañón I., Jaureguibeitia A., Virto R., Pagán R., Botello-Morte L. (2022). The significance of cheese sampling in the determination of histamine concentration: distribution pattern of histamine in ripened cheeses. LWT. 171: 114099. [DOI: 10.1016/j.lwt.2022.114099]
Muthukumar J., Selvasekaran P., Lokanadham M., Chidambaram R. (2020). Food and food products associated with food allergy and food intolerance – an overview. Food Research International. 138: 109780. [DOI: 10.1016/j.foodres.2020.109780]
OECD/FAO. (2019). OECD-FAO agricultural outlook 2019-2028. OECD Publishing, Paris/Food and Agriculture Organization of the United Nations, Rome. [DOI: 10.1787/agr_outlook-2019-en]
Omer A.K., Mohammed R.R., Ameen P.S.M., Abas Z.A., Ekici K. (2021). Presence of biogenic amines in food and their public health implications: a review. Journal of Food Protection. 84: 1539-1548. [DOI: 10.4315/jfp-21-047]
Önal A. (2007). A review: current analytical methods for the determination of biogenic amines in foods. Food Chemistry. 103: 1475-1486. [DOI: 10.1016/j.foodchem.2006.08.028]
Pastorino A., Hansen C., Mcmahon D.J. (2003). Effect of salt on structure-function relationships of cheese. Journal of Dairy Science. 86: 60-69. [DOI: 10.3168/jds.S0022-0302(03)73584-X]
Poveda J., Molina G., Gómez-Alonso S. (2016). Variability of biogenic amine and free amino acid concentrations in regionally produced goat milk cheeses. Journal of Food Composition and Analysis. 51: 85-92. [DOI: 10.1016/j.jfca.2016.06.012]
Razavi Rohani S.M., Aliakbarlu J., Ehsani A., Hassanzadazar H. (2013). Biogenic amines determination in some traditional cheeses in West Azerbaijan province of Iran. Veterinary Research Forum. 4:115-118.
Renes E., Fernández D., Abarquero D., Ladero V., Álvarez M., Tornadijo M., Fresno J.M. (2021). Effect of forage type, season, and ripening time on selected quality properties of sheep milk cheese. Journal of Dairy Science. 104: 2539-2552. [DOI: 10.3168/jds.2020-19036]
Schirone M., Visciano P., Conte F., Paparella A. (2022). Formation of biogenic amines in the cheese production chain: favouring and hindering factors. International Dairy Journal. 133: 105420. [DOI: 10.1016/j.idairyj.2022.105420]
Selim A.M., Elsabagh Y.A., El-Sawalhi M.M., Ismail N.A., Senousy M.A. (2023). Association of integrin-β2 polymorphism and expression with the risk of rheumatoid arthritis and osteoarthritis in Egyptian patients. BMC Medical Genomics. 16: 204. [DOI: 10.1186/s12920-023-01635-3]
Tapingkae W., Tanasupawat S., Parkin K.L., Benjakul S., Visessanguan W. (2010). Degradation of histamine by extremely halophilic archaea isolated from high salt-fermented fishery products. Enzyme and Microbial Technology. 46: 92-99. [DOI: 10.1016/j.enzmictec.2009.10.011]
Thangam E.B., Jemima E.A., Singh H., Baig M.S., Khan M., Mathias C.B., Church M.K., Saluja R. (2018). The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Frontiers in Immunology. 9: 1873. [DOI: 10.3389/fimmu.2018.01873]
Zhang Y., Bi P., Hiller J.E. (2010). Climate variations and Salmonella infection in Australian subtropical and tropical regions. The Science of the Total Environment. 408: 524-530. [DOI: 10.1016/j.scitotenv.2009.10.068]
The immune response to histamine depends on the receptor and the cell type (Thangam et al., 2018). The most common symptoms of poisoning with different receptors (gastric, respiratory, etc.) include diarrhea and vomiting, heart palpitations, low blood pressure, headache, respiratory, and asthma attacks and skin rashes(Annunziata et al., 2022).
Histamine poisoning occurs when consuming foods with high histamine content, such as fish and fermented cheeses. Recently, the presence and high concentration of BAs in foods have been one of the foremost issues in food safety (Omer et al., 2021). Many Gram-positive and Gram-negative bacteria are capable of producing histamine, some of which are common food contaminants (EFSA Panel on Biological Hazards, 2011). The growth and activity of BAs-producing microorganisms in milk and cheese are affected by factors such as water activity, salt concentration, pH, and redox potential (Schirone et al., 2022). Ripe cheese is one of the most studied fermented foods, as this product is commonly associated with histamine poisoning (Muthukumar et al., 2020). Cheese is the second most consumed dairy product in Europe, North America, and the Pacific, and its per capita consumption is increasing (OECD/FAO, 2019). Unfortunately, accurate and clear information about the permissible limit of histamine or BAs concentration in cheese is not available in any domestic or international standard. Considering the high consumption of processed cheeses over the past decade, no research has been done in this field in Iran. Therefore, this research was conducted to measure the amount of histamine in processed cheeses produced and supplied by some dairy factories in Tehran.
