Volume 11, Issue 3 (September 2024)
J. Food Qual. Hazards Control 2024, 11(3): 177-185 |
Back to browse issues page
Ethics code: Not applicable
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Varela-Rangel Y, Guillén L, Cuadra-Sánchez C, Araque M. Molecular Typing of Potentially Pathogenic Escherichia coli Isolated from Fresh Pasta Filata Venezuelan Cheeses. J. Food Qual. Hazards Control 2024; 11 (3) :177-185
URL: http://jfqhc.ssu.ac.ir/article-1-1204-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1204-en.html
Molecular Microbiology Laboratory, Faculty of Pharmacy and Bioanalysis, University of The Andes, Mérida 5101, Venezuela , araquemc@ula.ve
Full-Text [PDF 565 kb]
(448 Downloads)
| Abstract (HTML) (1168 Views)
Full-Text: (166 Views)
Molecular Typing of Potentially Pathogenic Escherichia coli Isolated from Fresh Pasta Filata Venezuelan Cheeses
Y.Y. Varela-Rangel 1 , L. Guillén 1, C. Cuadra-Sánchez 1,2, M. Araque 1[*]*
1. Molecular Microbiology Laboratory, Faculty of Pharmacy and Bioanalysis, University of The Andes, Mérida 5101, Venezuela.
2. Corpogen Clinical Laboratory, Managua 14027, Nicaragua.
HIGHLIGHTS
To cite: Varela-Rangel Y.Y., Guillén L., Cuadra-Sánchez C., Araque M. (2024). Molecular typing of potentially pathogenic Escherichia coli isolated from fresh pasta filata Venezuelan cheeses. Journal of Food Quality and Hazards Control. 11: 177-185.
Introduction
Table 4: Distribution of the number and profile of virulence genes according to phylogenetic group of Escherichia coli from pasta filata cheeses
UC*=Unclassified
Table 5: Distribution of phylogenetic groups and presence of virulence genes of Escherichia coli according to the type of pasta filata cheese
PAI=Pathogenicity Island; UC*=Unclassified
Table 6: Genetic characteristics of the six clonally related strains of Escherichia coli (A-I, A-II and B-I)
PCR=Polymerase Chain Reaction
Ombarak R.A., Hinenoya A., Awasthi S.P., Iguchi A., Shima A., Elbagory A.R.M., Yamasaki S. (2016). Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt. International Journal of Food Microbiology. 221: 69-76. [DOI: 10/1016/j. ijfoodmicro.2016.01.009]
Ovi F., Zhang L., Nabors H., Jia L., Adhikari P. (2023). A compilation of virulence-associated genes that are frequently reported in avian pathogenic Escherichia coli (APEC) compared to other E.coli. Journal of Applied Microbiology. 134: 1-20. [DOI: 10.1093/jambio/lxad014]
Perdomo C., Gutiérrez F., García O., Acevedo I., Bastidas Z., Kowalski A. (2015). Physicochemical and bacteriological characterization of white artisan cheese in Buria parish, Lara state, Venezuela. Gaceta de Ciencias Veterinarias. 20: 35-44. [Spanish with English abstract]
Pineda A.P.A, Campos G.Z., Pimentel-Filho N.J., Franco B.D.G.d.M., Pinto U.M. (2021). Brazilian artisanal cheeses: diversity, microbiological safety, and challenges for the sector. Frontiers in Microbiology. 12: 666922. [DOI: 10.3389/fmicb.2021.666922]
Quijada-Martínez P., Flores-Carrero A., Labrador I., Millán Y., Araque M. (2017). Microbiological profile and molecular characterization of multidrug-resistant gram-negative bacilli producing catheter–associated urinary tract infections in the internal medicine services of a Venezuelan university hospital. Austin Journal of Infectious Diseases. 4: 1030.
