Volume 11, Issue 1 (March 2024)
J. Food Qual. Hazards Control 2024, 11(1): 59-68 |
Back to browse issues page
Ethics code: (NASRI) grant R&D_2022/17
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mato L, Damani Z, Spahiu J, Halimi E, Seiti B, Topi D. High Prevalence of Mycotoxigenic Fungi and Aflatoxin B1 Contamination in Corn and Wheat Grains Grown to Albania: Implications for Food Safety. J. Food Qual. Hazards Control 2024; 11 (1) :59-68
URL: http://jfqhc.ssu.ac.ir/article-1-1165-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1165-en.html
University of Tirana, Natural Sciences Faculty, Chemistry Department, Blvd. Zog 1, Tirana, 1,016, Albania , dritan.topi@unitir.edu.al
Full-Text [PDF 785 kb]
(1219 Downloads)
| Abstract (HTML) (1446 Views)
 (2)
(2)
Table 1: The mycological contamination of wheat and corn samples a Number of positive samples/ Incidence(%).
b Arithmetic mean/median of positive samples (×103 CFU/g).
c Maximum level observed (×103 CFU/g).
d Total count of positive samples on a specific genus.
CFU=Colony Forming Unit
Table 2: Aflatoxin B1 (AFB1) in maize and wheat from the harvesting year 2022 (Mean±SD)
Table 3: Aflatoxin B1 (AFB1) incidence and risk assessment concerning corn application as food Maximum Residue Level (MRL) (5 μg/kg) and feed MRL (20 μg/kg) (EC, 2023)
Table 4: Estimating Daily Intake (EDI) of adult Albanians to Aflatoxin B1 (AFB1) from wheat and corn consumption
EDImax calculated according to the maximum concentration in maize or wheat samples.
α  =0.05, we reject the null hypothesis and accept the alternative hypothesis in both cases (p1=0.0001 and p2=0.0001). Also, after comparing two independent samples, 1 and 2, we again reject the null hypothesis for the equality of the two means, as the p-value is smaller than the significance level (p=0.001). While, regarding the amount of AFB1 in wheat, the results are the opposite of corn. So, after performing a t-test in two samples with size n=61 elements, as the p-value is greater than the significance level
=0.05, we reject the null hypothesis and accept the alternative hypothesis in both cases (p1=0.0001 and p2=0.0001). Also, after comparing two independent samples, 1 and 2, we again reject the null hypothesis for the equality of the two means, as the p-value is smaller than the significance level (p=0.001). While, regarding the amount of AFB1 in wheat, the results are the opposite of corn. So, after performing a t-test in two samples with size n=61 elements, as the p-value is greater than the significance level α  =0.05, we can't reject the null hypothesis in both cases (p1=0.092 and p2=0.091). Also, after comparing two independent samples, 1 and 2, again, we can't reject the null hypothesis for the equality of the two means, as the p-value is greater than the significance level (p=0.098).
=0.05, we can't reject the null hypothesis in both cases (p1=0.092 and p2=0.091). Also, after comparing two independent samples, 1 and 2, again, we can't reject the null hypothesis for the equality of the two means, as the p-value is greater than the significance level (p=0.098).
Cereals constitute an essential part of Albanians' daily consumption of approx. 350 g grain and grain-based products, wheat comprises 445 g, and corn 5 g (Héraud et al., 2013). Considering mean values of AFB1 in wheat and corn during this harvesting year, it was found that the highest exposure in adult Albanians originated from corn consumption, EDI, 1.411 ng/kgbw/day in comparison with wheat consumption of 0.086 ng/kgbw/day. Meanwhile, considering the maximum AFB1 levels detected in corn and wheat, these values demonstrate that consumption has a higher risk for adults, 5.650 and 2.304 ng/kgbw/day, respectively. This value is triple higher as compared to data from the literature (Udovicki et al., 2021). This investigation indicates that a more detailed consumption survey is required to perform.
Conclusions
This paper analyzes mycotoxigenic contamination in corn and wheat harvested in Albania in 2022. The AFB1 values indicate that the contamination rate of corn commodities is a substantial issue. None of the wheat samples exceeded the MRL set by the European :union:. In contrast, 41.18% of corn samples were higher than the MRL of 5 μg/kg intended for use as food, and 32.25% exceeded the MRL of 20 μg/kg as well. The research reveals a small occurrence of AFB1 contamination in the wheat grain. The study provides substantial data for the assessment of hazards. Measuring the frequency of AFB1, the central AF of interest, includes health institutions and organizations with information regarding the likelihood of adverse health consequences for consumers. Regulatory authorities can employ these data to prioritize surveillance of mycotoxin contamination in the nation's main crops and recommend appropriate measures based on the identified risks. They must collaborate with farmers to implement appropriate agricultural practices, storage techniques, and other preventive measures to prevent crop contamination.
Additionally, this partnership should encompass the formulation of suggestions. Regulatory bodies must effectively address growing concerns, particularly in the ever-changing food industry, due to the impacts of climate change. The EDI indicates that the adult population's exposure mainly originates from wheat while identifying higher values than data from neighboring countries.
Author Contributions
L.M. and J.S. worked on formal analysis and validation methods; L.M. wrote the manuscript; D.T. and Z.D. designed the study, manuscript review, and editing; B.S. and E.H. engaged in resources, data curation, and visualization; D.T. funding acquisition and involved in supervision. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This study received financial support from the Albanian National Agency for Scientific Research and Innovation (NASRI) grant R&D_2022/17.
Ethical Consideration
The authors conducted this study according to the Code of Ethics approved by the Senate of the University of Tirana. It is part of the first author's Ph.D. thesis.
Funding
This study received financial support from the Albanian National Agency for Scientific Research and Innovation (NASRI) grant R&D_2022/17.
Full-Text: (9 Views)
High Prevalence of Mycotoxigenic Fungi and Aflatoxin B1 Contamination in Corn and Wheat Grains Grown to Albania: Implications for Food Safety
L. Mato 1, Z. Damani 2, J. Spahiu 3, E. Halimi 4, B. Seiti 1, D. Topi 1**
1. University of Tirana, Natural Sciences Faculty, Chemistry Department, Blvd. Zog 1, Tirana, 1,016, Albania
2. Medicine University of Tirana, Medical & Technical Sciences Faculty, Diagnostics and Rehabilitation Department, QSU “Nënë Tereza"; 'Kongresi i Manastirit' str., Tirana, Albania
3. Food and Veterinary Agency, Zona Industriale, Fushë Kosovë, 1,000 Pristine, Kosovo
4. University of Tirana, Natural Sciences Museum, ‘Petro Nini Luarasi’ str., Tirana, Albania
HIGHLIGHTS
L. Mato 1, Z. Damani 2, J. Spahiu 3, E. Halimi 4, B. Seiti 1, D. Topi 1**
1. University of Tirana, Natural Sciences Faculty, Chemistry Department, Blvd. Zog 1, Tirana, 1,016, Albania
2. Medicine University of Tirana, Medical & Technical Sciences Faculty, Diagnostics and Rehabilitation Department, QSU “Nënë Tereza"; 'Kongresi i Manastirit' str., Tirana, Albania
3. Food and Veterinary Agency, Zona Industriale, Fushë Kosovë, 1,000 Pristine, Kosovo
4. University of Tirana, Natural Sciences Museum, ‘Petro Nini Luarasi’ str., Tirana, Albania
- Fungi of five genera, Alternaria, Aspergillus, Cladosporium, Fusarium, and Penicillium, were isolated in corn and wheat from Albania.
- The most abundant resulted the Penicillium (77.89%), followed by Fusarium (74.73%) and Aspergillus genera (72.63%).
- The maximum Aflatoxin B1 level in corn was found to be 69.12 μg/kg, with an incidence of 88.23%; in contrast, wheat, 0.402 μg/kg, and incidence of 4.91%.
- Referring to the corn consumed as food, 41.18% of samples exceeded the European :union: Maximum Residue Level (5 μg/kg), and intended as feed, 32.25% exceeded the European :union: Maximum Residue Level (20 μg/kg).
- Estimated Daily Intake indicates that Aflatoxin B1 chronic exposure from contaminated corn is considerably higher than wheat consumption by Albanians.
| Article type Original article |
ABSTRACT Background: Today, mycotoxins are considered critical contaminants in foodstuffs produced by fungi, highlighting the importance of food safety to human health. The toxigenic fungi invasion and mycotoxin production are highly variable and depend on climate, plant, and agronomic practices. Among these, Aflatoxins (AFs) are considered the most potent toxins. This study investigated the fungi presence and AFB1 contamination in corn and wheat grown in Albania during the 2022 harvesting year. Methods: Wheat samples were collected during the summer, while corn during the autumn, and further analyzed. Mycological contamination assessment applied the Verband Deutscher Landëirtschaftlicher Untersuchungs ̶ und Forschungsanstalten (VDLUFA) procedures. The AFB1 levels were measured using the Enzyme-Linked Immunosorbent Assay (ELISA) method. The MATLAB R2016b software was applied to perform statistical analysis. The Estimated Daily Intake on AFB1 was calculated to evaluate human exposure. Results: The genera Alternaria, Aspergillus, Cladosporium, Fusarium, and Penicillium were isolated, with higher rates of contamination in corn and the highest frequency Penicillium genus (77.89%). The Korça region presented a higher fungal load, 104 Colony Forming Unit (CFU)/g in corn. The AFB1 incidence (88.23%) in corn, was significantly higher than in wheat (4.91%). Additionally, the maximum level in corn was found 69.120 μg/kg, while in wheat, only 0.402 μg/kg. None of the wheat samples, in contrast to the 41.18% of corn samples, exceeded the threshold when referring to the respective Maximum Residue Levels. Conclusions: Our observation indicates a higher rate of AFB1 contamination in corn than in wheat. The high concentration levels and contamination incidence in corn require targeted interventions to reduce the AFB1 amounts. Strengthened regulation based on scientific evidence can reduce contamination outbreaks, economic implications, and potential benefits, such as increased consumer trust. Our study indicates that the exposure to AFB1 originates from corn consumption among the adult population. © 2024, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Food Safety Zea Mays Triticum Aflatoxin B1 Enzyme-Linked Immunosorbent Assay |
||
| Article history Received: 6 Nov 2023 Revised: 13 Jan 2024 Accepted: 20 Mar 2024 |
||
| Acronyms and abbreviations AF=Aflatoxin CFU= Colony Forming Unit EDI=Estimated Daily Intake ELISA=Enzyme-Linked Immunosorbent Assay MRL=Maximum Residue Level |
To cite: Mato L., Damani Z., Spahiu J., Halimi E., Seiti B., Topi D. (2024). High prevalence of mycotoxigenic fungi and aflatoxin B1 contamination in corn and wheat grains grown to Albania: implications for food safety. Journal of Food Quality and Hazards Control. 11: 59-68.
Introduction
Introduction
Trade globalization has emphasized the significance of food and feed safety issues for human and animal health, respectively (Bricher, 2010). Monitoring food contaminants and implementing safety standards are essential tasks carried out worldwide. Consumers are at a higher risk of exposure to food contaminants, including mycotoxins, particularly in developing countries compared to developed countries, due to differences in food safety standards (Nazhand et al., 2020; Patial et al., 2018). Aflatoxins (AFs), Fumonisins (FBs), Deoxynivalenol (DON), Ochratoxin A (OTA), and Zearalenone (ZEN) are the mycotoxins that are most frequently detected. The primary mycotoxin-producing fungi usually belong to the Aspergillus, Fusarium, and Penicillium genera (Luo et al., 2021).
Out of all the identified mycotoxins, AFs are the most toxic and carcinogenic naturally occurring compounds. Among 20 compounds described so far as AFs, AFB1, AFB2, AFG1, and AFG2 are known to be the most potent toxicants, posing a higher threat to human health (IARC, 2012). The main aflatoxigenic-producing species, Aspergillus flavus and A. parasiticus, are observed in peanuts, oilseeds, maize, soybeans, and wheat (Liu and Wu, 2010). Manifested only in tropical areas in the past, they presented food safety concerns only at a regional level; nowadays, the AFs contamination is regarded as a global health problem (Alameri et al., 2023; Groopman and Wogan, 2016). Between 2011 and 2021, AFs were involved in 95% of notifications and border rejections, according to the Rapid Alert System for Food and Feed (RASFF) (Owolabi et al., 2023). High temperatures, drought, water activity (aw), storage conditions, and concurrent microbiota affect grains degree of mycotoxin contamination (Pitt et al., 2013).
Fungal contamination can occur from pre- to post-harvest stages. In stored grain, the incidence and prevalence of mycotoxigenic fungi are influenced by the grain type, environmental conditions, and biological factors. Temperature and aw are the main ecological factors affecting fungi and mycotoxin levels in stored grains (Mannaa and Kim, 2017). The Hazard Analysis and Critical Control Points (HACCP) approach is applied as a prevention strategy for mycotoxin contamination in the case of grains (Chulze, 2010). AFs mitigation in the post-harvest stage includes physical methods involving the application of sorting, dehulling, wet milling, dry milling, heat treatment, and irradiation; chemical methods contain chemical agents such as adsorbents, acids, and bases; microbiological methods involve intervention with microbiological agents; finally, the methods of genetic engineering rely on the regulatory mechanism of AFs biosynthesis in the A. flavus strain (Mahato et al., 2019).