Materials and methods
Materials
The histamine standard was purchased from Sigma (Science Park Drive, Singapore). Copper (II) sulfate hexahydrate (CuSO4(6H2O)), potassium dichromate (K2Cr2O7), silver nitrate (AgNO3), phosphate buffered saline pH 7 (PBS), and alizarin red S were purchased from Sigma-Aldrich, US. Methanol was of pure analytical grade. Whatman filter paper 52 from Merck, Darmstadt, Germany was used.
Instrumentation
A UV-visible spectrophotometer (HACH DR 5,000, Germany) was used to measure the absorption of the formed complexes (Miftakhul et al., 2019). A pH meter (7,020, Richmond, Surrey) was used to measure the sample's pH (ISIRI, 2022).
Sampling
Four types of processed cheese (n=68) were purchased from January to March 2021 (from different production dates) from supermarkets located in Tehran. The number of each type of cheese was 25 Mozzarellas, 15 Goudas, 15 Cheddar, and 13 Parmesans. All samples were transported to the laboratory under standard conditions and kept in the freezer (at -21 °C) until the test.
Reagent preparation
The reagents were prepared as follows: 0.3929 g of CuSO4.6H2O and 0.1 g of alizarin red S were dissolved in distilled water. Each reagent reached a volume of 100 ml in a volumetric flask.
Preparation of mixed solution of alizarin red S- (Cu+2)
Alizarin red S solution (0.25 ml), one ml of sulfate solution (Cu+2), and one ml of phosphate buffer solution (pH 7) were added to a 10 ml volumetric flask. Afterward, methanol was added over 15 min at room temperature up to the mark.
Preparation of mixed solution of histamine alizarin red S-(Cu+2)
Alizarin red S solution (0.25 ml) was slowly transferred to a 10 ml volumetric flask, and then (Cu+2) sulfate (one ml), histamine (1.25 ml), and phosphate buffer (one ml) solutions were added. Methanol was added over 15 min at room temperature up to the mark.
Calibration curve
Histamine (0.1 g) was weighed, and dissolved in methanol. The solution in a volumetric flask with methanol reached a volume of 100 ml. Concentrations of 100, 250, 500, 750, and 1,000 mg/kg were prepared to draw the calibration curve. The concentration of histamine in the samples was measured 10 min after preparation and complex formation with a UV-vis spectrophotometer at a wavelength of 600 nm.
Samples preparation and extraction
All test steps were performed using Jannatin et al.'s method with slight modifications based on other articles (Jannatin et al., 2017; Karami et al., 2020; Miftakhul et al., 2019). Five g of each cheese sample was weighed, finely chopped, and transferred to a 50 ml centrifuge tube, then 20 ml of methanol is added to it. Next, it was rapidly mixed for five min and then homogenized for 10 min using an ultrasonic bath (TAT, China). Then the sample (Universal 320, PIT Company, Iran) was centrifuged at 4,500 rpm for 15 min. One and twenty-five hundredths ml of the supernatant was transferred to a beaker, then one ml of CuSO4.6H2O, one ml of buffer solution, and 0.25 ml of alizarin red S were added. The spectrophotometer was zeroed each time before loading the samples with a blank (methanol) and then the absorption wavelength was read. The optimal response time was seen 10 min after preparing the solution. The absorption of the complex was measured by UV-vis spectrophotometer at 600 nm wavelength.
Measuring the amount of salt
The measurement of salt in the samples was done by Mohr's method (titration with silver nitrate).
pH measurement
According to the national standard of Iran (ISIRI, 2022), the pH value of the cheeses was determined with an electric pH meter.
Statistical analysis
In statistical analysis, the normality of data distribution was checked by the Kolmogorov-Smirnov test. Using a one-way ANOVA test, the difference in histamine concentration between different groups of cheese was examined. The Pearson correlation coefficient test was used to investigate the effect of salt and pH variables on the increase of histamine concentration in the samples. All analyses were performed in SPSS software version 24.