Rodríguez C., Caldas L., Ogeerally P. (2009). Sanitary conditions of hand-made “telita” type cheese in Upata, Bolivar State, Venezuela. Revista de la Sociedad Venezolana de Microbiología. 29: 98-102. [Spanish with English abstract]
Sarowska J., Futoma-Koloch B., Jama-Kmiecik A., Frej-Madrzak M., Ksiazczyk M., Bugla-Ploskonska G., Choroszy-Krol I. (2019). Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathogens. 11: 10. [DOI: 10.1186/s13099-019-0290-0]
Sebastianski M., Bridger N.A., Featherstone R.M., Robinson J.L. (2022). Diseases outbreaks linked to pasteurized and unpasteurized dairy products in Canada and the United States: a systematic review. Canadian Journal of Public Health. 113: 569-578. [DOI: 10.17269/s41997-022-00614-y]
Venezuelan Industrial Standards Commission (COVENIN). (1989). Foodstuffs. Identification and sample preparation for microbiological analysis. Standards Nº 1126-89. URL: https://www.scribd.com/doc/50579844/1126-89. Accessed 10 January 2024.
Versalovic J., Koeuth T., Lupski J.R. (1991). Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Research. 19: 6823-6831. [DOI: 10.1093/nar/19.24.6823]
Y.Y. Varela-Rangel 1
1. Molecular Microbiology Laboratory, Faculty of Pharmacy and Bioanalysis, University of The Andes, Mérida 5101, Venezuela.
2. Corpogen Clinical Laboratory, Managua 14027, Nicaragua.
- All strains of Escherichia coli carried virulence genes and were susceptible to the antibiotics tested.
- E. coli strains carried at least two virulence genes, fimH (type 1 fimbriae), and fyuA (yersiniabactin receptor).
- The profile of virulence factors of E. coli was similar to the pathotypes Uropathogenic E. coli.
- About 80% of the E. coli strains were grouped within the phylogroups A and D.
| Article type Original article |
ABSTRACT Background. Fresh pasta filata cheese is considered as one of the most important foods in the Venezuelan diet. It is typically produced by small-scale producers using raw milk. The objective of this research was to molecularly characterize the pathogenic potential of Escherichia coli strains isolated from pasta filata cheese manufactured and marketed in Venezuela. Methodology. In the period between January and March of 2019, a total of 36 strains of E. coli were isolated from a variety of pasta filata cheeses including 17 samples of mozzarella, 16 of telita, and 3 of guayanés. These strains were isolated according to the Venezuelan Commission of Industrial Standards (COVENIN) and identified by conventional methods (biochemical and phenotypic tests). Antimicrobial susceptibility was determined using the disk diffusion technique. Phylogenetic grouping and detection of virulence genes were performed by Polymerase Chain Reaction amplification. Diversity and genetic relationships were determined by Rep-PCR. Results: All strains were susceptible to the tested antibiotics. Phylogroup A (n=19) was the most frequent (52.8%), followed by groups D (n=11; 30.6%), and B1 (n=2; 5.6%). The majority of isolates carried at least two virulence genes, one coding for adhesion mechanisms (fimH) and the other for iron uptake (fyuA). Only one strain of phylogroup A presented a profile consisting of four virulence genes (fimH, fyuA, kpsMT II, and papAH). Four strains that could not be classified according to Clermont's scheme carried resistance genes as well. A heterogeneous population structure was observed by Rep-PCR of the strains. Conclusion: Results support the hypothesis that the E. coli strains isolated from the three types of pasta filata cheeses manufactured and marketed in Venezuela have identical characteristics and virulence factors to Extraintestinal Pathogenic E. coli strains observed in animals and humans, posing a potential health risk. Therefore, it is essential to improve hygienic and sanitary controls at all stages of cheese production and to implement measures for epidemiological surveillance of potentially pathogenic bacterial strains present in Venezuelan, artisanal pasta filata cheeses. © 2024, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Escherichia coli Food Safety Cheese Virulence Genetic Variation |
||
| Article history Received: 13 Mar 2024 Revised: 10 Jun 2024 Accept: 05 Sep 2024 |
||
| Acronyms and abbreviations ExPEC=Extraintestinal Pathogenic Escherichia coli PAI=Pathogenicity Island PCR=Polymerase Chain Reaction |
Introduction
Pasta filata cheese, also referred to as spun paste or stretched-curd cheese is one of the most popular and significant food in the Venezuelan diet. It is typically made by small-scale producers who frequently consume raw milk, which contains the optimal microbiota that contributes to the organoleptic characteristics of the final product (Perdomo et al., 2015). Fresh pasta filata cheeses, containing telita, guayanés, and mozzarella, are firmly embedded in artisanal traditions that have been transmitted from one generation to the next. These practices exhibit considerable regional variation, resulting in notable differences in the texture, flavor, and organoleptic characteristics of the resulting cheeses. For instance, alterations in the stretching technique and coagulation temperature may influence the ultimate quality of the product. The production process is greatly influenced by the climatic and environmental conditions in each region. Other factors including temperature, humidity, and the quality of the raw milk, exert an influence on the natural microbiota and, consequently, on the fermentation and ripening of the cheese (Maldonado Gómez et al., 2011). These cheeses are in high demand due to their ready availability for human consumption following artisanal production, delicate flavor, medium fat content, and smooth texture. Furthermore, these cheeses have high pH and humidity levels with a low salt content (Márquez and García R, 2007; Rodríguez et al., 2009).