AFs can cause severe public health issues as potent mutagens, immunosuppressants, liver toxins, and carcinogens (Eaton et al., 2018; Magnussen and Parsi, 2013). Exposure to AFB1, in particular, is linked to liver cancer in humans due to its hepatocarcinogenic and hepatotoxic properties (CONTAM et al., 2020). It has been categorized as a Group 1 carcinogenic toxin (IARC, 2012). Reports indicate that Afs can accumulate in various organs, including the kidneys, lungs, heart, and brain (Eaton et al., 2018; Liu and Wu, 2010). Depending on their chemical structure, they display varying degrees of carcinogenicity, mutagenicity, and toxicity, with AFB1 and AFG1 being the most harmful. The order of toxicity and carcinogenicity of AFs is AFB1>G1>B2>G2 (IARC, 2012; JECFA, 2018).
The corn (Zea mays L.) and wheat (Triticum aestivum L.) cultivation has been one of Albania's most important agricultural activities. The country's favorable climate and fertile soil allow for high yields of maize and wheat grains. Climate change is expected to impact the security of staple crops. The Mediterranean basin, including Albania, will inevitably be affected (Leggieri et al., 2021). Global CO2 emissions, rising temperatures, and drought in Europe have influenced crop yields and AFs contamination (Perrone et al., 2020).
AFs contamination in corn is a worldwide concern, and recently in southern Europe, with A. flavus being the primary species responsible. There exists limited information regarding AFs crop contamination in Albania, belonging to this region, except a study conducted in 2014-2015 uncovered high levels of contamination in maize, ranging from 0.32 to 3,550 µg/kg (Topi et al., 2023). This study aimed to comprehensively examine the fungi and mycotoxin contamination in corn and wheat crops grown in Albania, taking into account the various factors that may contribute to the presence of these harmful substances. Investigating the presence and levels of mycotoxins in these crops is essential to comprehend the potential health risks associated with their consumption in Albanians.
Materials and methods
Chemicals and reagents
The solvents, Methanol (MeOH) and Acetonitrile (AcCN) of High-Performance Liquid Chromatography (HPLC)-grade were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water was produced by the Sartorius Arius Mini purification system (Sartorius Italy S.r.l., Varedo, Italy). Microbiological terrains, malt extract, agar, yeast extract, glucose, C, oxytetracycline, and rose bengal were purchased from Sigma-Aldrich (St. Louis, MO, USA). The AFB1 stock solution standard was purchased by RomerLab (Viena, Austria). Enzyme-Linked Immunosorbent Assay (ELISA) was performed using MaxSignal® AFB1 test kit was purchased from PerkinElmer (Waltham, Massachusetts, USA).
Grain sample collection
Corn (68) and wheat (61) samples were collected throughout the primary producing districts of the nation throughout the 2022 harvesting year. The wheat samples were obtained during the summer, particularly June-July, while the corn sample collection was in the autumn, specifically September-October, to Durrësi, Elbasan, Fieri, and Kavaja. Meanwhile, sampling in the Korça region was proceeded in November due to differences in climate. Located in the western part of the country, Durrësi, Elbasan, Fieri, and Kavaja belong to the identical plateau, alongside the Adriatic sea, a substantial water body of the Mediterranean sea, situated at a very low altitude Above Sea Level (ASL) and characterized by a typical subtropical climate. In contrast, a typical continental climate characterizes the Korça district in the eastern region, at a high altitude of 850 m ASL. The EU regulation 2023/915 sampling procedures were applied during the sample collection to ensure randomization and representativeness (EC, 2023). The samples were then submitted to the Laboratory of Toxic Substances and Biomolecules of the Department of Chemistry for analysis. A random sampling strategy was employed to ensure representation from different geographic regions. A total of 60 farms were randomly selected, collecting samples for each crop commodity. The selection process involved identifying factors such as farm size and crop type. This approach allowed for a comprehensive perception of the overall fungal population while minimizing bias in the sample collection process. Ultimately, the samples were stored in dark conditions under low humidity and at 4 oC until mycological and analytical analyses were completed.
For each sample, 1,000 g of grain was milled using a Perten Lab Mill 120 (PerkinElmer, MA, USA). Finally, a representative flour sample (100 g) was placed in a plastic jar in a dark and dry place at 4 °C (FAO, 2004).
Mycological analysis
Fungi isolation and identification were performed
using the Verband Deutscher Landëirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) procedures (VDLUFA, 2007a, b, c). To begin with, a 20 g sample was ground and added to 180 ml of peptone/water (0.5%). The mixture was then diluted to final concentrations of 10-2, 10-3, and 10-4. Next, aliquots of 1 ml were taken from each dilution and spread onto parallel plates on a solid medium surface consisting of deionized water (1,000 ml), malt extract (40 g), agar (12 g), yeast extract (2 g), glucose (2 g), marlophen 810 (1 ml), oxytetracycline (60 mg), and Bengal rose (60 mg). The inoculated petri dishes were incubated for three days at 25 °C, kept in a dark and standard atmosphere, and stored at room temperature. The entire process, including the initial incubation period, took around five days. Eventually, the colonies were counted, and the results were expressed as a mean of the Colony Forming Unit (CFU) in thousands per g of sample (103 CFU/g) using equation (1) (VDLUFA, 2007a).
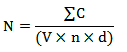 (1)
(1)
Out of all the identified mycotoxins, AFs are the most toxic and carcinogenic naturally occurring compounds. Among 20 compounds described so far as AFs, AFB1, AFB2, AFG1, and AFG2 are known to be the most potent toxicants, posing a higher threat to human health (IARC, 2012). The main aflatoxigenic-producing species, Aspergillus flavus and A. parasiticus, are observed in peanuts, oilseeds, maize, soybeans, and wheat (Liu and Wu, 2010). Manifested only in tropical areas in the past, they presented food safety concerns only at a regional level; nowadays, the AFs contamination is regarded as a global health problem (Alameri et al., 2023; Groopman and Wogan, 2016). Between 2011 and 2021, AFs were involved in 95% of notifications and border rejections, according to the Rapid Alert System for Food and Feed (RASFF) (Owolabi et al., 2023). High temperatures, drought, water activity (aw), storage conditions, and concurrent microbiota affect grains degree of mycotoxin contamination (Pitt et al., 2013).
Fungal contamination can occur from pre- to post-harvest stages. In stored grain, the incidence and prevalence of mycotoxigenic fungi are influenced by the grain type, environmental conditions, and biological factors. Temperature and aw are the main ecological factors affecting fungi and mycotoxin levels in stored grains (Mannaa and Kim, 2017). The Hazard Analysis and Critical Control Points (HACCP) approach is applied as a prevention strategy for mycotoxin contamination in the case of grains (Chulze, 2010). AFs mitigation in the post-harvest stage includes physical methods involving the application of sorting, dehulling, wet milling, dry milling, heat treatment, and irradiation; chemical methods contain chemical agents such as adsorbents, acids, and bases; microbiological methods involve intervention with microbiological agents; finally, the methods of genetic engineering rely on the regulatory mechanism of AFs biosynthesis in the A. flavus strain (Mahato et al., 2019).
AFs can cause severe public health issues as potent mutagens, immunosuppressants, liver toxins, and carcinogens (Eaton et al., 2018; Magnussen and Parsi, 2013). Exposure to AFB1, in particular, is linked to liver cancer in humans due to its hepatocarcinogenic and hepatotoxic properties (CONTAM et al., 2020). It has been categorized as a Group 1 carcinogenic toxin (IARC, 2012). Reports indicate that Afs can accumulate in various organs, including the kidneys, lungs, heart, and brain (Eaton et al., 2018; Liu and Wu, 2010). Depending on their chemical structure, they display varying degrees of carcinogenicity, mutagenicity, and toxicity, with AFB1 and AFG1 being the most harmful. The order of toxicity and carcinogenicity of AFs is AFB1>G1>B2>G2 (IARC, 2012; JECFA, 2018).
The corn (Zea mays L.) and wheat (Triticum aestivum L.) cultivation has been one of Albania's most important agricultural activities. The country's favorable climate and fertile soil allow for high yields of maize and wheat grains. Climate change is expected to impact the security of staple crops. The Mediterranean basin, including Albania, will inevitably be affected (Leggieri et al., 2021). Global CO2 emissions, rising temperatures, and drought in Europe have influenced crop yields and AFs contamination (Perrone et al., 2020).
AFs contamination in corn is a worldwide concern, and recently in southern Europe, with A. flavus being the primary species responsible. There exists limited information regarding AFs crop contamination in Albania, belonging to this region, except a study conducted in 2014-2015 uncovered high levels of contamination in maize, ranging from 0.32 to 3,550 µg/kg (Topi et al., 2023). This study aimed to comprehensively examine the fungi and mycotoxin contamination in corn and wheat crops grown in Albania, taking into account the various factors that may contribute to the presence of these harmful substances. Investigating the presence and levels of mycotoxins in these crops is essential to comprehend the potential health risks associated with their consumption in Albanians.
Materials and methods
Chemicals and reagents
The solvents, Methanol (MeOH) and Acetonitrile (AcCN) of High-Performance Liquid Chromatography (HPLC)-grade were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water was produced by the Sartorius Arius Mini purification system (Sartorius Italy S.r.l., Varedo, Italy). Microbiological terrains, malt extract, agar, yeast extract, glucose, C, oxytetracycline, and rose bengal were purchased from Sigma-Aldrich (St. Louis, MO, USA). The AFB1 stock solution standard was purchased by RomerLab (Viena, Austria). Enzyme-Linked Immunosorbent Assay (ELISA) was performed using MaxSignal® AFB1 test kit was purchased from PerkinElmer (Waltham, Massachusetts, USA).
Grain sample collection
Corn (68) and wheat (61) samples were collected throughout the primary producing districts of the nation throughout the 2022 harvesting year. The wheat samples were obtained during the summer, particularly June-July, while the corn sample collection was in the autumn, specifically September-October, to Durrësi, Elbasan, Fieri, and Kavaja. Meanwhile, sampling in the Korça region was proceeded in November due to differences in climate. Located in the western part of the country, Durrësi, Elbasan, Fieri, and Kavaja belong to the identical plateau, alongside the Adriatic sea, a substantial water body of the Mediterranean sea, situated at a very low altitude Above Sea Level (ASL) and characterized by a typical subtropical climate. In contrast, a typical continental climate characterizes the Korça district in the eastern region, at a high altitude of 850 m ASL. The EU regulation 2023/915 sampling procedures were applied during the sample collection to ensure randomization and representativeness (EC, 2023). The samples were then submitted to the Laboratory of Toxic Substances and Biomolecules of the Department of Chemistry for analysis. A random sampling strategy was employed to ensure representation from different geographic regions. A total of 60 farms were randomly selected, collecting samples for each crop commodity. The selection process involved identifying factors such as farm size and crop type. This approach allowed for a comprehensive perception of the overall fungal population while minimizing bias in the sample collection process. Ultimately, the samples were stored in dark conditions under low humidity and at 4 oC until mycological and analytical analyses were completed.
For each sample, 1,000 g of grain was milled using a Perten Lab Mill 120 (PerkinElmer, MA, USA). Finally, a representative flour sample (100 g) was placed in a plastic jar in a dark and dry place at 4 °C (FAO, 2004).
Mycological analysis
Fungi isolation and identification were performed
using the Verband Deutscher Landëirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) procedures (VDLUFA, 2007a, b, c). To begin with, a 20 g sample was ground and added to 180 ml of peptone/water (0.5%). The mixture was then diluted to final concentrations of 10-2, 10-3, and 10-4. Next, aliquots of 1 ml were taken from each dilution and spread onto parallel plates on a solid medium surface consisting of deionized water (1,000 ml), malt extract (40 g), agar (12 g), yeast extract (2 g), glucose (2 g), marlophen 810 (1 ml), oxytetracycline (60 mg), and Bengal rose (60 mg). The inoculated petri dishes were incubated for three days at 25 °C, kept in a dark and standard atmosphere, and stored at room temperature. The entire process, including the initial incubation period, took around five days. Eventually, the colonies were counted, and the results were expressed as a mean of the Colony Forming Unit (CFU) in thousands per g of sample (103 CFU/g) using equation (1) (VDLUFA, 2007a).
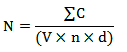 (1)
(1)Where: N: number of CFU/g of sample; ΣC: the sum of colon counts on the count plate; V: volume of the dilution transferred into the counting plate, measured in ml; n: number of count plates that may be assessed; and d: the dilution factor.
AFB1 analysis
The ELISA screening method for AFB1 was conducted following the MaxSignal® AFB1 (#1055-04) of PerkinElmer (Waltham, MA, United States) kit manufacturer instructions was applied to extract wheat and maize samples. In brief, 5.0 g of ground material was placed into a 50 ml conical test tube, adding 25.0 ml of MeOH:H2O (70:30 v/v). The mixture was shaken for 10 min using a rack fixed in an orbital shaker IKA Rocker 2D basic (Staufen, Germany). The solution was centrifuged at room temperature at 2,000×g for 10 min in Hettich centrifuge Universal 320R (Tuttlingen, Germany). Subsequently, 300 μl of the acquired supernatant was transferred to a 2 ml tube filled with 900 μl extraction solution C (MeOH/extraction phosphate buffer, 7:23 v/v). Manual vortex was performed to sample for 1 min at maximum speed in Vortex 2, IKA (Steufen, Germany).