Results and Discussion
Cheese provides a favorable environment for the production of BAs. Histamine can produce during the production, ripening, and storage of cheese. Histamine production is mainly due to the microbial decarboxylation of the amino acid histidine or the catalysis of the decarboxylation reaction caused by decarboxylase enzymes (Fernandes et al., 2001; Halasz et al., 1994). Linear regression analysis was analyzed according to Figure 1; the linear regression equation was y=0. 0012 x+0.6291, and R2=0.9918. The recovery percentage was obtained about 96.25% for the Cu+2 complex is shown in Table 1. Histamine concentration in 68 processed cheese samples was investigated. The range of histamine levels was between 143.3-979.9 mg/kg. The highest and lowest amount of histamine was found in parmesan cheese (646.07±219.52 mg/kg) and mozzarella cheese (359.78±174.84 mg/kg), respectively. The description of primary characteristics such as the total amount, mean, and standard deviation of histamine are described separately in different types of cheese according to Table 2. The highest amount of histamine was observed in parmesan cheese (Figure 2). A supplementary one-way ANOVA test was performed to determine the significant difference between the groups. The p-value of Parmesan cheese with Mozzarella, Gouda, and Cheddar were 0, 0.014, and 0.018, respectively. No significant difference was observed between the groups except for Parmesan cheese and other cheeses. The p-value of Parmesan cheese with Mozzarella was zero and p<0.05 (Table 3).
Table 1: The recovery of method
| Actual concentrations (mg/Kg) |
Recovery concentrations (mg/Kg) |
Recovery (%) |
| 200 | 966.583 | 105.94 |
| 300 | 850.750 | 93.24 |
| 400 | 817.417 | 89.59 |
Table 2: Histamine concentration in various type of cheese (mg/kg)
| Cheese type | N | Mean±SD | Min | Max |
| Mozzarella | 25 | 359.78±174.84 | 144.92 | 692.42 |
| Gouda | 15 | 439.69±267.47 | 143.25 | 978.25 |
| Cheddar | 15 | 446.42±220.94 | 174.08 | 814.08 |
| Parmesan | 13 | 646.07±219.52 * | 402.42 | 979.92 |
| Total | 68 | 472.99±220.69 | 143.25 | 979.92 |
Table 3: Amount of Salt and pH in various type of cheese
| Type of cheeses | Salt (%) | pH | ||||
| Mean | Min | Max | Mean | Min | Max | |
| Mozzarella | 0.10 | 0.03 | 0.23 | 5.9 | 5 | 7.1 |
| Gouda | 0.15 | 0.05 | 0.30 | 5.6 | 5.2 | 6.3 |
| Cheddar | 0.14 | 0.08 | 0.24 | 5.5 | 5.0 | 6.2 |
| Parmesan | 0.20 * | 0.06 | 0.46 | 5.8 * | 5.4 | 6.2 |

Figure 1: Calibration curve for the spectrophotometric determination of copper (II) (Cu+2)

Figure 2: The amount of histamine in various type of cheese
In the present study, histamine was detected in Parmesan, Gouda, Cheddar, and Mozzarella cheeses. The average histamine level in all cheese groups was higher than the limit of histamine concentration in fermented foods in the European :union: (200 mg/kg). The European Food Safety Authority (EFSA) has declared the maximum histamine amount in fresh and hard cheeses to be about 119 and 1,240 mg/kg, respectively (EFSA Panel on Biological Hazards, 2011). In a research, common BAs in ripened cheeses were determined in Slovakia using the High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) method. Results showed that histamine was detected in 54.5% of the cheeses, but its concentration was not more than 68.7 mg/kg (in the sample of hard cheese ripened for 18 months). The lowest and highest concentrations of histamine were 9.60 and 2.1 mg/kg, respectively (Jakabová et al., 2023). These findings were in contrary to our study, where histamine was found in all samples and its amount was high in most cheeses. In our study, the highest concentration of histamine was found in Parmesan cheese (hard-ripened) which was consistent with the mentioned study.
In another study, the BAs of ripened cheeses produced in different seasons from pasteurized sheep's milk were measured by the Ultra-HPLC method. The average histamine was 204.9 mg/kg in hard cheeses and 38.3 mg/kg in semi-hard cheeses (Renes et al., 2021). In our research, the mean level of histamine was high in different groups of cheese. Histamine levels in Parmesan cheeses (as hard cheese) were higher than Cheddar and Gouda cheeses (as semi-hard). The higher concentration of histamine in semi-hard and hard cheeses is justified due to the longer ripening period.
Mayer and Fiechter (2018) measured BAs in 151 commercial cheese samples using the UHPLC. Histamine was present in 79% of the samples. Its maximum concentration was up to 116 mg/100 g in hard cheeses, but only 5% of samples had histamine levels higher than 17 mg/100 g (Mayer and Fiechter, 2018). The maximum concentration of histamine in the sample from this study was higher than in our samples, but our study had a greater number of samples with high histamine concentration.