Artisanal pasta filata cheeses are typically observed in markets, open-air food fairs or from street vendors. They are frequently immersed in whey, which can promote the growth of microorganisms (Maldonado Gómez et al., 2011; Rodríguez et al., 2009). Additionally, the consumption of raw milk, as well as unsophisticated manufacturing processes, transportation, and storage, are critical points that can result in contamination of the product with harmful microorganisms (Perdomo et al., 2015). Previous studies have suggested that consumption of artisanal cheeses is associated with an increase in food-borne disease outbreaks (Koski et al., 2022; Sebastianski et al., 2022).
Escherichia coli is a microorganism commonly observed in the intestines of humans and animals as part of the normal microbiota. Its presence in food is typically indicative of direct or indirect fecal contamination, which may occasionally be accompanied by other intestinal pathogens (Bujnáková et al., 2021). However, the genomic plasticity of E. coli allows it to survive and evolve in various environments, casing it adaptable to different ecological niches, including food, and enabling its presence at any stage of the food chain. Detection and quantification of E. coli is an important factor in assessing food hygiene standards (Ombarak et al., 2016; Sarowska et al., 2019).
Although E. coli is a natural member of the human intestinal microbiota, its interactions with the host and the presence of virulence factors permit it to be classified into three main groups: commensal, Diarrheagenic E. coli (DEC), and Extraintestinal Pathogenic E. coli (ExPEC) (Braz et al., 2020). Phylogenetically, E. coli can be divided into eight phylogroups: A, B1, B2, C, D, E, F, and the cryptic clade I, which can be utilized to estimate its pathogenic potential (Clermont et al., 2013). However, the majority of E. coli strains are classified in groups A, B1, B2, and D (Beghain et al., 2018). Commensal strains are regarded to be of low virulence and comprise phylogroups A and B1, whereas ExPEC are principally grouped in phylogroups B2 and D, which commonly carry genes encoding virulence factors that act on a wide range of cellular processes (Sarowska et al., 2019).
Several studies conducted in Latin America have reported the prevalence of ExPEC in various types of cheese made with unpasteurized milk (Guillén et al., 2014; Pineda et al., 2021). In Venezuela, fresh pasta filata cheese is one of the most common carriers of food-borne diseases due to its poor microbiological quality (Maldonado Gómez et al., 2011; Márquez and García R, 2007; Perdomo et al., 2015; Rodríguez et al., 2009). However, studies describing the genetic characterization of ExpEC in artisanal dairy products for human consumption in the country are scarce (Guillén et al., 2014; Millán et al., 2018). In this regard, the purpose of this research was to molecularly characterize pathogenic E. coli strains isolated from fresh pasta filata cheeses manufactured and marketed in Venezuela.
Materials and methods
This study employed an observational, cross-sectional, descriptive methodology which was conducted between January and March 2019.
Sampling and E. coli isolation
A total of 75 E. coli strains were isolated from a variety of pasta filata cheeses. Of these, 36 strains were randomly selected for analysis. This sampling approach was based on proportionality for each of the selected products and product availability during sampling. The selected sample included unpasteurized soft pasta filata cheeses. Mozzarella, telita, and guayanés were collected from local markets and informal vendors in the urban area of the Caroní municipality of the city of Puerto Ordaz, Bolívar State, Venezuela.
Two hundred and fifty g of the substance were collected into a sterile sample collection bag, transferred to the laboratory in a chilled container, and processed within 24 h of collection.