The AFB1 analysis was conducted using the MaxSignal® AFB1 Kit, which utilizes a competitive ELISA method. This kit includes 96-well microtiter plates that have been sensitized with a monoclonal antibody that targets explicitly AFB1. Ultimately, 50 μl of each AFB1 standard solution (0.1, 1.0, 5.0, 10, 20, 50, 100 ng/ml), arranged in ascending order of concentration, together with the diluted supernatant, were added twice to the wells of the microtiter plate. Afterward, 100 μl of AFB1-Horseradish Peroxidase conjugate (HRP) was introduced into each plate well. The plate was agitated for 1 min and placed in an environment with ambient temperature for 30 min. Following incubation, the microtiter well plate was fully emptied, rinsed three times with 250 ml of the 1× wash solution during each rinse, and dried by tapping on a layer of paper towel many times. The unconjugated substance was eliminated during the washing process. After agitating a 100 μl solution of Tetramethylbenzidine (TMB) substrate, for 1 min, was left to react at room temperature for 15 min. To stop the reaction, 100 μl of the enzyme reaction inhibition buffer (stop solution) was added, and the absorbance was measured at 450 nm using a TECAN Microplate reader (Infinite 200 Pro, Nanoquant, Vienna, Austria).
Quality assurance
The analytical methods were validated based on linearity, Limit of Detection (LOD), Limit of Quantification (LOQ), and percentage of recovery according to the screening and confirmatory methods set by Regulation EC/401/2006 (EC, 2006). For Certified Reference Materials (CRM), the validation parameters were expressed as the sum of 3 replicates for each spiking level.
Health risk assessment
Classified as a Group 1 carcinogenic toxin, AFB1 induce Hepatocellular Carcinoma (HCC) as the main human adverse health effect (IARC, 2012). Risk prediction of developing AFB1-induced HCC is based on the Estimation of Daily Intake (EDI) on a chronic basis. In this paper, the AFB1 exposure in the adult population was assessed by using the daily wheat and corn consumption data extracted by the World Health Organization/Global Environment Monitoring System (WHO/GEMS). Based on each data, the daily consumption of grain and grain-based products was calculated at 350 g/day, with wheat and corn at 345 and 4.9 g, respectively. The EDI values were expressed as ng/kg body weight (bw)/day, referring to 60 kg as adult body weight in equation 2 (CONTAM, 2020).
AFB1 analysis
The ELISA screening method for AFB1 was conducted following the MaxSignal® AFB1 (#1055-04) of PerkinElmer (Waltham, MA, United States) kit manufacturer instructions was applied to extract wheat and maize samples. In brief, 5.0 g of ground material was placed into a 50 ml conical test tube, adding 25.0 ml of MeOH:H2O (70:30 v/v). The mixture was shaken for 10 min using a rack fixed in an orbital shaker IKA Rocker 2D basic (Staufen, Germany). The solution was centrifuged at room temperature at 2,000×g for 10 min in Hettich centrifuge Universal 320R (Tuttlingen, Germany). Subsequently, 300 μl of the acquired supernatant was transferred to a 2 ml tube filled with 900 μl extraction solution C (MeOH/extraction phosphate buffer, 7:23 v/v). Manual vortex was performed to sample for 1 min at maximum speed in Vortex 2, IKA (Steufen, Germany).
The AFB1 analysis was conducted using the MaxSignal® AFB1 Kit, which utilizes a competitive ELISA method. This kit includes 96-well microtiter plates that have been sensitized with a monoclonal antibody that targets explicitly AFB1. Ultimately, 50 μl of each AFB1 standard solution (0.1, 1.0, 5.0, 10, 20, 50, 100 ng/ml), arranged in ascending order of concentration, together with the diluted supernatant, were added twice to the wells of the microtiter plate. Afterward, 100 μl of AFB1-Horseradish Peroxidase conjugate (HRP) was introduced into each plate well. The plate was agitated for 1 min and placed in an environment with ambient temperature for 30 min. Following incubation, the microtiter well plate was fully emptied, rinsed three times with 250 ml of the 1× wash solution during each rinse, and dried by tapping on a layer of paper towel many times. The unconjugated substance was eliminated during the washing process. After agitating a 100 μl solution of Tetramethylbenzidine (TMB) substrate, for 1 min, was left to react at room temperature for 15 min. To stop the reaction, 100 μl of the enzyme reaction inhibition buffer (stop solution) was added, and the absorbance was measured at 450 nm using a TECAN Microplate reader (Infinite 200 Pro, Nanoquant, Vienna, Austria).
Quality assurance
The analytical methods were validated based on linearity, Limit of Detection (LOD), Limit of Quantification (LOQ), and percentage of recovery according to the screening and confirmatory methods set by Regulation EC/401/2006 (EC, 2006). For Certified Reference Materials (CRM), the validation parameters were expressed as the sum of 3 replicates for each spiking level.
Health risk assessment
Classified as a Group 1 carcinogenic toxin, AFB1 induce Hepatocellular Carcinoma (HCC) as the main human adverse health effect (IARC, 2012). Risk prediction of developing AFB1-induced HCC is based on the Estimation of Daily Intake (EDI) on a chronic basis. In this paper, the AFB1 exposure in the adult population was assessed by using the daily wheat and corn consumption data extracted by the World Health Organization/Global Environment Monitoring System (WHO/GEMS). Based on each data, the daily consumption of grain and grain-based products was calculated at 350 g/day, with wheat and corn at 345 and 4.9 g, respectively. The EDI values were expressed as ng/kg body weight (bw)/day, referring to 60 kg as adult body weight in equation 2 (CONTAM, 2020).
 (2)
(2)Statistical analysis
The statistical analysis was implemented using MATLAB R2016b. The t-test was performed, comparing the mean value with the defined value, considering the null hypothesis asH o : μ = μ o H o : μ d =0
The statistical analysis was implemented using MATLAB R2016b. The t-test was performed, comparing the mean value with the defined value, considering the null hypothesis as
Results
Mycological contamination
During the pre- and post-harvest stages, various biological and environmental factors interact complexly, affecting fungal growth and mycotoxin synthesis. According to the findings, the collected wheat and corn samples manifested fungi contamination belonging to different genera, such as Alternaria, Aspergillus, Cladosporium, Fusarium, and Penicillium (Table 1). The studied samples indicated a similar distribution of mold infection patterns for the three main genera: Penicillium spp. (77.89%), Fusarium spp. (74.73%), and Aspergillus spp. (72.63%). However, for the genera Alternaria and Cladosporium, contamination was recorded in only 13.68% of samples in each case, displaying a very different result than the data for the three main genera mentioned previously.
Concerning the regions, we detected that the incidence of various genera was not uniform. Five fungi genera were observed in wheat samples from western Albania, characterized by low-altitude geography and a Mediterranean climate. Wheat samples manifested identical contamination patterns (approx. 103 CFU/g), regardless of sampling site or mold genera (Table 1).
Mycological contamination
During the pre- and post-harvest stages, various biological and environmental factors interact complexly, affecting fungal growth and mycotoxin synthesis. According to the findings, the collected wheat and corn samples manifested fungi contamination belonging to different genera, such as Alternaria, Aspergillus, Cladosporium, Fusarium, and Penicillium (Table 1). The studied samples indicated a similar distribution of mold infection patterns for the three main genera: Penicillium spp. (77.89%), Fusarium spp. (74.73%), and Aspergillus spp. (72.63%). However, for the genera Alternaria and Cladosporium, contamination was recorded in only 13.68% of samples in each case, displaying a very different result than the data for the three main genera mentioned previously.
Concerning the regions, we detected that the incidence of various genera was not uniform. Five fungi genera were observed in wheat samples from western Albania, characterized by low-altitude geography and a Mediterranean climate. Wheat samples manifested identical contamination patterns (approx. 103 CFU/g), regardless of sampling site or mold genera (Table 1).
Table 1: The mycological contamination of wheat and corn samples a Number of positive samples/ Incidence(%).
b Arithmetic mean/median of positive samples (×103 CFU/g).
c Maximum level observed (×103 CFU/g).
d Total count of positive samples on a specific genus.
CFU=Colony Forming Unit
Concerning corn contamination, the highest incidence of Penicillium sp. referring to the region was recorded in corn samples from the Korça region (500×104 CFU/g), followed by Fusarium sp. in samples from the Fieri region (100×104 CFU/g), and the Korça region (80×104 CFU/g). The third-most prevalent mold belonged to the Aspergillus genera, with the highest incidence in the Elbasan region (26×104 CFU/g), compared to 20-24×104 CFU/g in the Fieri, Korça, and Durrësi regions.
To wheat contamination, the Fusarium spp., Aspergillus spp., and Penicillium spp. were identified on the enormous scale, respectively Aspergillus genera (34×103 CFU/g), followed by Penicillium (10×103 CFU/g). Regarding the region, the highest contamination levels observed in wheat samples belonged to the Fieri region. Moreover, Alternaria genus was present in samples from this region at a level of 1×103 CFU/g. Interestingly, wheat from the Korça region exhibited a different fungi contamination pattern, with the highest counts recorded for the Fusarium genus (5×102 CFU/g).
AFB1 contamination
The AFB1 concentration was determined by calculating the average of two replicates. The mycotoxin presence in winter wheat samples (61) harvested during the summer season and corn (68) harvested in autumn from the 2022 harvesting year was investigated (Table 2). The contamination rate (88.23%) in corn samples was much higher (p<0.05) than in wheat samples (4.91%). A key role is related to climate conditions, with wheat harvested during the summer and corn harvested in autumn. AFB1 contamination levels in corn varied from 0.389-69.295 μg/kg, with only eight samples not contaminated (11.77%). The AFB1 contamination in wheat samples was detected in three out of 61 samples, and the maximum value 0.401 μg/kg.
The AFB1 exposure assessment for Albanians was calculated based on the EDI (ng/kg bw/day), which refers to the mean value for wheat and maize consumption (Table 4).
To wheat contamination, the Fusarium spp., Aspergillus spp., and Penicillium spp. were identified on the enormous scale, respectively Aspergillus genera (34×103 CFU/g), followed by Penicillium (10×103 CFU/g). Regarding the region, the highest contamination levels observed in wheat samples belonged to the Fieri region. Moreover, Alternaria genus was present in samples from this region at a level of 1×103 CFU/g. Interestingly, wheat from the Korça region exhibited a different fungi contamination pattern, with the highest counts recorded for the Fusarium genus (5×102 CFU/g).
AFB1 contamination
The AFB1 concentration was determined by calculating the average of two replicates. The mycotoxin presence in winter wheat samples (61) harvested during the summer season and corn (68) harvested in autumn from the 2022 harvesting year was investigated (Table 2). The contamination rate (88.23%) in corn samples was much higher (p<0.05) than in wheat samples (4.91%). A key role is related to climate conditions, with wheat harvested during the summer and corn harvested in autumn. AFB1 contamination levels in corn varied from 0.389-69.295 μg/kg, with only eight samples not contaminated (11.77%). The AFB1 contamination in wheat samples was detected in three out of 61 samples, and the maximum value 0.401 μg/kg.
The AFB1 exposure assessment for Albanians was calculated based on the EDI (ng/kg bw/day), which refers to the mean value for wheat and maize consumption (Table 4).
Table 2: Aflatoxin B1 (AFB1) in maize and wheat from the harvesting year 2022 (Mean±SD)
| Corn | Wheat | |
| Number of studied samples | 68 | 61 |
| Positive samples | 60 | 3 |
| Incidence (%) | 88.23 | 4.91 |
| Mean value (µg/kg) | 17.379±0.161 | 0.015±0.000 |
| Median value (µg/kg) | 0.920±0.017 | 0.000±0.000 |
| Minimum value (µg/kg) | 0.389±0.003 | 0.221±0.003 |
| Maximum value (µg/kg) | 69.295±0.244 | 0.402±0.001 |
Table 3: Aflatoxin B1 (AFB1) incidence and risk assessment concerning corn application as food Maximum Residue Level (MRL) (5 μg/kg) and feed MRL (20 μg/kg) (EC, 2023)
| Interval (μg/kg) | No. of samples (Total number) | Incidence (%) |
| 0 | 8 (68) | 11.76 |
| 0-5.00 | 32 (68) | 47.06 |
| Over 5.00 (MRL, food) | 28 (68) | 41.18 |
| 5.00-10.00 | 2 (68) | 2.94 |
| Over 10.00 | 26 (68) | 38.23 |
| Over 20.00 (MRL, feed) | 22 (68) | 32.35 |
Table 4: Estimating Daily Intake (EDI) of adult Albanians to Aflatoxin B1 (AFB1) from wheat and corn consumption
| Corn | Wheat | |||
| Mean (ng/g) |
Maximum (ng/g) |
Mean (ng/g) |
Maximum (ng/g) |
|
| 17.374 | 69.295 | 0.015 | 0.402 | |
| EDI (n.d=0) | 1.4112 | - | 0.0862 | - |
| EDImax | - | 5.6497 | - | 2.3047 |
Discussion
This study presents mycological contamination on two main grains grown in the country while also giving their AFB1 contamination. Battilani et al. (2016) and Leggieri et al. (2021) have proposed prediction models on AF corn contamination for southern European countries such as Italy. According to studies, A. flavus and A. parasiticus are the two primary species associated with AF contamination in crops. A. flavus isolates generally produce AFBs and occasionally Cyclopiazonic Acid (CPA), while most A. parasiticus strains produce both AFBs and AFGs but fail to produce CPA. The toxin production in A. flavus varies significantly and depends on genotype, substrate, geographic origin, climate change, and agronomic practice (Shabeer et al., 2022).