A study was conducted in Basrah city in 2018 on nine groups of traditional and imported cheeses using the Enzyme-Linked Immunosorbent Assay (ELISA) method. The results showed that most traditional cheeses have small amounts of histamine. The average concentration of histamine in local cheese samples was 0.2725 and 23.5025 mg/kg in Arabic cheese and Dhafayer cheese, respectively. The average in six imported cheese samples from low to high was 0.6875, 1.9250, 2.5775, 4.4100, 4.7600, 6.8275, 10.5325 mg/kg, which is the highest amount related to Goody cheese imported from Saudi Arabia (Issa et al., 2018). Poveda et al. (2016) measured amino acid concentration and BAs content in goat milk cheeses using HPLC. Low concentrations of BAs were observed, the highest of which were tyramine and histamine with values between 4.2 and 50.7 and 10.2 and 60.5 mg/kg, which are considered less than dangerous for humans. Low histamine concentrations in cheeses were in contrast to the observations of our present study.
A study in Poland on samples of moldy cheese and hard cheeses was investigated using RP-HPLC. The highest concentration in Gorgonzola Piccante cheese (moldy cheese ripened for 42 days) was 730.47 mg/kg. The highest average concentration of histamine in cheeses ripened at 4 °C was 405.21 mg/kg in Camembert cheese. The concentration of histamine in some types of cheeses was higher than the toxic threshold dose (Madejska et al., 2018). The high concentration of histamine in ripened cheeses confirms the results of our study.
Razavi Rohani et al. (2013) conducted research on the presence of BA in western Iranian cheese. Eighty-five cheese samples were evaluated using a modified technique of HPLC. In Koopeh cheese, the average concentrations of putrescine, cadaverine, histamine, and tyramine were 156.09, 282.34, 70.80, and 8.48 ppm, respectively. For Liqvan cheese, these values were 53.277, 342.74, 37.58, and 351.12 ppm, and for Salmas red cheese, they were 438.03, 701.05, 105.21, and 182.62 ppm, respectively (Razavi Rohani et al., 2013). Our study aligns with other research in highlighting the potential health risks associated with high histamine levels in certain cheese samples (Madejeska et al., 2018; Razavi Rohani et al., 2013).
Based on statistical analysis, there was a significant relationship between pH and high concentration of histamine in the samples (p<0.05). Correlation was also observed between salt amounts and histamine concentration (p<0.05). The details of the measured amount of salt and pH for each sample group are described in Table 3. Histamine accumulation in cheese is influenced by the type of milk, heat treatment, ripening time, and storage conditions (Møller et al., 2020). The use of salt in cheese with high humidity content (4.7-5.7%) and ripening at low temperature can be associated with suppressing the growth of undesirable microorganisms and thus it reducing the risk of histamine formation (Guinee and Sutherland, 2011). As a result, the high concentration of histamine is found in low-salt cheeses (Møller et al., 2020). The salt level is directly related to increasing pH and can lead to a decrease in the activity of microorganisms, especially reducing lactic acid production in high salt concentration (Madadlou et al., 2007; Pastorino et al., 2003). While in the findings of the present research, the relationship between the increase of salt and the high concentration of histamine in the samples was significant.
Zhang et al. (2010) reported that the amount of BAs decreases with increasing salt concentration and it can be due to the reduction of microorganisms in fresh cheese samples. While in ripened cheese samples, increasing the brine concentration has a positive effect on BAs accumulation and it possibly leads to progressively greater enzymatic decarboxylation. Considering that our study was carried out on ripened cheeses, the positive correlation of high salt and increased histamine concentration confirms the results of Zhang et al. (2010) study. In another study, it was also noted that there is higher accumulation of histamine in saltier, wetter, and less oxidized parts of cheese, which is consistent with our findings in cheeses with higher salt contents (Moniente et al., 2022). The spectrophotometric method used in this study can be used quickly, cheaply, and effectively for monitoring in a short time, although it has lower accuracy compared to expensive and time-consuming methods such as HPLC.
Conclusion
The average histamine in all cheese groups was found higher than the limit of histamine concentration in fermented foods according to the European :union: limit. Considering the high concentration of histamine in cheeses, it is suggested to monitor histamine in cheeses and conduct further research on its health and safety implications for consumers. Additionally, there is a need for broader studies on other BAs and factors such as temperature, starter bacteria, ripening duration, etc. to address the safety concerns associated with histamine in cheese.
Author contributions
T.H., G.J.K., P.S., and N.S. designed and edited the study; T.H. conducted the experimental work, analyzed the data, and wrote the manuscript; all authors read and approved the final manuscript.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study is a part of a master's thesis in the field of food safety and hygiene at the School of Public Health with financial support from Tehran University of Medical Sciences, Tehran, Iran (Project no.1400-1-99-52389).