For E. coli isolation, 10 g of each cheese were homogenized in 90 ml of 0.1% peptone water (diluted to 10-1) following the procedures established by the Venezuelan Commission of Industrial Standards (COVENIN), (1989). Briefly, the homogenized solutions were subsequently incubated at 36 °C for 2 h to enhance the detection of E. coli strains. Three dilutions (10-2 to 10-4) were prepared from this solution and 1 ml from each dilution was inoculated onto rehydratable Petrifilm-type E. coli/coliform plates (3MTM, USA) and incubated at 35 °C for 18 to 24 h, according to the supplier's recommendations (Guillén et al., 2014). All plates indicating growth between 4 to 10 Colony Forming Units (CFU), suggestive of E. coli, were selected. These colonies were recognized by their blue color and gas production which manifested as bubbles. Four colonies from each plate were randomly selected and plated in Brain Heart Infusion (BHI) broth (BBL, Cockeysville, Md, USA) and incubated at 36 °C for 18-24 h. The subcultures were then plated on Levine or MacConkey agar (Himedia, Mumbai, India) and incubated at 36 °C for 18-24 h. Lactose-fermenting colonies were collected, and those with a morphology typical of E. coli were identified by conventional methods (biochemical and phenotypic tests).
Although we were not aware of any clinical cases associated with the consumption of the dairy products under investigation during the course of this study, we additionally investigated the presence of E. coli O157:H7. All E. coli strains were tested for sorbitol fermentation using biochemical methods. Sorbitol-negative phenotypic variants were subjected to agglutination assays with particular antisera (AntiColi O157:K-, Sifin Berlin, Germany). E. coli ATCC 25922 and E. coli O157:H7 (CVCM1931) were utilized as control strains according to the supplier's recommendations (Guillén et al., 2014; Millán et al., 2018).
Antimicrobial susceptibility tests
The disk diffusion method was applied to consider the antimicrobial susceptibility profiles of the isolates and the data were interpreted based on the breakpoint values presented in the Clinical and Laboratory Standards Institute (CLSI, 2023) guidelines. Fifteen antimicrobial agents (Oxoid Ltd., Basingstoke, UK) were tested: ampicillin (10 μg), amoxicillin/clavulanate (20/10 μg), cefazolin (30 μg), ceftazidime (30 μg), cefotaxime (30 μg), aztreonam (30 μg), imipenem (10 μg), meropenem (10 μg), ertapenem (10 μg), gentamicin (10 μg), tobramycin (10 μg), amikacin (30 μg), ciprofloxacin (5 μg), tetracycline (30 μg), and trimethoprim-sulfamethoxazole (1.25 μg/23.75 μg). E. coli ATCC 25922 was applied as a quality control strain (CLSI, 2023).
DNA preparation
For DNA extraction, the strains were primarily streaked onto Trypticase Soy Agar (TSA; Oxoid, Ltd., Basingstoke, UK) and incubated for 24 h at 36 0C. A single colony was suspended in 100 μl of sterile deionized water and placed in a thermal block (Eppendorf, Germany) at 100 0C for 10 min. The suspension was frozen and centrifuged at 14,000 rpm for 5 min, the DNA-containing supernatant was collected and 1 μl was utilized as a DNA template (Guillén et al., 2014; Millán et al., 2018).
Detection of virulence genes
All 36 E. coli isolates were subjected to conventional Polymerase Chain Reaction (PCR) screening for genetic markers of virulence related to ExPEC, employing primers and conditions previously depicted (Johnson and Stell, 2000). The selected genes were: papAH (P fimbriae structural subunit), kpsMT II (group 2 capsular polysaccharide units), fimH (D-mannose specific adhesin, type 1 fimbriae), fyuA (yersiniabactin receptor), usp (uropathogenic specific protein), and Pathogenicity Island (PAI; GenBank Nº AF003742). These six virulence genes were selected on the basis of their expression in ExPEC strains circulating in the region. Previous studies have identified the prevalence of these genes in strains isolated from dairy products and clinical samples, denoting a potential connection with outbreaks of infections in humans and animals, as reported by our team since 2014 in various investigations (Guillén et al., 2014; Millán et al., 2018). E. coli LMM/E02-ULA (fimH+,fyuA+,kpsMTII+, and PAI+), E. coli LMM/Sc03-ULA (papAH+), and E. coli LMM/E02-ULA (usp+) were used as positive controls strain.