Global literature data on the contamination of wheat and corn reveal that fungal diseases significantly impede cereal yield, causing yield reduction, estimating 15-20%. In situations as fungal growth and infections are predominant, the losses can escalate to as high as 50% (Różewicz et al., 2021). High levels of mycological contamination in wheat from Poland was detected. The major fungi genera was Fusarium spp. present in 95.5% of samples, Aspergillus spp. (81.8%), Penicillium spp. (72.3%), Alternaria spp. (22.7%) (Čonková et al., 2006). The results are entirely different in the mycological contamination of wheat from northern Africa, with the most common fungi species in wheat, Eurotium spp. (62.5%) followed by Cladosporium spp. (29.17%), and Rhizopus spp. (4.17%), meanwhile, corn revealed higher levels of Aspergillus spp. (76.19%), being the most prevalent; however, other genera have a much lower frequency, Penicillium spp. (38.10%) or Fusarium spp. (19.05%) (Jedidi et al., 2018). Similar to North Africa, the wheat grain from Iran exhibited contamination rates with Aspergillus spp. (32.1%), Alternaria spp. (26.7%), and Fusarium spp. (17.8%), most prevalent (Joshaghani et al., 2013).
Our study indicates a higher rate of AFB1 contamination in corn compared with wheat (p<0.05). An identical pattern distribution was encountered in both crops for the three main mold genera, Fusarium, Penicillium, and Aspergillus. However, a different situation for the Alternaria and Cladosporium genera was observed; wheat samples were more likely to be affected than corn, with 19.7 and 2.90%, respectively. In the pre-harvest stage, the main contamination factors are high temperatures, water stresses, and insect damage differently, in the post-harvest stage, temperature and aw are the main determining factors (Mahato et al., 2019; Mannaa and Kim, 2017).
Numerous methods are tested globally to decrease pre-harvest AFs contamination. Recently, using toxigenic strains of A. flavus for biological control in agricultural fields has demonstrated significant effectiveness (Bandyopadhyay et al., 2016; Cotty et al., 2007). This method of competitive exclusion, where the toxigenic strains outperform the toxigenic A. flavus strains, is considered as the most efficient measure to mitigate the risk of AFs. The efficacy of toxigenic A. flavus species as a biocontrol agent has been proven, resulting in a 70 to 99% reduction of AF levels in treated crops (Bhatnagar-Mathur et al., 2015). Monitoring the contamination levels will provide crucial evidence for the proposed prediction models for aflatoxigenic fungi that grow in temperate regions. This evidence will be necessary for implementing effective strategies to reduce the impact of climate change on agricultural production and food safety in Albania.
Considered one of the most critical contaminants in foodstuffs due to their adverse health effects on humans and animals, EU Regulation No 2023/915 addresses mycotoxin contamination by setting Maximum Residue Level (MRL) for specific contaminants in food (EC, 2023). Climate change, manifested in increased temperatures and extreme weather, has raised the AFB1 contamination incidence in corn, especially in southern Europe (Piva et al., 2006). In Europe, the hot and dry conditions necessary for A. flavus infestation of corn mainly prevail at latitudes below 45° N (Battilani et al., 2016). Not a concern till the end of the 20th century (Leggieri et al., 2021; Perrone et al., 2020), the first report on AF corn contamination in Italy was reported in 2003 (Battilani et al., 2016). In southern Europe, climate change scenarios propose an increased probability of AF contamination from low to medium due to a temperature rise of only 2 °C (CONTAM et al., 2020; Luo et al., 2021).
According to the EU Regulation 2023/915, the AFB1 MRL in corn intended for human consumption (5 µg/kg), the analysed corn samples revealed 28 or 41.18 %, out of 68 samples, were found about this threshold (Table 3). In addition, considering corn as feed, 22 samples, or 32.35 %, presented levels above the MRL (20 μg/kg). In contrast, in European feed, just 2.1% of corn samples exceeded this level (Gruber-Dorninger et al., 2019). The AFB1 maximum level detected in this commodity was 69.295±0.244 µg/kg. Data from Albania reveal that the AFB1 occurrence from the 2014 and 2015 harvested seasons, corn samples had a mean value of 464 and 55.7 µg/kg, respectively. AFB1 contamination in corn samples from 2023 resulted in a much lower prevalence than the 2014 harvesting year but similar to 2015 (Topi et al., 2023).
Seasonal climate differences impact aflatoxin production during plant growth and could explain the pronounced year-to-year variation in mycotoxin levels (Leggieri et al., 2021). Gruber-Dorninger et al. (2019) investigated that, globally, the incidence of AFB1 in corn was 24%, and a significant percentage (64%) exerting co-contamination by two or more mycotoxins. The data on the European continent indicates that AFB1 contamination in southern Europe (28.9%) is more common than in other regions (5.9-17.0%) (Gruber-Dorninger et al., 2019). Furthermore, this pattern is present when discussed with corn in China (Sun et al., 2017).
The first AF contamination incidence in the Balkans was reported during the 2013 harvesting season, while different mycotoxins’ occurrence in different crops from this region has been documented during last decade (De Rijk et al., 2015; Gagiu et al., 2018; Janić Hajnal et al., 2017; Kos et al., 2014; Kovač et al., 2022; Pleadin et al., 2015; Topi et al., 2019, 2022, 2023). Crop contamination with mycotoxins is not considered a critical issue, only referring to the adverse effects on human health, but it also endangers the animals’ health, as in the case of corn, which is widely applied as feed in livestock (Topi et al., 2022, 2023).
The results on AFB1 contamination in wheat indicate a better situation, with lower incidence, in comparison with publications from the region, e.g. wheat from Romania (45.4%) (Gagiu et al., 2018), while a similar pattern was observed when compared to the contamination incidence in Serbia (Kos et al., 2014), Croatia (Kovač et al., 2022), and Southern Europe, such as Italy (Alkadri et al., 2014)., Considering the global data on contamination level, referred median (1.0 μg/kg), or maximum values (161 μg/kg), our finding indicates a better situation.
The incidence of AFB1 contamination (88.23%) in corn in our survey denotes a higher incidence compared to the corn from Serbia, 57.2% (Janić Hajnal et al., 2017), Romania, 45.4% (Gagiu et al., 2018), Croatia 31.4% (Pleadin et al., 2015), and 8.7% (Kovač et al., 2022). This difference is further present in comparison with the data from the South Mediterranean, 16% (Abdallah et al., 2017) and 24% globally (Gruber-Dorninger et al., 2019). At maximum levels, this study detected similarity in cron from Serbia, 88.8 µg/kg (Janić Hajnal et al., 2017), Romania, 82.94 µg/kg (Gagiu et al., 2018), but lower value compared to South Mediterranean, 197.5 µg/kg (Abdallah et al., 2017). These studies manifest a considerable variation in corn contamination, with levels ranging from 2,072 µg/kg belonging to the year 2013 in Croatia (Pleadin et al., 2015), up to 4,822 μg/kg (Topi et al., 2023), The global survey data, including China, reveal that in our study, the occurrence levels are higher (Gruber-Dorninger et al., 2019; Jiang et al., 2019).
The AFB1 incidence (4.9%) in wheat displays the same pattern as data from two countries, Croatia and Serbia, where an incidence range of 0-19% was reported (Kos et al., 2014; Kovač et al., 2022; Pleadin et al., 2015), but different in case of Romania, 45.4% (Gagiu et al., 2018). The maximum level in wheat samples is 0.402 µg/kg) was much lower than the data in AFB1 contamination from this commodity in the region, Croatia, 5.41 µg/kg (Pleadin et al., 2015) or 16.20 µg/kg (Kovač et al., 2022), Romania 82.94 µg/kg (Gagiu et al., 2018); the Mediterranean area, 66.7 µg/kg (Serrano et al., 2012), and globally, 161 µg/kg (Gruber-Dorninger et al., 2019).
In positive samples, concentrations ranged from 0.223-0.402 μg/kg, with a mean of 0.015±0.000 μg/kg, indicating no wheat samples exceeded the MRL (2 μg/kg). Considering the region, the contaminated samples related to the Fieri region, geographically part of the western plain, with a typical Mediterranean climate (Table 1). Similar contamination rates are identified with reported data from 2014 and 2015, indicating 6.0 and 0.0%, respectively (Topi et al., 2023).
Considering the significant differences in AFB1 contamination levels among corn and wheat, The climate conditions are considered as the main factor, especially in the pre-harvesting stage, when wheat commodities cropped in the summer. In contrast, corn harvesting during the autumn imposes higher exposure to climate factors, which favors fungi growth.
Regarding the amount of AFB1 in corn, after performing a t-test in two samples with size n=68 elements, as the p-value is smaller than the significance level
This study presents mycological contamination on two main grains grown in the country while also giving their AFB1 contamination. Battilani et al. (2016) and Leggieri et al. (2021) have proposed prediction models on AF corn contamination for southern European countries such as Italy. According to studies, A. flavus and A. parasiticus are the two primary species associated with AF contamination in crops. A. flavus isolates generally produce AFBs and occasionally Cyclopiazonic Acid (CPA), while most A. parasiticus strains produce both AFBs and AFGs but fail to produce CPA. The toxin production in A. flavus varies significantly and depends on genotype, substrate, geographic origin, climate change, and agronomic practice (Shabeer et al., 2022).
Global literature data on the contamination of wheat and corn reveal that fungal diseases significantly impede cereal yield, causing yield reduction, estimating 15-20%. In situations as fungal growth and infections are predominant, the losses can escalate to as high as 50% (Różewicz et al., 2021). High levels of mycological contamination in wheat from Poland was detected. The major fungi genera was Fusarium spp. present in 95.5% of samples, Aspergillus spp. (81.8%), Penicillium spp. (72.3%), Alternaria spp. (22.7%) (Čonková et al., 2006). The results are entirely different in the mycological contamination of wheat from northern Africa, with the most common fungi species in wheat, Eurotium spp. (62.5%) followed by Cladosporium spp. (29.17%), and Rhizopus spp. (4.17%), meanwhile, corn revealed higher levels of Aspergillus spp. (76.19%), being the most prevalent; however, other genera have a much lower frequency, Penicillium spp. (38.10%) or Fusarium spp. (19.05%) (Jedidi et al., 2018). Similar to North Africa, the wheat grain from Iran exhibited contamination rates with Aspergillus spp. (32.1%), Alternaria spp. (26.7%), and Fusarium spp. (17.8%), most prevalent (Joshaghani et al., 2013).
Our study indicates a higher rate of AFB1 contamination in corn compared with wheat (p<0.05). An identical pattern distribution was encountered in both crops for the three main mold genera, Fusarium, Penicillium, and Aspergillus. However, a different situation for the Alternaria and Cladosporium genera was observed; wheat samples were more likely to be affected than corn, with 19.7 and 2.90%, respectively. In the pre-harvest stage, the main contamination factors are high temperatures, water stresses, and insect damage differently, in the post-harvest stage, temperature and aw are the main determining factors (Mahato et al., 2019; Mannaa and Kim, 2017).
Numerous methods are tested globally to decrease pre-harvest AFs contamination. Recently, using toxigenic strains of A. flavus for biological control in agricultural fields has demonstrated significant effectiveness (Bandyopadhyay et al., 2016; Cotty et al., 2007). This method of competitive exclusion, where the toxigenic strains outperform the toxigenic A. flavus strains, is considered as the most efficient measure to mitigate the risk of AFs. The efficacy of toxigenic A. flavus species as a biocontrol agent has been proven, resulting in a 70 to 99% reduction of AF levels in treated crops (Bhatnagar-Mathur et al., 2015). Monitoring the contamination levels will provide crucial evidence for the proposed prediction models for aflatoxigenic fungi that grow in temperate regions. This evidence will be necessary for implementing effective strategies to reduce the impact of climate change on agricultural production and food safety in Albania.
Considered one of the most critical contaminants in foodstuffs due to their adverse health effects on humans and animals, EU Regulation No 2023/915 addresses mycotoxin contamination by setting Maximum Residue Level (MRL) for specific contaminants in food (EC, 2023). Climate change, manifested in increased temperatures and extreme weather, has raised the AFB1 contamination incidence in corn, especially in southern Europe (Piva et al., 2006). In Europe, the hot and dry conditions necessary for A. flavus infestation of corn mainly prevail at latitudes below 45° N (Battilani et al., 2016). Not a concern till the end of the 20th century (Leggieri et al., 2021; Perrone et al., 2020), the first report on AF corn contamination in Italy was reported in 2003 (Battilani et al., 2016). In southern Europe, climate change scenarios propose an increased probability of AF contamination from low to medium due to a temperature rise of only 2 °C (CONTAM et al., 2020; Luo et al., 2021).
According to the EU Regulation 2023/915, the AFB1 MRL in corn intended for human consumption (5 µg/kg), the analysed corn samples revealed 28 or 41.18 %, out of 68 samples, were found about this threshold (Table 3). In addition, considering corn as feed, 22 samples, or 32.35 %, presented levels above the MRL (20 μg/kg). In contrast, in European feed, just 2.1% of corn samples exceeded this level (Gruber-Dorninger et al., 2019). The AFB1 maximum level detected in this commodity was 69.295±0.244 µg/kg. Data from Albania reveal that the AFB1 occurrence from the 2014 and 2015 harvested seasons, corn samples had a mean value of 464 and 55.7 µg/kg, respectively. AFB1 contamination in corn samples from 2023 resulted in a much lower prevalence than the 2014 harvesting year but similar to 2015 (Topi et al., 2023).