Funding
This study was funded by Tehran University of Medical Sciences, Tehran, Iran.
Ethical consideration
Ethical considerations were taken into account at all stages of the research. Certificate Identification Number: IR.TUMS.REC.1399.321.
References
Annunziata L., Schirone M., Campana G., De Massis M.R., Scortichini G., Visciano P. (2022). Histamine in fish and fish products: an 8-year survey. Follow up and official control activities in the Abruzzo region (Central Italy). Food Control. 133: 108651. [DOI: 10.1016/j.foodcont.2021.108651]
EFSA Panel on Biological Hazards (BIOHAZ). (2011). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA Journal. 9: 2393. [DOI: 10.2903/j.efsa.2011.2393]
Erdag D., Merhan O., Yildiz B. (2018). Biochemical and pharmacological properties of biogenic amines. Biogenic Amines. 8: 1-14. [DOI: 10.5772/intechopen.81569]
Fernandes J.O., Judas I.C., Oliveira M.B., Ferreira I.M.P.L.V.O., Ferreira M.A. (2001). A GC–MS method for quantitation of histamine and other biogenic amines in beer. Chromatography. 53: S-327–S-331.
Guinee T.P., Sutherland B.J. (2011). Cheese | salting of cheese. In: Fuquay J.W. (editor). Encyclopedia of dairy sciences. 2nd Edition. Academic Press, San Diego. pp: 595-606.
Halasz A., Barath A., Simon-sarkadi L., Holzapfel W. (1994). Biogenic amines and their production by microorganisms in food. Trends of Food Science and Technology. 5: 42-49. [DOI: 10.1016/0924-2244(94)90070-1]
Institute of Standards and Industrial Research of Iran (ISIRI). (2022). Milk and milk products- determination of titrable acidity and pH - test method. National Standard No. 2852. URL: https://standard.inso.gov.ir/StandardView.aspx?Id=57037. Accessed 8 September 2024.
Issa A.H., Saewan S.A., Abdulrahman B.A. (2018). Studying of histamine concentration and quality parameters in some local soft cheese and imported processed cheese types in basrah markets. Journal of Zankoy Sulaimani, 2nd International Conference of Agricultural Sciences. 555-560.
Jakabová S., Árvay J., Benešová L., Zajác P., Čapla J., Čurlej J., Golian J. (2023). Evaluation of biogenic amines in goat and sheep cheeses of Slovak origin. Journal of Microbiology, Biotechnology and Food Sciences. 13: 1-5. [DOI: 10.55251/jmbfs.10000]
Jannatin M., Supriyanto G., Pudjiastuti P. (2017). A novel spectrophotometric method for determination of histamine based on its complex reaction with ni (ii) and alizarin red s. Indonesian Journal of Chemistry. 17: 139-143. [DOI: 10.22146/ijc.23621]
Karami M., Alizadeh-Sani M., Sadighara P., Tajdar-Oranj B., Shariatifar N., Molaee-Aghaee E., Peivasteh-Roudsari L. (2020). Rapid determination of histamine levels in canned tuna by a novel spectrophotometric method. Journal of Food Safety and Hygiene. 6: 198-204.
Madadlou A., Khosrowshahi A., Mousavi M.E., Farmani J. (2007). The influence of brine concentration on chemical composition and texture of Iranian white cheese. Journal of Food Engineering. 81: 330-335. [DOI: 10.1016/j.jfoodeng. 2006.11.010]
Madejska A., Michalski M., Pawul-Gruba M., Osek J. (2018). Histamine content in rennet ripening cheeses during storage at different temperatures and times. Journal of Veterinary Research. 62: 65-69. [DOI: 10.1515/jvetres-2018-0009]
Mayer H. K., Fiechter G. (2018). UHPLC analysis of biogenic amines in different cheese varieties. Food Control. 93: 9-16. [DOI: 10.1016/j.foodcont.2018.05.040]
Miftakhul J., Ayu Nabila I.L., Sri W., Superiyanto G., Ibrahim W.A.W. (2019). Rapid spectrophotometric method for histamine determination in fish using alizarin red s and metal. Malaysian Journal of Analytical Sciences. 23: 505-515. [DOI: 10.17576/mjas-2019-2303-15]
Møller C.D.A., Ücok E., Rattray F. (2020). Histamine forming behaviour of bacterial isolates from aged cheese. Food Research International. 128: 108719. [DOI: 10.1016/j.foodres.2019. 108719]