Phylogenetic grouping
Phylogenetic grouping of E. coli strains was determined by multiplex PCR, following the procedure outlined by Beghain et al. (2018). Isolates were classified into eight major E. coli phylogenetic groups (A, B1, B2, C, D, E, F, and clade I) in accordance with the presence or absence of genes (chuA, yjaA, arpa, trpA, and a non-coding DNA fragment (TspE4.C2). The strains E. coli AO38-ULA (arpA and yjaA), UPEC 09-ULA (chuA, yjaA, and TspE4.C2), and E. coli SC20-ULA (trpA) were used as positive controls.
Repetitive Element sequence-based PCR (Rep-PCR) typing
Rep-PCR analysis was conducted using the primers Rep-PCR1 (5'-IIIG CGC CGI CAT CAG GC- 3') and Rep-PCR2 (5'-ACG TCT TAT CAG GCC TAC-3') according to the established protocols described by Versalovic et al. (1991). Briefly, the amplification was performed in 25 µl of reaction mixture including 5 μl of the DNA template, 2.5 µl of buffer (10X; Bioneer, Daejeon, Korea), 2.5 µl of MgCl2 (50 Mm; Bioneer, Daejeon, Korea), 3 µL of dNTPs (10 Mm; Bioneer, Daejeon, Korea), 3 µl of each of the primers (10 pmol/µl), 0.5 µl of Taq polymerase (5 U/µL; Bioneer, Daejeon, Korea), and 5.5 µl of sterile milli-Q water. The resulting Rep-PCR patterns were analyzed using the TreeCon 1.3b software (http://bioinformatics.psb. ugent.be/ software/details/TREECON). A minimum of 95% genetic similarity was exploited to classify strains as genetically related and to assign them to the identical cluster.
All DNA amplifications were executed in a thermocycler Master cycler (Eppendorf, Germany). The PCR products were separated by horizontal electrophoresis through 1.5% (w/v) agarose gels (Sigma-Aldrich Co. St. Louis, MO, USA), stained with ethidium bromide (Sigma-Aldrich, Co. St. Louis, MO, USA) and documented by using the UVP Biodoc-it system (California, USA). Amplicon sizes were compared with a 100-bp DNA ladder (Bioneer, Daejeon, Korea).
Statistical analysis
Data were analyzed using the IBM SPSS Statistics software, version 21 (IBM Corporation, NY, USA). Continuous variables were characterized using mean and Standard Deviation (SD), whereas nominal and ordinal variables were expressed as percentages. The Chi-square test was employed to ascertain associations between categorical variables. A p-value<0.05 was considered statistically significant.
Results
A total of 75 E. coli strains were isolated from three different types of fresh pasta filata cheese: mozzarella, telita, and guayanés. From these strains, 36 E. coli isolates were randomly selected and distributed as illustrated in Table 1. None of the selected strains tested positive for the pathogenic serotype E. coli O157:H7. All strains were susceptible to the 15 antibiotics tested (Table 2).
Table 1: Distribution of 36 selected strains of Escherichia coli* isolated from the pasta filata cheeses
* The total E. coli population was 75 strains.
Table 2: Susceptibility of 36 strains of Escherichia coli against 15 antimicrobial agents isolated from mozzarella, telita, and guayanés cheeses
Table 3 presents the phylogenetic grouping of the E. coli strains found in pasta filata cheeses. Approximately half of the strains were classified in phylogroup A, followed by groups D and B1. Four strains isolated from telita cheese could not be distributed. The E. coli strains of phylogroup A were observed in all studied cheeses, while those of group D were discovered in mozzarella and telita cheeses, and group B1 in telita and guayanés cheeses. Phylogroups B2, C, E, F, and I clade were not detected.