Seasonal climate differences impact aflatoxin production during plant growth and could explain the pronounced year-to-year variation in mycotoxin levels (Leggieri et al., 2021). Gruber-Dorninger et al. (2019) investigated that, globally, the incidence of AFB1 in corn was 24%, and a significant percentage (64%) exerting co-contamination by two or more mycotoxins. The data on the European continent indicates that AFB1 contamination in southern Europe (28.9%) is more common than in other regions (5.9-17.0%) (Gruber-Dorninger et al., 2019). Furthermore, this pattern is present when discussed with corn in China (Sun et al., 2017).
The first AF contamination incidence in the Balkans was reported during the 2013 harvesting season, while different mycotoxins’ occurrence in different crops from this region has been documented during last decade (De Rijk et al., 2015; Gagiu et al., 2018; Janić Hajnal et al., 2017; Kos et al., 2014; Kovač et al., 2022; Pleadin et al., 2015; Topi et al., 2019, 2022, 2023). Crop contamination with mycotoxins is not considered a critical issue, only referring to the adverse effects on human health, but it also endangers the animals’ health, as in the case of corn, which is widely applied as feed in livestock (Topi et al., 2022, 2023).
The results on AFB1 contamination in wheat indicate a better situation, with lower incidence, in comparison with publications from the region, e.g. wheat from Romania (45.4%) (Gagiu et al., 2018), while a similar pattern was observed when compared to the contamination incidence in Serbia (Kos et al., 2014), Croatia (Kovač et al., 2022), and Southern Europe, such as Italy (Alkadri et al., 2014)., Considering the global data on contamination level, referred median (1.0 μg/kg), or maximum values (161 μg/kg), our finding indicates a better situation.
The incidence of AFB1 contamination (88.23%) in corn in our survey denotes a higher incidence compared to the corn from Serbia, 57.2% (Janić Hajnal et al., 2017), Romania, 45.4% (Gagiu et al., 2018), Croatia 31.4% (Pleadin et al., 2015), and 8.7% (Kovač et al., 2022). This difference is further present in comparison with the data from the South Mediterranean, 16% (Abdallah et al., 2017) and 24% globally (Gruber-Dorninger et al., 2019). At maximum levels, this study detected similarity in cron from Serbia, 88.8 µg/kg (Janić Hajnal et al., 2017), Romania, 82.94 µg/kg (Gagiu et al., 2018), but lower value compared to South Mediterranean, 197.5 µg/kg (Abdallah et al., 2017). These studies manifest a considerable variation in corn contamination, with levels ranging from 2,072 µg/kg belonging to the year 2013 in Croatia (Pleadin et al., 2015), up to 4,822 μg/kg (Topi et al., 2023), The global survey data, including China, reveal that in our study, the occurrence levels are higher (Gruber-Dorninger et al., 2019; Jiang et al., 2019).
The AFB1 incidence (4.9%) in wheat displays the same pattern as data from two countries, Croatia and Serbia, where an incidence range of 0-19% was reported (Kos et al., 2014; Kovač et al., 2022; Pleadin et al., 2015), but different in case of Romania, 45.4% (Gagiu et al., 2018). The maximum level in wheat samples is 0.402 µg/kg) was much lower than the data in AFB1 contamination from this commodity in the region, Croatia, 5.41 µg/kg (Pleadin et al., 2015) or 16.20 µg/kg (Kovač et al., 2022), Romania 82.94 µg/kg (Gagiu et al., 2018); the Mediterranean area, 66.7 µg/kg (Serrano et al., 2012), and globally, 161 µg/kg (Gruber-Dorninger et al., 2019).
In positive samples, concentrations ranged from 0.223-0.402 μg/kg, with a mean of 0.015±0.000 μg/kg, indicating no wheat samples exceeded the MRL (2 μg/kg). Considering the region, the contaminated samples related to the Fieri region, geographically part of the western plain, with a typical Mediterranean climate (Table 1). Similar contamination rates are identified with reported data from 2014 and 2015, indicating 6.0 and 0.0%, respectively (Topi et al., 2023).
Considering the significant differences in AFB1 contamination levels among corn and wheat, The climate conditions are considered as the main factor, especially in the pre-harvesting stage, when wheat commodities cropped in the summer. In contrast, corn harvesting during the autumn imposes higher exposure to climate factors, which favors fungi growth.
Regarding the amount of AFB1 in corn, after performing a t-test in two samples with size n=68 elements, as the p-value is smaller than the significance level
 =0.05, we reject the null hypothesis and accept the alternative hypothesis in both cases (p1=0.0001 and p2=0.0001). Also, after comparing two independent samples, 1 and 2, we again reject the null hypothesis for the equality of the two means, as the p-value is smaller than the significance level (p=0.001). While, regarding the amount of AFB1 in wheat, the results are the opposite of corn. So, after performing a t-test in two samples with size n=61 elements, as the p-value is greater than the significance level
=0.05, we reject the null hypothesis and accept the alternative hypothesis in both cases (p1=0.0001 and p2=0.0001). Also, after comparing two independent samples, 1 and 2, we again reject the null hypothesis for the equality of the two means, as the p-value is smaller than the significance level (p=0.001). While, regarding the amount of AFB1 in wheat, the results are the opposite of corn. So, after performing a t-test in two samples with size n=61 elements, as the p-value is greater than the significance level  =0.05, we can't reject the null hypothesis in both cases (p1=0.092 and p2=0.091). Also, after comparing two independent samples, 1 and 2, again, we can't reject the null hypothesis for the equality of the two means, as the p-value is greater than the significance level (p=0.098).
=0.05, we can't reject the null hypothesis in both cases (p1=0.092 and p2=0.091). Also, after comparing two independent samples, 1 and 2, again, we can't reject the null hypothesis for the equality of the two means, as the p-value is greater than the significance level (p=0.098).Cereals constitute an essential part of Albanians' daily consumption of approx. 350 g grain and grain-based products, wheat comprises 445 g, and corn 5 g (Héraud et al., 2013). Considering mean values of AFB1 in wheat and corn during this harvesting year, it was found that the highest exposure in adult Albanians originated from corn consumption, EDI, 1.411 ng/kgbw/day in comparison with wheat consumption of 0.086 ng/kgbw/day. Meanwhile, considering the maximum AFB1 levels detected in corn and wheat, these values demonstrate that consumption has a higher risk for adults, 5.650 and 2.304 ng/kgbw/day, respectively. This value is triple higher as compared to data from the literature (Udovicki et al., 2021). This investigation indicates that a more detailed consumption survey is required to perform.
Conclusions
This paper analyzes mycotoxigenic contamination in corn and wheat harvested in Albania in 2022. The AFB1 values indicate that the contamination rate of corn commodities is a substantial issue. None of the wheat samples exceeded the MRL set by the European :union:. In contrast, 41.18% of corn samples were higher than the MRL of 5 μg/kg intended for use as food, and 32.25% exceeded the MRL of 20 μg/kg as well. The research reveals a small occurrence of AFB1 contamination in the wheat grain. The study provides substantial data for the assessment of hazards. Measuring the frequency of AFB1, the central AF of interest, includes health institutions and organizations with information regarding the likelihood of adverse health consequences for consumers. Regulatory authorities can employ these data to prioritize surveillance of mycotoxin contamination in the nation's main crops and recommend appropriate measures based on the identified risks. They must collaborate with farmers to implement appropriate agricultural practices, storage techniques, and other preventive measures to prevent crop contamination.
Additionally, this partnership should encompass the formulation of suggestions. Regulatory bodies must effectively address growing concerns, particularly in the ever-changing food industry, due to the impacts of climate change. The EDI indicates that the adult population's exposure mainly originates from wheat while identifying higher values than data from neighboring countries.
Author Contributions
L.M. and J.S. worked on formal analysis and validation methods; L.M. wrote the manuscript; D.T. and Z.D. designed the study, manuscript review, and editing; B.S. and E.H. engaged in resources, data curation, and visualization; D.T. funding acquisition and involved in supervision. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This study received financial support from the Albanian National Agency for Scientific Research and Innovation (NASRI) grant R&D_2022/17.
Ethical Consideration
The authors conducted this study according to the Code of Ethics approved by the Senate of the University of Tirana. It is part of the first author's Ph.D. thesis.
Funding
This study received financial support from the Albanian National Agency for Scientific Research and Innovation (NASRI) grant R&D_2022/17.
References
Abdallah M.F., Girgin G., Baydar T., Krska R., Sulyok M. (2017). Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS. Journal of Science of Food and Agriculture. 97: 4419-4428. [DOI: 10.1002/jsfa.8293]
Alameri M.M., Kong A.S.-Y., Aljaafari M.N., Al Ali H., Eid K., Al Sallagi M., Cheng W.-H., Abushelaibi A., Erin Lim S.-H., Loh J.-Y., Lai K.-S. (2023). Aflatoxin contamination: an overview on health issues, detection and management strategies. Toxins. 15: 246. [DOI: 10.3390/toxins15040246]
Alkadri D., Rubert J., Prodi A., Pisi A., Mañes J., Soler C. (2014). Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chemistry. 157: 111-118. [DOI: 10.1016/j.foodchem.2014.01.052]
Bandyopadhyay R., Ortega-Beltran A., Akande A., Mutegi C., Atehnkeng J., Kaptoge L., Senghor A.L., Adhikari B.N., Cotty P.J. (2016). Biological control of aflatoxins in Africa: current status and potential challenges in the face of climate change. World Mycotoxin Journal. 9: 771-789. [DOI: 10.3920/ WMJ2016.2130]
Battilani P., Toscano P., Van Der Fels-Klerx H.J., Moretti A., Camardo Leggieri M., Brera C., Rortais A., Goumperis T., Robinson T. (2016). Aflatoxin B1 contamination in maize in Europe increases due to climate change. Scientific Reports. 6: 24328. [DOI: 10.1038/srep24328]
Bhatnagar-Mathur P., Sunkara S., Bhatnagar-Panwar M., Waliyar F., Kumar Sharma K. (2015). Biotechnological advances for combating Aspergillus flavus and aflatoxin contamination in crops. Plant Science. 234: 119-132. [DOI: 10.1016/ j.plantsci.2015.02.009]
Bricher J.L. (2010). Ensuring global food safety‒a public health priority and a global responsibility. In: Boisrobert C.E., Stjepanovic A., Oh S., Lelieveld H.L. (Editors). Ensuring global food safety. Academic Press, Cambridge, Massachusetts, USA. pp: 1-4.
Chulze S.N. (2010). Strategies to reduce mycotoxin levels in maize during storage: a review. Food Additives and Contaminants: Part A. 27: 651-657. [DOI: 10.1080/ 19440040903573032]
Čonková E., Laciaková A., Štyriak I., Czerwiecki L., Wilczyńska G. (2006). Fungal contamination and the levels of mycotoxins (DON and OTA) in cereal samples from Poland and East Slovakia. Czech Journal of Food Sciences. 24: 33-40. [DOI: 10.17221/3291-CJFS]
Cotty P.J., Antilla L., Wakelyn P.J. (2007). Competitive exclusion of aflatoxin producers: farmer driven research and development. In: Vincent C., Goettel N., Lazarovits G. (Editors). Biological control: a global perspective. CAB International, London, UK. pp: 241-253. [DOI: 10.1079/ 9781845932657.0241]
De Rijk T.C., Van Egmond H.P., Van Der Fels-Klerx H.J., Herbes R., De Nijs M., Samson R., Slate A.B., Van Der Spiegel M. (2015). A study of the 2013 western European issue of aflatoxin contamination of maize from the Balkan area. World Mycotoxin Journal. 8: 641-651. [DOI: 10.3920/ WMJ2015.1903]
Eaton D.L., Beima K.M., Bammler T.K., Riley R.T., Voss K.A. (2018). Hepatotoxic mycotoxins. In: McQueen C.A. (Editor). Comprehensive toxicology, 3rd Edition. Elsevier, Oxford, U.K. pp: 483-521. [DOI: 10.1016/B978-0-12-801238-3.64337-4]
ESFA Panel on Contaminants in the Food Chain (CONTAM)., Schrenk D., Bignami M., Bodin L., Chipman J.K., Mazo J.D., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.-C., Nebbia C.S., Nielsen E., et.al. (2020). Risk assessment of aflatoxins in food. EFSA Journal. 18: 6040. [DOI: 10.2903/j.efsa.2020.6040]
European Commission (EC). (2006). Commission regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Office Journal of European :union:. L 70: 12-34. (Accessed 28 August 2023).
European Commission (EC). (2023). Commission regulation (EU) 2023/915 of 25 April 2023 on maximum levels for specific contaminants in food and repealing Regulation (EC) No 1881/2006. Office Journal of European :union:. L 119: 103-157. (Accessed 28 August 2023).
Food and Agriculture Organization (FAO). (2004). Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Rome, Italy. URL: https://www.fao.org/3/y5499e/y5499e00.htm.