Moniente M., García-Gonzalo D., Llamas-Arriba M.G., Garate J., Ontañón I., Jaureguibeitia A., Virto R., Pagán R., Botello-Morte L. (2022). The significance of cheese sampling in the determination of histamine concentration: distribution pattern of histamine in ripened cheeses. LWT. 171: 114099. [DOI: 10.1016/j.lwt.2022.114099]
Muthukumar J., Selvasekaran P., Lokanadham M., Chidambaram R. (2020). Food and food products associated with food allergy and food intolerance – an overview. Food Research International. 138: 109780. [DOI: 10.1016/j.foodres.2020.109780]
OECD/FAO. (2019). OECD-FAO agricultural outlook 2019-2028. OECD Publishing, Paris/Food and Agriculture Organization of the United Nations, Rome. [DOI: 10.1787/agr_outlook-2019-en]
Omer A.K., Mohammed R.R., Ameen P.S.M., Abas Z.A., Ekici K. (2021). Presence of biogenic amines in food and their public health implications: a review. Journal of Food Protection. 84: 1539-1548. [DOI: 10.4315/jfp-21-047]
Önal A. (2007). A review: current analytical methods for the determination of biogenic amines in foods. Food Chemistry. 103: 1475-1486. [DOI: 10.1016/j.foodchem.2006.08.028]
Pastorino A., Hansen C., Mcmahon D.J. (2003). Effect of salt on structure-function relationships of cheese. Journal of Dairy Science. 86: 60-69. [DOI: 10.3168/jds.S0022-0302(03)73584-X]
Poveda J., Molina G., Gómez-Alonso S. (2016). Variability of biogenic amine and free amino acid concentrations in regionally produced goat milk cheeses. Journal of Food Composition and Analysis. 51: 85-92. [DOI: 10.1016/j.jfca.2016.06.012]
Razavi Rohani S.M., Aliakbarlu J., Ehsani A., Hassanzadazar H. (2013). Biogenic amines determination in some traditional cheeses in West Azerbaijan province of Iran. Veterinary Research Forum. 4:115-118.
Renes E., Fernández D., Abarquero D., Ladero V., Álvarez M., Tornadijo M., Fresno J.M. (2021). Effect of forage type, season, and ripening time on selected quality properties of sheep milk cheese. Journal of Dairy Science. 104: 2539-2552. [DOI: 10.3168/jds.2020-19036]
Schirone M., Visciano P., Conte F., Paparella A. (2022). Formation of biogenic amines in the cheese production chain: favouring and hindering factors. International Dairy Journal. 133: 105420. [DOI: 10.1016/j.idairyj.2022.105420]
Selim A.M., Elsabagh Y.A., El-Sawalhi M.M., Ismail N.A., Senousy M.A. (2023). Association of integrin-β2 polymorphism and expression with the risk of rheumatoid arthritis and osteoarthritis in Egyptian patients. BMC Medical Genomics. 16: 204. [DOI: 10.1186/s12920-023-01635-3]
Tapingkae W., Tanasupawat S., Parkin K.L., Benjakul S., Visessanguan W. (2010). Degradation of histamine by extremely halophilic archaea isolated from high salt-fermented fishery products. Enzyme and Microbial Technology. 46: 92-99. [DOI: 10.1016/j.enzmictec.2009.10.011]
Thangam E.B., Jemima E.A., Singh H., Baig M.S., Khan M., Mathias C.B., Church M.K., Saluja R. (2018). The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Frontiers in Immunology. 9: 1873. [DOI: 10.3389/fimmu.2018.01873]
Zhang Y., Bi P., Hiller J.E. (2010). Climate variations and Salmonella infection in Australian subtropical and tropical regions. The Science of the Total Environment. 408: 524-530. [DOI: 10.1016/j.scitotenv.2009.10.068]
* Corresponding author (G. Jahed Khaniki)
* E-mail: ghjahed@sina.tums.ac.ir
ORCID ID: https://orcid.org/0000-0001-9983-4838
* E-mail: ghjahed@sina.tums.ac.ir
ORCID ID: https://orcid.org/0000-0001-9983-4838
Type of Study: Original article |
Subject:
Special
Received: 23/12/28 | Accepted: 24/10/21 | Published: 24/12/30
Received: 23/12/28 | Accepted: 24/10/21 | Published: 24/12/30
References
1. Annunziata L., Schirone M., Campana G., De Massis M.R., Scortichini G., Visciano P. (2022). Histamine in fish and fish products: an 8-year survey. Follow up and official control activities in the Abruzzo region (Central Italy). Food Control. 133: 108651. [DOI: 10.1016/j.foodcont.2021.108651] [DOI:10.1016/j.foodcont.2021.108651]