Table 3: Distribution of phylogenetic groups of Escherichia coli according to the type of pasta filata cheese
UC*=Unclassified
The relationship between phylogenetic group and virulence gene profile of E. coli strains is illustrated in Table 4. All strains had at least one virulence gene, with fimH being the most abundant. Regardless of the phylogenetic group, most of the strains unveiled various virulence genes associations. The majority of profiles were formed by the combination of two or three virulence genes. Only one strain from phylogroup A presented a profile consisting of four virulence genes (fimH, fyuA, kpsMT II, and papAH). Table 5 reveals the distribution of phylogroups and virulence factors according to the type of cheese analyzed. Although most E. coli strains were isolated from mozzarella cheese, two phylogroups, A (10) and D (7), were exclusively identified. The strains isolated from telita cheese uncovered the greatest diversity of phylogroups, including A, B1, and D, and the four unclassified strains. The B1 phylogroup was detected in strains isolated from telita and guayanés cheeses, whereas D group was observed in strains from mozzarella and telita cheeses. Regardless of the type of cheese analyzed, 91.7% of the strains carried the virulence gene fimH, which was the only statistically significant virulence factor (p=0.008). The frequency of fyuA was greater than 50% in the strains isolated from these cheeses. kpsMT II was not detected in any of the strains isolated from guayanés cheese, nor in group D strains from mozzarella cheese or in E. coli phylogroups B1 and D isolated from telita cheese. Furthermore, the PAI was absent in all the studied strains.
Artisanal pasta filata cheeses are typically observed in markets, open-air food fairs or from street vendors. They are frequently immersed in whey, which can promote the growth of microorganisms (Maldonado Gómez et al., 2011; Rodríguez et al., 2009). Additionally, the consumption of raw milk, as well as unsophisticated manufacturing processes, transportation, and storage, are critical points that can result in contamination of the product with harmful microorganisms (Perdomo et al., 2015). Previous studies have suggested that consumption of artisanal cheeses is associated with an increase in food-borne disease outbreaks (Koski et al., 2022; Sebastianski et al., 2022).
Escherichia coli is a microorganism commonly observed in the intestines of humans and animals as part of the normal microbiota. Its presence in food is typically indicative of direct or indirect fecal contamination, which may occasionally be accompanied by other intestinal pathogens (Bujnáková et al., 2021). However, the genomic plasticity of E. coli allows it to survive and evolve in various environments, casing it adaptable to different ecological niches, including food, and enabling its presence at any stage of the food chain. Detection and quantification of E. coli is an important factor in assessing food hygiene standards (Ombarak et al., 2016; Sarowska et al., 2019).
Although E. coli is a natural member of the human intestinal microbiota, its interactions with the host and the presence of virulence factors permit it to be classified into three main groups: commensal, Diarrheagenic E. coli (DEC), and Extraintestinal Pathogenic E. coli (ExPEC) (Braz et al., 2020). Phylogenetically, E. coli can be divided into eight phylogroups: A, B1, B2, C, D, E, F, and the cryptic clade I, which can be utilized to estimate its pathogenic potential (Clermont et al., 2013). However, the majority of E. coli strains are classified in groups A, B1, B2, and D (Beghain et al., 2018). Commensal strains are regarded to be of low virulence and comprise phylogroups A and B1, whereas ExPEC are principally grouped in phylogroups B2 and D, which commonly carry genes encoding virulence factors that act on a wide range of cellular processes (Sarowska et al., 2019).
Several studies conducted in Latin America have reported the prevalence of ExPEC in various types of cheese made with unpasteurized milk (Guillén et al., 2014; Pineda et al., 2021). In Venezuela, fresh pasta filata cheese is one of the most common carriers of food-borne diseases due to its poor microbiological quality (Maldonado Gómez et al., 2011; Márquez and García R, 2007; Perdomo et al., 2015; Rodríguez et al., 2009). However, studies describing the genetic characterization of ExpEC in artisanal dairy products for human consumption in the country are scarce (Guillén et al., 2014; Millán et al., 2018). In this regard, the purpose of this research was to molecularly characterize pathogenic E. coli strains isolated from fresh pasta filata cheeses manufactured and marketed in Venezuela.
Materials and methods
This study employed an observational, cross-sectional, descriptive methodology which was conducted between January and March 2019.
Sampling and E. coli isolation
A total of 75 E. coli strains were isolated from a variety of pasta filata cheeses. Of these, 36 strains were randomly selected for analysis. This sampling approach was based on proportionality for each of the selected products and product availability during sampling. The selected sample included unpasteurized soft pasta filata cheeses. Mozzarella, telita, and guayanés were collected from local markets and informal vendors in the urban area of the Caroní municipality of the city of Puerto Ordaz, Bolívar State, Venezuela.