Gagiu V., Mateescu E., Armeanu I., Dobre A.A., Smeu I., Cucu M.E., Oprea O.A., Iorga E., Belc N. (2018). Post-harvest contamination with mycotoxins in the context of the geographic and agroclimatic conditions in Romania. Toxins. 10: 533. [DOI: 10.3390/toxins10120533]
Groopman J.D., Wogan G.N. (2016). Aflatoxins: a global public health problem. In: Caballero B., Finglas P.M., Toldrá F. (Editors). Encyclopedia of food and health. Elsevier, Oxford, U.K. pp: 68-72. [DOI: 10.1016/B978-0-12-384947-2.00015-5]
Gruber-Dorninger C., Jenkins T., Schatzmayr G. (2019). Global mycotoxin occurrence in feed: a ten-year survey. Toxins. 11: 375. [DOI: 10.3390/toxins11070375]
Héraud F., Barraj L.M., Moy G.G. (2013). GEMS/food consumption cluster diets. In: Moy G., Vannoort R.W (Editors). Total diet studies. Springer, New York. pp: 427-434. [DOI: 10.1007/978-1-4419-7689-5_43]
International Agency for Research on Cancer (IARC). (2012). Chemical agents and related occupations. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans. 100F. Lyon, France. URL: www.ncbi.nlm.nih.gov/books/NBK304416/.
Janić Hajnal E., Kos J., Krulj J., Krstović S., Jajić I., Pezo L., Šarić B., Nedeljković N. (2017). Aflatoxins contamination of maize in Serbia: the impact of weather conditions in 2015. Food Additives and Contaminants: Part A. 34: 1999-2010. [DOI: 10.1080/19440049.2017.1331047]
Jedidi I., Soldevilla C., Lahouar A., Marín P., González-Jaén M.T., Said S. (2018). Mycoflora isolation and molecular characterization of aspergillus and fusarium species in Tunisian cereals. Saudi Journal of Biological Sciences. 25: 868-874. [DOI: 10.1016/j.sjbs.2017.11.050]
Jiang D., Li F., Zheng F., Zhou J., Li L., Shen F., Chen J., Li W. (2019). Occurrence and dietary exposure assessment of multiple mycotoxins in corn-based food products from Shandong, China. Food Additives and Contaminants: Part B. 12: 10-17. [DOI: 10.1080/19393210.2018.1503341]
Joint Expert Committee on Food Additives (JECFA). (2018). Safety evaluation of specific contaminants in food: prepared by the eighty-third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series, Rome, Italy, 74, pp: 3-280.[URL: https://www.who.int/publications/i/item/9789241660747]
Joshaghani H., Namjoo M., Rostami M., Kohsar F., Niknejad F. (2013). Mycoflora of fungal contamination in wheat storage (silos) in Golestan province, north of Iran. Jundishapur Journal of Microbiology. 6: e6334. [DOI: 10.5812/jjm.6334]
Kos J.J., Škrinjar M.M., Mandić A.I., Mišan A.Č., Bursić V.P., Šarić B.M., Janić Hajnal E.P. (2014). Presence of aflatoxins in cereals from Serbia. Food and Feed Research. 41: 31-38. [DOI: 10.5937/FFR1401031K]
Kovač M., Bulaič M., Nevistič A., Rot T., Babič J., Panjičko M., Kovač T., Šarkanj B. (2022). Regulated mycotoxin occurrence and co-occurrence in Croatian cereals. Toxins. 14: 112. [DOI: 10.3390/toxins14020112]
Leggieri M.C., Toscano P., Battilani P. (2021). Predicted aflatoxin B1 increase in Europe due to climate change: actions and reactions at global level. Toxins. 13: 292. [DOI: 10.3390/ toxins13040292]
Liu Y., Wu F. (2010). Global burden of aflatoxin‒induced hepatocellular carcinoma: a risk assessment. Environmental Health Perspective. 118: 818-824. [DOI: 10.1289/ehp. 0901388]
Luo S., Du H., Kebede H., Liu Y., Xing F. (2021). Contamination status of major mycotoxins in agricultural products and foodstuff in Europe. Food Control. 127: 108120. [DOI: 10.1016/j.foodcont.2021.108120]
Magnussen A., Parsi M.A. (2013). Aflatoxins, hepatocellular carcinoma and public health. World Journal of Gastroenterology. 19: 1508-1512. [DOI: 10.3748/wjg.v19. i10.1508]
Mahato D.K., Lee K.E., Kamle M., Devi S., Dewangan K.N., Kumar P., Kang S.G. (2019). Aflatoxins in food and feed: an overview on prevalence, detection and control strategies. Frontiers in Microbiology. 10: 2266. [DOI: 10.3389/ fmicb.2019.02266]
Mannaa M., Kim K.D. (2017). Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology. 45: 240-254. [DOI: 10.5941/ MYCO.2017.45.4.240]
Nazhand A., Durazzo A., Lucarini M., Souto E.B., Santini A. (2020). Characteristics, occurrence, detection, and detoxification of aflatoxins in foods and feeds. Foods. 9: 644. [DOI: 10.3390/foods9050644]
Owolabi I.O., Karoonuthaisiri N., Elliott C.T., Petchkongkaew A. (2023). A 10-year analysis of RASFF notifications for mycotoxins in nuts. Trend in key mycotoxins and impacted countries. Food Research International. 172: 112915. [DOI: 10.1016/ j.foodres.2023.112915]
Patial V., Asrani R.K., Thakur M. (2018). Foodborne mycotoxicosis: pathologies and public health impact. In: Holban A.M., Grumezescu A.M. (Editors). Handbook of food bioengineering, foodborne diseases. 15. Academic Press, Cambridge, Massachusetts, USA. pp: 239-274.
Perrone G., Ferrara M., Medina A., Pascale M., Magan N. (2020). Toxigenic fungi and mycotoxins in a climate change scenario: ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms. 8: 1496. [DOI: 10.3390/ microorganisms8101496]
Pitt J.I., Taniwaki M.H., Cole M.B. (2013). Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement food safety objectives. Food Control. 32: 205-215. [DOI: 10.1016/j.foodcont.2012.11.023]
Piva G., Battilani P., Pietri A. (2006). Emerging issues in southern Europe: aflatoxins in Italy. In: Barug D., Bhatnagar D., Van Egmond H.P. Van Der Kamp J.W., Van Osenbruggen W.A., Visconti A. (Editors). The mycotoxin factbook. Food and Feed Topics; Wageningen Academic Publishers, Wageningen, The Netherlands. pp. 139-153.
Pleadin J., Vulić A., Perši N., Škrivanko M., Capek B., Cvetnić Ž. (2015). Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control. 47: 221-225. [DOI: 10.1016/j.foodcont.2014.07.017]
Różewicz M., Wyzińska M., Grabiński J. (2021). The most important fungal diseases of cereals—problems and possible solutions. Agronomy. 11: 714. [DOI: 10.3390/agronomy11040714]
Serrano A.B., Font G., Ruiz M.J., Ferrer E. (2012). Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chemistry. 135: 423-429. [DOI: 10.1016/j.foodchem.2012.03.064]
Shabeer S., Asad S., Jamal A., Ali A. (2022). Aflatoxin contamination, its impact and management strategies: an updated review. Toxins. 14: 307. [DOI: 10.3390/ toxins14050307]
Sun X. D., Su P., Shan H. (2017). Mycotoxin contamination of maize in China. Comprehensive Review in Food Science and Food Safety. 16: 835-849. [DOI: 10.1111/1541-4337.12286]
Topi D., Babic J., Jakovac-Strajn B., Tavčar-Kalcher G. (2023). Incidence of aflatoxins and ochratoxin A in wheat and corn from Albania. Toxins. 15: 567. [DOI: 10.3390/ toxins15090567]
Topi D., Spahiu J., Rexhepi A., Marku N. (2022). Two-year survey of aflatoxin M1 in milk marketed in Albania and human exposure assessment. Food Control. 136: 108831. [DOI: 10.1016/j.foodcont.2022.108831]
Topi D., Tavčar-Kalcher G., Pavšič-Vrtač K., Babič J., Jakovac-Strajn B. (2019). Alternaria mycotoxins in grains from Albania: alternariol, alternariol monomethyl ether, tenuazonic acid, and tentoxin. World Mycotoxin Journal. 12: 89-99. [DOI: 10.3920/WMJ2018.2342]
Udovicki B., Tomic N., Spirovic Trifunovic B., Despotovic S., Jovanovic J., Jacxsens L., Rajkovic A. (2021). Risk assessment of dietary exposure to aflatoxin B1 in Serbia. Food and Chemical Toxicology. 151: 112116. [DOI: 10.1016/j.fct.2021.112116]
VDLUFA (2007a). Standard operating procedure for identifying bacteria, yeasts, moulds, and dematiaceae as product-typical and spoilage-indicating microorganisms in feeds. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.3., 11). VDLUFA, Darmstadt, Germany.
VDLUFA (2007b). Standard operation procedure for the enumeration of microorganisms using solid culture media. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.1., 14). VDLUFA, Darmstadt, Germany.
VDLUFA (2007c). Standard operation procedure to enumerate bacteria, yeasts, moulds, and Dematiaceae. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.2., 18). VDLUFA, Darmstadt, Germany
Abdallah M.F., Girgin G., Baydar T., Krska R., Sulyok M. (2017). Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS. Journal of Science of Food and Agriculture. 97: 4419-4428. [DOI: 10.1002/jsfa.8293]
Alameri M.M., Kong A.S.-Y., Aljaafari M.N., Al Ali H., Eid K., Al Sallagi M., Cheng W.-H., Abushelaibi A., Erin Lim S.-H., Loh J.-Y., Lai K.-S. (2023). Aflatoxin contamination: an overview on health issues, detection and management strategies. Toxins. 15: 246. [DOI: 10.3390/toxins15040246]
Alkadri D., Rubert J., Prodi A., Pisi A., Mañes J., Soler C. (2014). Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chemistry. 157: 111-118. [DOI: 10.1016/j.foodchem.2014.01.052]
Bandyopadhyay R., Ortega-Beltran A., Akande A., Mutegi C., Atehnkeng J., Kaptoge L., Senghor A.L., Adhikari B.N., Cotty P.J. (2016). Biological control of aflatoxins in Africa: current status and potential challenges in the face of climate change. World Mycotoxin Journal. 9: 771-789. [DOI: 10.3920/ WMJ2016.2130]
Battilani P., Toscano P., Van Der Fels-Klerx H.J., Moretti A., Camardo Leggieri M., Brera C., Rortais A., Goumperis T., Robinson T. (2016). Aflatoxin B1 contamination in maize in Europe increases due to climate change. Scientific Reports. 6: 24328. [DOI: 10.1038/srep24328]
Bhatnagar-Mathur P., Sunkara S., Bhatnagar-Panwar M., Waliyar F., Kumar Sharma K. (2015). Biotechnological advances for combating Aspergillus flavus and aflatoxin contamination in crops. Plant Science. 234: 119-132. [DOI: 10.1016/ j.plantsci.2015.02.009]
Bricher J.L. (2010). Ensuring global food safety‒a public health priority and a global responsibility. In: Boisrobert C.E., Stjepanovic A., Oh S., Lelieveld H.L. (Editors). Ensuring global food safety. Academic Press, Cambridge, Massachusetts, USA. pp: 1-4.
Chulze S.N. (2010). Strategies to reduce mycotoxin levels in maize during storage: a review. Food Additives and Contaminants: Part A. 27: 651-657. [DOI: 10.1080/ 19440040903573032]
Čonková E., Laciaková A., Štyriak I., Czerwiecki L., Wilczyńska G. (2006). Fungal contamination and the levels of mycotoxins (DON and OTA) in cereal samples from Poland and East Slovakia. Czech Journal of Food Sciences. 24: 33-40. [DOI: 10.17221/3291-CJFS]
Cotty P.J., Antilla L., Wakelyn P.J. (2007). Competitive exclusion of aflatoxin producers: farmer driven research and development. In: Vincent C., Goettel N., Lazarovits G. (Editors). Biological control: a global perspective. CAB International, London, UK. pp: 241-253. [DOI: 10.1079/ 9781845932657.0241]
De Rijk T.C., Van Egmond H.P., Van Der Fels-Klerx H.J., Herbes R., De Nijs M., Samson R., Slate A.B., Van Der Spiegel M. (2015). A study of the 2013 western European issue of aflatoxin contamination of maize from the Balkan area. World Mycotoxin Journal. 8: 641-651. [DOI: 10.3920/ WMJ2015.1903]
Eaton D.L., Beima K.M., Bammler T.K., Riley R.T., Voss K.A. (2018). Hepatotoxic mycotoxins. In: McQueen C.A. (Editor). Comprehensive toxicology, 3rd Edition. Elsevier, Oxford, U.K. pp: 483-521. [DOI: 10.1016/B978-0-12-801238-3.64337-4]
ESFA Panel on Contaminants in the Food Chain (CONTAM)., Schrenk D., Bignami M., Bodin L., Chipman J.K., Mazo J.D., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.-C., Nebbia C.S., Nielsen E., et.al. (2020). Risk assessment of aflatoxins in food. EFSA Journal. 18: 6040. [DOI: 10.2903/j.efsa.2020.6040]
European Commission (EC). (2006). Commission regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Office Journal of European :union:. L 70: 12-34. (Accessed 28 August 2023).