2. EFSA Panel on Biological Hazards (BIOHAZ). (2011). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA Journal. 9: 2393. [DOI: 10.2903/j.efsa.2011.2393] [DOI:10.2903/j.efsa.2011.2393]
3. Erdag D., Merhan O., Yildiz B. (2018). Biochemical and pharmacological properties of biogenic amines. Biogenic Amines. 8: 1-14. [DOI: 10.5772/intechopen.81569] [DOI:10.5772/intechopen.81569]
4. Fernandes J.O., Judas I.C., Oliveira M.B., Ferreira I.M.P.L.V.O., Ferreira M.A. (2001). A GC-MS method for quantitation of histamine and other biogenic amines in beer. Chromatography. 53: S-327-S-331. [DOI:10.1007/BF02490351]
5. Guinee T.P., Sutherland B.J. (2011). Cheese | salting of cheese. In: Fuquay J.W. (editor). Encyclopedia of dairy sciences. 2nd Edition. Academic Press, San Diego. pp: 595-606. [DOI:10.1016/B978-0-12-374407-4.00074-1]
6. Halasz A., Barath A., Simon-sarkadi L., Holzapfel W. (1994). Biogenic amines and their production by microorganisms in food. Trends of Food Science and Technology. 5: 42-49. [DOI: 10.1016/0924-2244(94)90070-1] [DOI:10.1016/0924-2244(94)90070-1]
7. Institute of Standards and Industrial Research of Iran (ISIRI). (2022). Milk and milk products- determination of titrable acidity and pH - test method. National Standard No. 2852. URL: https://standard.inso.gov.ir/StandardView.aspx?Id=57037. Accessed 8 September 2024.
8. Issa A.H., Saewan S.A., Abdulrahman B.A. (2018). Studying of histamine concentration and quality parameters in some local soft cheese and imported processed cheese types in basrah markets. Journal of Zankoy Sulaimani, 2nd International Conference of Agricultural Sciences. 555-560. [DOI:10.17656/jzs.10702]
9. Jakabová S., Árvay J., Benešová L., Zajác P., Čapla J., Čurlej J., Golian J. (2023). Evaluation of biogenic amines in goat and sheep cheeses of Slovak origin. Journal of Microbiology, Biotechnology and Food Sciences. 13: 1-5. [DOI: 10.55251/jmbfs.10000] [DOI:10.55251/jmbfs.10000]
10. Jannatin M., Supriyanto G., Pudjiastuti P. (2017). A novel spectrophotometric method for determination of histamine based on its complex reaction with ni (ii) and alizarin red s. Indonesian Journal of Chemistry. 17: 139-143. [DOI: 10.22146/ijc.23621] [DOI:10.22146/ijc.23621]
11. Karami M., Alizadeh-Sani M., Sadighara P., Tajdar-Oranj B., Shariatifar N., Molaee-Aghaee E., Peivasteh-Roudsari L. (2020). Rapid determination of histamine levels in canned tuna by a novel spectrophotometric method. Journal of Food Safety and Hygiene. 6: 198-204. [DOI:10.18502/jfsh.v6i4.7565]
12. Madadlou A., Khosrowshahi A., Mousavi M.E., Farmani J. (2007). The influence of brine concentration on chemical composition and texture of Iranian white cheese. Journal of Food Engineering. 81: 330-335. [DOI: 10.1016/j.jfoodeng. 2006.11.010] [DOI:10.1016/j.jfoodeng.2006.11.010]
13. Madejska A., Michalski M., Pawul-Gruba M., Osek J. (2018). Histamine content in rennet ripening cheeses during storage at different temperatures and times. Journal of Veterinary Research. 62: 65-69. [DOI: 10.1515/jvetres-2018-0009] [DOI:10.2478/jvetres-2018-0009] [PMID] [PMCID]
14. Mayer H. K., Fiechter G. (2018). UHPLC analysis of biogenic amines in different cheese varieties. Food Control. 93: 9-16. [DOI: 10.1016/j.foodcont.2018.05.040] [DOI:10.1016/j.foodcont.2018.05.040]
15. Miftakhul J., Ayu Nabila I.L., Sri W., Superiyanto G., Ibrahim W.A.W. (2019). Rapid spectrophotometric method for histamine determination in fish using alizarin red s and metal. Malaysian Journal of Analytical Sciences. 23: 505-515. [DOI: 10.17576/mjas-2019-2303-15] [DOI:10.17576/mjas-2019-2303-15]
16. Møller C.D.A., Ücok E., Rattray F. (2020). Histamine forming behaviour of bacterial isolates from aged cheese. Food Research International. 128: 108719. [DOI: 10.1016/j.foodres.2019. 108719] [DOI:10.1016/j.foodres.2019.108719] [PMID]