Two hundred and fifty g of the substance were collected into a sterile sample collection bag, transferred to the laboratory in a chilled container, and processed within 24 h of collection.
For E. coli isolation, 10 g of each cheese were homogenized in 90 ml of 0.1% peptone water (diluted to 10-1) following the procedures established by the Venezuelan Commission of Industrial Standards (COVENIN), (1989). Briefly, the homogenized solutions were subsequently incubated at 36 °C for 2 h to enhance the detection of E. coli strains. Three dilutions (10-2 to 10-4) were prepared from this solution and 1 ml from each dilution was inoculated onto rehydratable Petrifilm-type E. coli/coliform plates (3MTM, USA) and incubated at 35 °C for 18 to 24 h, according to the supplier's recommendations (Guillén et al., 2014). All plates indicating growth between 4 to 10 Colony Forming Units (CFU), suggestive of E. coli, were selected. These colonies were recognized by their blue color and gas production which manifested as bubbles. Four colonies from each plate were randomly selected and plated in Brain Heart Infusion (BHI) broth (BBL, Cockeysville, Md, USA) and incubated at 36 °C for 18-24 h. The subcultures were then plated on Levine or MacConkey agar (Himedia, Mumbai, India) and incubated at 36 °C for 18-24 h. Lactose-fermenting colonies were collected, and those with a morphology typical of E. coli were identified by conventional methods (biochemical and phenotypic tests).
Although we were not aware of any clinical cases associated with the consumption of the dairy products under investigation during the course of this study, we additionally investigated the presence of E. coli O157:H7. All E. coli strains were tested for sorbitol fermentation using biochemical methods. Sorbitol-negative phenotypic variants were subjected to agglutination assays with particular antisera (AntiColi O157:K-, Sifin Berlin, Germany). E. coli ATCC 25922 and E. coli O157:H7 (CVCM1931) were utilized as control strains according to the supplier's recommendations (Guillén et al., 2014; Millán et al., 2018).
Antimicrobial susceptibility tests
The disk diffusion method was applied to consider the antimicrobial susceptibility profiles of the isolates and the data were interpreted based on the breakpoint values presented in the Clinical and Laboratory Standards Institute (CLSI, 2023) guidelines. Fifteen antimicrobial agents (Oxoid Ltd., Basingstoke, UK) were tested: ampicillin (10 μg), amoxicillin/clavulanate (20/10 μg), cefazolin (30 μg), ceftazidime (30 μg), cefotaxime (30 μg), aztreonam (30 μg), imipenem (10 μg), meropenem (10 μg), ertapenem (10 μg), gentamicin (10 μg), tobramycin (10 μg), amikacin (30 μg), ciprofloxacin (5 μg), tetracycline (30 μg), and trimethoprim-sulfamethoxazole (1.25 μg/23.75 μg). E. coli ATCC 25922 was applied as a quality control strain (CLSI, 2023).
DNA preparation
For DNA extraction, the strains were primarily streaked onto Trypticase Soy Agar (TSA; Oxoid, Ltd., Basingstoke, UK) and incubated for 24 h at 36 0C. A single colony was suspended in 100 μl of sterile deionized water and placed in a thermal block (Eppendorf, Germany) at 100 0C for 10 min. The suspension was frozen and centrifuged at 14,000 rpm for 5 min, the DNA-containing supernatant was collected and 1 μl was utilized as a DNA template (Guillén et al., 2014; Millán et al., 2018).
Detection of virulence genes
All 36 E. coli isolates were subjected to conventional Polymerase Chain Reaction (PCR) screening for genetic markers of virulence related to ExPEC, employing primers and conditions previously depicted (Johnson and Stell, 2000). The selected genes were: papAH (P fimbriae structural subunit), kpsMT II (group 2 capsular polysaccharide units), fimH (D-mannose specific adhesin, type 1 fimbriae), fyuA (yersiniabactin receptor), usp (uropathogenic specific protein), and Pathogenicity Island (PAI; GenBank Nº AF003742). These six virulence genes were selected on the basis of their expression in ExPEC strains circulating in the region. Previous studies have identified the prevalence of these genes in strains isolated from dairy products and clinical samples, denoting a potential connection with outbreaks of infections in humans and animals, as reported by our team since 2014 in various investigations (Guillén et al., 2014; Millán et al., 2018). E. coli LMM/E02-ULA (fimH+,fyuA+,kpsMTII+, and PAI+), E. coli LMM/Sc03-ULA (papAH+), and E. coli LMM/E02-ULA (usp+) were used as positive controls strain.