European Commission (EC). (2023). Commission regulation (EU) 2023/915 of 25 April 2023 on maximum levels for specific contaminants in food and repealing Regulation (EC) No 1881/2006. Office Journal of European :union:. L 119: 103-157. (Accessed 28 August 2023).
Food and Agriculture Organization (FAO). (2004). Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Rome, Italy. URL: https://www.fao.org/3/y5499e/y5499e00.htm.
Gagiu V., Mateescu E., Armeanu I., Dobre A.A., Smeu I., Cucu M.E., Oprea O.A., Iorga E., Belc N. (2018). Post-harvest contamination with mycotoxins in the context of the geographic and agroclimatic conditions in Romania. Toxins. 10: 533. [DOI: 10.3390/toxins10120533]
Groopman J.D., Wogan G.N. (2016). Aflatoxins: a global public health problem. In: Caballero B., Finglas P.M., Toldrá F. (Editors). Encyclopedia of food and health. Elsevier, Oxford, U.K. pp: 68-72. [DOI: 10.1016/B978-0-12-384947-2.00015-5]
Gruber-Dorninger C., Jenkins T., Schatzmayr G. (2019). Global mycotoxin occurrence in feed: a ten-year survey. Toxins. 11: 375. [DOI: 10.3390/toxins11070375]
Héraud F., Barraj L.M., Moy G.G. (2013). GEMS/food consumption cluster diets. In: Moy G., Vannoort R.W (Editors). Total diet studies. Springer, New York. pp: 427-434. [DOI: 10.1007/978-1-4419-7689-5_43]
International Agency for Research on Cancer (IARC). (2012). Chemical agents and related occupations. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans. 100F. Lyon, France. URL: www.ncbi.nlm.nih.gov/books/NBK304416/.
Janić Hajnal E., Kos J., Krulj J., Krstović S., Jajić I., Pezo L., Šarić B., Nedeljković N. (2017). Aflatoxins contamination of maize in Serbia: the impact of weather conditions in 2015. Food Additives and Contaminants: Part A. 34: 1999-2010. [DOI: 10.1080/19440049.2017.1331047]
Jedidi I., Soldevilla C., Lahouar A., Marín P., González-Jaén M.T., Said S. (2018). Mycoflora isolation and molecular characterization of aspergillus and fusarium species in Tunisian cereals. Saudi Journal of Biological Sciences. 25: 868-874. [DOI: 10.1016/j.sjbs.2017.11.050]
Jiang D., Li F., Zheng F., Zhou J., Li L., Shen F., Chen J., Li W. (2019). Occurrence and dietary exposure assessment of multiple mycotoxins in corn-based food products from Shandong, China. Food Additives and Contaminants: Part B. 12: 10-17. [DOI: 10.1080/19393210.2018.1503341]
Joint Expert Committee on Food Additives (JECFA). (2018). Safety evaluation of specific contaminants in food: prepared by the eighty-third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series, Rome, Italy, 74, pp: 3-280.[URL: https://www.who.int/publications/i/item/9789241660747]
Joshaghani H., Namjoo M., Rostami M., Kohsar F., Niknejad F. (2013). Mycoflora of fungal contamination in wheat storage (silos) in Golestan province, north of Iran. Jundishapur Journal of Microbiology. 6: e6334. [DOI: 10.5812/jjm.6334]
Kos J.J., Škrinjar M.M., Mandić A.I., Mišan A.Č., Bursić V.P., Šarić B.M., Janić Hajnal E.P. (2014). Presence of aflatoxins in cereals from Serbia. Food and Feed Research. 41: 31-38. [DOI: 10.5937/FFR1401031K]
Kovač M., Bulaič M., Nevistič A., Rot T., Babič J., Panjičko M., Kovač T., Šarkanj B. (2022). Regulated mycotoxin occurrence and co-occurrence in Croatian cereals. Toxins. 14: 112. [DOI: 10.3390/toxins14020112]
Leggieri M.C., Toscano P., Battilani P. (2021). Predicted aflatoxin B1 increase in Europe due to climate change: actions and reactions at global level. Toxins. 13: 292. [DOI: 10.3390/ toxins13040292]
Liu Y., Wu F. (2010). Global burden of aflatoxin‒induced hepatocellular carcinoma: a risk assessment. Environmental Health Perspective. 118: 818-824. [DOI: 10.1289/ehp. 0901388]
Luo S., Du H., Kebede H., Liu Y., Xing F. (2021). Contamination status of major mycotoxins in agricultural products and foodstuff in Europe. Food Control. 127: 108120. [DOI: 10.1016/j.foodcont.2021.108120]
Magnussen A., Parsi M.A. (2013). Aflatoxins, hepatocellular carcinoma and public health. World Journal of Gastroenterology. 19: 1508-1512. [DOI: 10.3748/wjg.v19. i10.1508]
Mahato D.K., Lee K.E., Kamle M., Devi S., Dewangan K.N., Kumar P., Kang S.G. (2019). Aflatoxins in food and feed: an overview on prevalence, detection and control strategies. Frontiers in Microbiology. 10: 2266. [DOI: 10.3389/ fmicb.2019.02266]
Mannaa M., Kim K.D. (2017). Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology. 45: 240-254. [DOI: 10.5941/ MYCO.2017.45.4.240]
Nazhand A., Durazzo A., Lucarini M., Souto E.B., Santini A. (2020). Characteristics, occurrence, detection, and detoxification of aflatoxins in foods and feeds. Foods. 9: 644. [DOI: 10.3390/foods9050644]
Owolabi I.O., Karoonuthaisiri N., Elliott C.T., Petchkongkaew A. (2023). A 10-year analysis of RASFF notifications for mycotoxins in nuts. Trend in key mycotoxins and impacted countries. Food Research International. 172: 112915. [DOI: 10.1016/ j.foodres.2023.112915]
Patial V., Asrani R.K., Thakur M. (2018). Foodborne mycotoxicosis: pathologies and public health impact. In: Holban A.M., Grumezescu A.M. (Editors). Handbook of food bioengineering, foodborne diseases. 15. Academic Press, Cambridge, Massachusetts, USA. pp: 239-274.
Perrone G., Ferrara M., Medina A., Pascale M., Magan N. (2020). Toxigenic fungi and mycotoxins in a climate change scenario: ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms. 8: 1496. [DOI: 10.3390/ microorganisms8101496]
Pitt J.I., Taniwaki M.H., Cole M.B. (2013). Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement food safety objectives. Food Control. 32: 205-215. [DOI: 10.1016/j.foodcont.2012.11.023]
Piva G., Battilani P., Pietri A. (2006). Emerging issues in southern Europe: aflatoxins in Italy. In: Barug D., Bhatnagar D., Van Egmond H.P. Van Der Kamp J.W., Van Osenbruggen W.A., Visconti A. (Editors). The mycotoxin factbook. Food and Feed Topics; Wageningen Academic Publishers, Wageningen, The Netherlands. pp. 139-153.
Pleadin J., Vulić A., Perši N., Škrivanko M., Capek B., Cvetnić Ž. (2015). Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control. 47: 221-225. [DOI: 10.1016/j.foodcont.2014.07.017]
Różewicz M., Wyzińska M., Grabiński J. (2021). The most important fungal diseases of cereals—problems and possible solutions. Agronomy. 11: 714. [DOI: 10.3390/agronomy11040714]
Serrano A.B., Font G., Ruiz M.J., Ferrer E. (2012). Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chemistry. 135: 423-429. [DOI: 10.1016/j.foodchem.2012.03.064]
Shabeer S., Asad S., Jamal A., Ali A. (2022). Aflatoxin contamination, its impact and management strategies: an updated review. Toxins. 14: 307. [DOI: 10.3390/ toxins14050307]
Sun X. D., Su P., Shan H. (2017). Mycotoxin contamination of maize in China. Comprehensive Review in Food Science and Food Safety. 16: 835-849. [DOI: 10.1111/1541-4337.12286]
Topi D., Babic J., Jakovac-Strajn B., Tavčar-Kalcher G. (2023). Incidence of aflatoxins and ochratoxin A in wheat and corn from Albania. Toxins. 15: 567. [DOI: 10.3390/ toxins15090567]
Topi D., Spahiu J., Rexhepi A., Marku N. (2022). Two-year survey of aflatoxin M1 in milk marketed in Albania and human exposure assessment. Food Control. 136: 108831. [DOI: 10.1016/j.foodcont.2022.108831]
Topi D., Tavčar-Kalcher G., Pavšič-Vrtač K., Babič J., Jakovac-Strajn B. (2019). Alternaria mycotoxins in grains from Albania: alternariol, alternariol monomethyl ether, tenuazonic acid, and tentoxin. World Mycotoxin Journal. 12: 89-99. [DOI: 10.3920/WMJ2018.2342]
Udovicki B., Tomic N., Spirovic Trifunovic B., Despotovic S., Jovanovic J., Jacxsens L., Rajkovic A. (2021). Risk assessment of dietary exposure to aflatoxin B1 in Serbia. Food and Chemical Toxicology. 151: 112116. [DOI: 10.1016/j.fct.2021.112116]
VDLUFA (2007a). Standard operating procedure for identifying bacteria, yeasts, moulds, and dematiaceae as product-typical and spoilage-indicating microorganisms in feeds. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.3., 11). VDLUFA, Darmstadt, Germany.
VDLUFA (2007b). Standard operation procedure for the enumeration of microorganisms using solid culture media. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.1., 14). VDLUFA, Darmstadt, Germany.
VDLUFA (2007c). Standard operation procedure to enumerate bacteria, yeasts, moulds, and Dematiaceae. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.2., 18). VDLUFA, Darmstadt, Germany
* Corresponding author (D. Topi)
* E-mail: dritan.topi@unitir.edu.al
ORCID ID: http://orcid.org/0000-0001-7852-5374
* E-mail: dritan.topi@unitir.edu.al
ORCID ID: http://orcid.org/0000-0001-7852-5374
Type of Study: Original article |
Subject:
Special
Received: 23/11/06 | Accepted: 24/03/20 | Published: 24/03/26
Received: 23/11/06 | Accepted: 24/03/20 | Published: 24/03/26
References
1. Abdallah M.F., Girgin G., Baydar T., Krska R., Sulyok M. (2017). Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS. Journal of Science of Food and Agriculture. 97: 4419-4428. [DOI: 10.1002/jsfa.8293] [DOI:10.1002/jsfa.8293] [PMID]
2. Alameri M.M., Kong A.S.-Y., Aljaafari M.N., Al Ali H., Eid K., Al Sallagi M., Cheng W.-H., Abushelaibi A., Erin Lim S.-H., Loh J.-Y., Lai K.-S. (2023). Aflatoxin contamination: an overview on health issues, detection and management strategies. Toxins. 15: 246. [DOI: 10.3390/toxins15040246] [DOI:10.3390/toxins15040246] [PMID] [PMCID]
3. Alkadri D., Rubert J., Prodi A., Pisi A., Mañes J., Soler C. (2014). Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chemistry. 157: 111-118. [DOI: 10.1016/j.foodchem.2014.01.052] [DOI:10.1016/j.foodchem.2014.01.052] [PMID]
4. Bandyopadhyay R., Ortega-Beltran A., Akande A., Mutegi C., Atehnkeng J., Kaptoge L., Senghor A.L., Adhikari B.N., Cotty P.J. (2016). Biological control of aflatoxins in Africa: current status and potential challenges in the face of climate change. World Mycotoxin Journal. 9: 771-789. [DOI: 10.3920/ WMJ2016.2130] [DOI:10.3920/WMJ2016.2130]
5. Battilani P., Toscano P., Van Der Fels-Klerx H.J., Moretti A., Camardo Leggieri M., Brera C., Rortais A., Goumperis T., Robinson T. (2016). Aflatoxin B1 contamination in maize in Europe increases due to climate change. Scientific Reports. 6: 24328. [DOI: 10.1038/srep24328] [DOI:10.1038/srep24328] [PMID] [PMCID]
6. Bhatnagar-Mathur P., Sunkara S., Bhatnagar-Panwar M., Waliyar F., Kumar Sharma K. (2015). Biotechnological advances for combating Aspergillus flavus and aflatoxin contamination in crops. Plant Science. 234: 119-132. [DOI: 10.1016/ j.plantsci.2015.02.009] [DOI:10.1016/j.plantsci.2015.02.009] [PMID]
7. Bricher J.L. (2010). Ensuring global food safety-a public health priority and a global responsibility. In: Boisrobert C.E., Stjepanovic A., Oh S., Lelieveld H.L. (Editors). Ensuring global food safety. Academic Press, Cambridge, Massachusetts, USA. pp: 1-4. [DOI:10.1016/B978-0-12-374845-4.00001-1]
8. Chulze S.N. (2010). Strategies to reduce mycotoxin levels in maize during storage: a review. Food Additives and Contaminants: Part A. 27: 651-657. [DOI: 10.1080/ 19440040903573032] [DOI:10.1080/19440040903573032] [PMID]
9. Čonková E., Laciaková A., Štyriak I., Czerwiecki L., Wilczyńska G. (2006). Fungal contamination and the levels of mycotoxins (DON and OTA) in cereal samples from Poland and East Slovakia. Czech Journal of Food Sciences. 24: 33-40. [DOI: 10.17221/3291-CJFS] [DOI:10.17221/3291-CJFS]
10. Cotty P.J., Antilla L., Wakelyn P.J. (2007). Competitive exclusion of aflatoxin producers: farmer driven research and development. In: Vincent C., Goettel N., Lazarovits G. (Editors). Biological control: a global perspective. CAB International, London, UK. pp: 241-253. [DOI: 10.1079/ 9781845932657.0241] [DOI:10.1079/9781845932657.0241]
11. De Rijk T.C., Van Egmond H.P., Van Der Fels-Klerx H.J., Herbes R., De Nijs M., Samson R., Slate A.B., Van Der Spiegel M. (2015). A study of the 2013 western European issue of aflatoxin contamination of maize from the Balkan area. World Mycotoxin Journal. 8: 641-651. [DOI: 10.3920/ WMJ2015.1903] [DOI:10.3920/WMJ2015.1903]
12. Eaton D.L., Beima K.M., Bammler T.K., Riley R.T., Voss K.A. (2018). Hepatotoxic mycotoxins. In: McQueen C.A. (Editor). Comprehensive toxicology, 3rd Edition. Elsevier, Oxford, U.K. pp: 483-521. [DOI: 10.1016/B978-0-12-801238-3.64337-4] [DOI:10.1016/B978-0-12-801238-3.64337-4]
13. ESFA Panel on Contaminants in the Food Chain (CONTAM)., Schrenk D., Bignami M., Bodin L., Chipman J.K., Mazo J.D., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.-C., Nebbia C.S., Nielsen E., et.al. (2020). Risk assessment of aflatoxins in food. EFSA Journal. 18: 6040. [DOI: 10.2903/j.efsa.2020.6040] [DOI:10.2903/j.efsa.2020.6040] [PMID]
14. European Commission (EC). (2006). Commission regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Office Journal of European :union:. L 70: 12-34. (Accessed 28 August 2023).