17. Moniente M., García-Gonzalo D., Llamas-Arriba M.G., Garate J., Ontañón I., Jaureguibeitia A., Virto R., Pagán R., Botello-Morte L. (2022). The significance of cheese sampling in the determination of histamine concentration: distribution pattern of histamine in ripened cheeses. LWT. 171: 114099. [DOI: 10.1016/j.lwt.2022.114099] [DOI:10.1016/j.lwt.2022.114099]
18. Muthukumar J., Selvasekaran P., Lokanadham M., Chidambaram R. (2020). Food and food products associated with food allergy and food intolerance - an overview. Food Research International. 138: 109780. [DOI: 10.1016/j.foodres.2020.109780] [DOI:10.1016/j.foodres.2020.109780] [PMID]
19. OECD/FAO. (2019). OECD-FAO agricultural outlook 2019-2028. OECD Publishing, Paris/Food and Agriculture Organization of the United Nations, Rome. [DOI: 10.1787/agr_outlook-2019-en] [DOI:10.1787/agr_outlook-2019-en]
20. Omer A.K., Mohammed R.R., Ameen P.S.M., Abas Z.A., Ekici K. (2021). Presence of biogenic amines in food and their public health implications: a review. Journal of Food Protection. 84: 1539-1548. [DOI: 10.4315/jfp-21-047] [DOI:10.4315/JFP-21-047] [PMID]
21. Önal A. (2007). A review: current analytical methods for the determination of biogenic amines in foods. Food Chemistry. 103: 1475-1486. [DOI: 10.1016/j.foodchem.2006.08.028] [DOI:10.1016/j.foodchem.2006.08.028]
22. Pastorino A., Hansen C., Mcmahon D.J. (2003). Effect of salt on structure-function relationships of cheese. Journal of Dairy Science. 86: 60-69. [DOI: 10.3168/jds.S0022-0302(03)73584-X] [DOI:10.3168/jds.S0022-0302(03)73584-X] [PMID]
23. Poveda J., Molina G., Gómez-Alonso S. (2016). Variability of biogenic amine and free amino acid concentrations in regionally produced goat milk cheeses. Journal of Food Composition and Analysis. 51: 85-92. [DOI: 10.1016/j.jfca.2016.06.012] [DOI:10.1016/j.jfca.2016.06.012]
24. Razavi Rohani S.M., Aliakbarlu J., Ehsani A., Hassanzadazar H. (2013). Biogenic amines determination in some traditional cheeses in West Azerbaijan province of Iran. Veterinary Research Forum. 4:115-118.
25. Renes E., Fernández D., Abarquero D., Ladero V., Álvarez M., Tornadijo M., Fresno J.M. (2021). Effect of forage type, season, and ripening time on selected quality properties of sheep milk cheese. Journal of Dairy Science. 104: 2539-2552. [DOI: 10.3168/jds.2020-19036] [DOI:10.3168/jds.2020-19036] [PMID]
26. Schirone M., Visciano P., Conte F., Paparella A. (2022). Formation of biogenic amines in the cheese production chain: favouring and hindering factors. International Dairy Journal. 133: 105420. [DOI: 10.1016/j.idairyj.2022.105420] [DOI:10.1016/j.idairyj.2022.105420]
27. Selim A.M., Elsabagh Y.A., El-Sawalhi M.M., Ismail N.A., Senousy M.A. (2023). Association of integrin-β2 polymorphism and expression with the risk of rheumatoid arthritis and osteoarthritis in Egyptian patients. BMC Medical Genomics. 16: 204. [DOI: 10.1186/s12920-023-01635-3] [DOI:10.1186/s12920-023-01635-3] [PMID] [PMCID]
28. Tapingkae W., Tanasupawat S., Parkin K.L., Benjakul S., Visessanguan W. (2010). Degradation of histamine by extremely halophilic archaea isolated from high salt-fermented fishery products. Enzyme and Microbial Technology. 46: 92-99. [DOI: 10.1016/j.enzmictec.2009.10.011] [DOI:10.1016/j.enzmictec.2009.10.011]
29. Thangam E.B., Jemima E.A., Singh H., Baig M.S., Khan M., Mathias C.B., Church M.K., Saluja R. (2018). The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Frontiers in Immunology. 9: 1873. [DOI: 10.3389/fimmu.2018.01873] [DOI:10.3389/fimmu.2018.01873] [PMID] [PMCID]
30. Zhang Y., Bi P., Hiller J.E. (2010). Climate variations and Salmonella infection in Australian subtropical and tropical regions. The Science of the Total Environment. 408: 524-530. [DOI: 10.1016/j.scitotenv.2009.10.068] [DOI:10.1016/j.scitotenv.2009.10.068] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