15. European Commission (EC). (2023). Commission regulation (EU) 2023/915 of 25 April 2023 on maximum levels for specific contaminants in food and repealing Regulation (EC) No 1881/2006. Office Journal of European :union:. L 119: 103-157. (Accessed 28 August 2023).
16. Food and Agriculture Organization (FAO). (2004). Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Rome, Italy. URL: https://www.fao.org/3/y5499e/y5499e00.htm.
17. Gagiu V., Mateescu E., Armeanu I., Dobre A.A., Smeu I., Cucu M.E., Oprea O.A., Iorga E., Belc N. (2018). Post-harvest contamination with mycotoxins in the context of the geographic and agroclimatic conditions in Romania. Toxins. 10: 533. [DOI: 10.3390/toxins10120533] [DOI:10.3390/toxins10120533] [PMID] [PMCID]
18. Groopman J.D., Wogan G.N. (2016). Aflatoxins: a global public health problem. In: Caballero B., Finglas P.M., Toldrá F. (Editors). Encyclopedia of food and health. Elsevier, Oxford, U.K. pp: 68-72. [DOI: 10.1016/B978-0-12-384947-2.00015-5] [DOI:10.1016/B978-0-12-384947-2.00015-5]
19. Gruber-Dorninger C., Jenkins T., Schatzmayr G. (2019). Global mycotoxin occurrence in feed: a ten-year survey. Toxins. 11: 375. [DOI: 10.3390/toxins11070375] [DOI:10.3390/toxins11070375] [PMID] [PMCID]
20. Héraud F., Barraj L.M., Moy G.G. (2013). GEMS/food consumption cluster diets. In: Moy G., Vannoort R.W (Editors). Total diet studies. Springer, New York. pp: 427-434. [DOI: 10.1007/978-1-4419-7689-5_43] [DOI:10.1007/978-1-4419-7689-5_43]
21. International Agency for Research on Cancer (IARC). (2012). Chemical agents and related occupations. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans. 100F. Lyon, France. URL: www.ncbi.nlm.nih.gov/books/NBK304416/.
22. Janić Hajnal E., Kos J., Krulj J., Krstović S., Jajić I., Pezo L., Šarić B., Nedeljković N. (2017). Aflatoxins contamination of maize in Serbia: the impact of weather conditions in 2015. Food Additives and Contaminants: Part A. 34: 1999-2010. [DOI: 10.1080/19440049.2017.1331047] [DOI:10.1080/19440049.2017.1331047] [PMID]
23. Jedidi I., Soldevilla C., Lahouar A., Marín P., González-Jaén M.T., Said S. (2018). Mycoflora isolation and molecular characterization of aspergillus and fusarium species in Tunisian cereals. Saudi Journal of Biological Sciences. 25: 868-874. [DOI: 10.1016/j.sjbs.2017.11.050] [DOI:10.1016/j.sjbs.2017.11.050] [PMID] [PMCID]
24. Jiang D., Li F., Zheng F., Zhou J., Li L., Shen F., Chen J., Li W. (2019). Occurrence and dietary exposure assessment of multiple mycotoxins in corn-based food products from Shandong, China. Food Additives and Contaminants: Part B. 12: 10-17. [DOI: 10.1080/19393210.2018.1503341] [DOI:10.1080/19393210.2018.1503341] [PMID]
25. Joint Expert Committee on Food Additives (JECFA). (2018). Safety evaluation of specific contaminants in food: prepared by the eighty-third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series, Rome, Italy, 74, pp: 3-280.[URL: https://www.who.int/publications/i/item/9789241660747]
26. Joshaghani H., Namjoo M., Rostami M., Kohsar F., Niknejad F. (2013). Mycoflora of fungal contamination in wheat storage (silos) in Golestan province, north of Iran. Jundishapur Journal of Microbiology. 6: e6334. [DOI: 10.5812/jjm.6334] [DOI:10.5812/jjm.6334]
27. Kos J.J., Škrinjar M.M., Mandić A.I., Mišan A.Č., Bursić V.P., Šarić B.M., Janić Hajnal E.P. (2014). Presence of aflatoxins in cereals from Serbia. Food and Feed Research. 41: 31-38. [DOI: 10.5937/FFR1401031K] [DOI:10.5937/FFR1401031K]
28. Kovač M., Bulaič M., Nevistič A., Rot T., Babič J., Panjičko M., Kovač T., Šarkanj B. (2022). Regulated mycotoxin occurrence and co-occurrence in Croatian cereals. Toxins. 14: 112. [DOI: 10.3390/toxins14020112] [DOI:10.3390/toxins14020112] [PMID] [PMCID]
29. Leggieri M.C., Toscano P., Battilani P. (2021). Predicted aflatoxin B1 increase in Europe due to climate change: actions and reactions at global level. Toxins. 13: 292. [DOI: 10.3390/ toxins13040292] [DOI:10.3390/toxins13040292] [PMID] [PMCID]
30. Liu Y., Wu F. (2010). Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environmental Health Perspective. 118: 818-824. [DOI: 10.1289/ehp. 0901388] [DOI:10.1289/ehp.0901388] [PMID] [PMCID]
31. Luo S., Du H., Kebede H., Liu Y., Xing F. (2021). Contamination status of major mycotoxins in agricultural products and foodstuff in Europe. Food Control. 127: 108120. [DOI: 10.1016/j.foodcont.2021.108120] [DOI:10.1016/j.foodcont.2021.108120]
32. Magnussen A., Parsi M.A. (2013). Aflatoxins, hepatocellular carcinoma and public health. World Journal of Gastroenterology. 19: 1508-1512. [DOI: 10.3748/wjg.v19. i10.1508] [DOI:10.3748/wjg.v19.i10.1508] [PMID] [PMCID]
33. Mahato D.K., Lee K.E., Kamle M., Devi S., Dewangan K.N., Kumar P., Kang S.G. (2019). Aflatoxins in food and feed: an overview on prevalence, detection and control strategies. Frontiers in Microbiology. 10: 2266. [DOI: 10.3389/ fmicb.2019.02266] [DOI:10.3389/fmicb.2019.02266]
34. Mannaa M., Kim K.D. (2017). Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology. 45: 240-254. [DOI: 10.5941/ MYCO.2017.45.4.240] [DOI:10.5941/MYCO.2017.45.4.240]
35. Nazhand A., Durazzo A., Lucarini M., Souto E.B., Santini A. (2020). Characteristics, occurrence, detection, and detoxification of aflatoxins in foods and feeds. Foods. 9: 644. [DOI: 10.3390/foods9050644] [DOI:10.3390/foods9050644] [PMID] [PMCID]
36. Owolabi I.O., Karoonuthaisiri N., Elliott C.T., Petchkongkaew A. (2023). A 10-year analysis of RASFF notifications for mycotoxins in nuts. Trend in key mycotoxins and impacted countries. Food Research International. 172: 112915. [DOI: 10.1016/ j.foodres.2023.112915] [DOI:10.1016/j.foodres.2023.112915] [PMID]
37. Patial V., Asrani R.K., Thakur M. (2018). Foodborne mycotoxicosis: pathologies and public health impact. In: Holban A.M., Grumezescu A.M. (Editors). Handbook of food bioengineering, foodborne diseases. 15. Academic Press, Cambridge, Massachusetts, USA. pp: 239-274. [DOI:10.1016/B978-0-12-811444-5.00009-9]
38. Perrone G., Ferrara M., Medina A., Pascale M., Magan N. (2020). Toxigenic fungi and mycotoxins in a climate change scenario: ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms. 8: 1496. [DOI: 10.3390/ microorganisms8101496] [DOI:10.3390/microorganisms8101496]
39. Pitt J.I., Taniwaki M.H., Cole M.B. (2013). Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement food safety objectives. Food Control. 32: 205-215. [DOI: 10.1016/j.foodcont.2012.11.023] [DOI:10.1016/j.foodcont.2012.11.023]
40. Piva G., Battilani P., Pietri A. (2006). Emerging issues in southern Europe: aflatoxins in Italy. In: Barug D., Bhatnagar D., Van Egmond H.P. Van Der Kamp J.W., Van Osenbruggen W.A., Visconti A. (Editors). The mycotoxin factbook. Food and Feed Topics; Wageningen Academic Publishers, Wageningen, The Netherlands. pp. 139-153. [DOI:10.3920/9789086865871_009]
41. Pleadin J., Vulić A., Perši N., Škrivanko M., Capek B., Cvetnić Ž. (2015). Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control. 47: 221-225. [DOI: 10.1016/j.foodcont.2014.07.017] [DOI:10.1016/j.foodcont.2014.07.017]
42. Różewicz M., Wyzińska M., Grabiński J. (2021). The most important fungal diseases of cereals-problems and possible solutions. Agronomy. 11: 714. [DOI: 10.3390/agronomy11040714] [DOI:10.3390/agronomy11040714]
43. Serrano A.B., Font G., Ruiz M.J., Ferrer E. (2012). Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chemistry. 135: 423-429. [DOI: 10.1016/j.foodchem.2012.03.064] [DOI:10.1016/j.foodchem.2012.03.064] [PMID]
44. Shabeer S., Asad S., Jamal A., Ali A. (2022). Aflatoxin contamination, its impact and management strategies: an updated review. Toxins. 14: 307. [DOI: 10.3390/ toxins14050307] [DOI:10.3390/toxins14050307] [PMID] [PMCID]
45. Sun X. D., Su P., Shan H. (2017). Mycotoxin contamination of maize in China. Comprehensive Review in Food Science and Food Safety. 16: 835-849. [DOI: 10.1111/1541-4337.12286] [DOI:10.1111/1541-4337.12286] [PMID]
46. Topi D., Babic J., Jakovac-Strajn B., Tavčar-Kalcher G. (2023). Incidence of aflatoxins and ochratoxin A in wheat and corn from Albania. Toxins. 15: 567. [DOI: 10.3390/ toxins15090567] [DOI:10.3390/toxins15090567] [PMID] [PMCID]
47. Topi D., Spahiu J., Rexhepi A., Marku N. (2022). Two-year survey of aflatoxin M1 in milk marketed in Albania and human exposure assessment. Food Control. 136: 108831. [DOI: 10.1016/j.foodcont.2022.108831] [DOI:10.1016/j.foodcont.2022.108831]
48. Topi D., Tavčar-Kalcher G., Pavšič-Vrtač K., Babič J., Jakovac-Strajn B. (2019). Alternaria mycotoxins in grains from Albania: alternariol, alternariol monomethyl ether, tenuazonic acid, and tentoxin. World Mycotoxin Journal. 12: 89-99. [DOI: 10.3920/WMJ2018.2342] [DOI:10.3920/WMJ2018.2342]
49. Udovicki B., Tomic N., Spirovic Trifunovic B., Despotovic S., Jovanovic J., Jacxsens L., Rajkovic A. (2021). Risk assessment of dietary exposure to aflatoxin B1 in Serbia. Food and Chemical Toxicology. 151: 112116. [DOI: 10.1016/j.fct.2021.112116] [DOI:10.1016/j.fct.2021.112116] [PMID]
50. VDLUFA (2007a). Standard operating procedure for identifying bacteria, yeasts, moulds, and dematiaceae as product-typical and spoilage-indicating microorganisms in feeds. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.3., 11). VDLUFA, Darmstadt, Germany.
51. VDLUFA (2007b). Standard operation procedure for the enumeration of microorganisms using solid culture media. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.1., 14). VDLUFA, Darmstadt, Germany.
52. VDLUFA (2007c). Standard operation procedure to enumerate bacteria, yeasts, moulds, and Dematiaceae. In VDLUFA Method Book III Suppl. No. 7 (chap. 28.1.2., 18). VDLUFA, Darmstadt, Germany
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