Phylogenetic grouping
Phylogenetic grouping of E. coli strains was determined by multiplex PCR, following the procedure outlined by Beghain et al. (2018). Isolates were classified into eight major E. coli phylogenetic groups (A, B1, B2, C, D, E, F, and clade I) in accordance with the presence or absence of genes (chuA, yjaA, arpa, trpA, and a non-coding DNA fragment (TspE4.C2). The strains E. coli AO38-ULA (arpA and yjaA), UPEC 09-ULA (chuA, yjaA, and TspE4.C2), and E. coli SC20-ULA (trpA) were used as positive controls.
Repetitive Element sequence-based PCR (Rep-PCR) typing
Rep-PCR analysis was conducted using the primers Rep-PCR1 (5'-IIIG CGC CGI CAT CAG GC- 3') and Rep-PCR2 (5'-ACG TCT TAT CAG GCC TAC-3') according to the established protocols described by Versalovic et al. (1991). Briefly, the amplification was performed in 25 µl of reaction mixture including 5 μl of the DNA template, 2.5 µl of buffer (10X; Bioneer, Daejeon, Korea), 2.5 µl of MgCl2 (50 Mm; Bioneer, Daejeon, Korea), 3 µL of dNTPs (10 Mm; Bioneer, Daejeon, Korea), 3 µl of each of the primers (10 pmol/µl), 0.5 µl of Taq polymerase (5 U/µL; Bioneer, Daejeon, Korea), and 5.5 µl of sterile milli-Q water. The resulting Rep-PCR patterns were analyzed using the TreeCon 1.3b software (http://bioinformatics.psb. ugent.be/ software/details/TREECON). A minimum of 95% genetic similarity was exploited to classify strains as genetically related and to assign them to the identical cluster.
All DNA amplifications were executed in a thermocycler Master cycler (Eppendorf, Germany). The PCR products were separated by horizontal electrophoresis through 1.5% (w/v) agarose gels (Sigma-Aldrich Co. St. Louis, MO, USA), stained with ethidium bromide (Sigma-Aldrich, Co. St. Louis, MO, USA) and documented by using the UVP Biodoc-it system (California, USA). Amplicon sizes were compared with a 100-bp DNA ladder (Bioneer, Daejeon, Korea).
Statistical analysis
Data were analyzed using the IBM SPSS Statistics software, version 21 (IBM Corporation, NY, USA). Continuous variables were characterized using mean and Standard Deviation (SD), whereas nominal and ordinal variables were expressed as percentages. The Chi-square test was employed to ascertain associations between categorical variables. A p-value<0.05 was considered statistically significant.
Results
A total of 75 E. coli strains were isolated from three different types of fresh pasta filata cheese: mozzarella, telita, and guayanés. From these strains, 36 E. coli isolates were randomly selected and distributed as illustrated in Table 1. None of the selected strains tested positive for the pathogenic serotype E. coli O157:H7. All strains were susceptible to the 15 antibiotics tested (Table 2).
Table 1: Distribution of 36 selected strains of Escherichia coli* isolated from the pasta filata cheeses
| Type | Number of Escherichia coli strains | % |
| Mozzarella | 17 | 47.2 |
| Telita | 16 | 44.4 |
| Guayanés | 3 | 8.3 |
| Total | 36 | 99.9 |
Table 2: Susceptibility of 36 strains of Escherichia coli against 15 antimicrobial agents isolated from mozzarella, telita, and guayanés cheeses
| Antibiotics | Antimicrobial susceptibility disk diffusion method |
| Ampicillin | sensible |
| Amoxicillin/clavulanate | sensible |
| Cefazolin | sensible |
| Cefotaxime | sensible |
| Ceftazidime | sensible |
| Aztreonam | sensible |
| Imipenem | sensible |
| Meropenem | sensible |
| Ertapenem | sensible |
| Amikacin | sensible |
| Gentamicin | sensible |
| Tobramycin | sensible |
| Ciprofloxacin | sensible |
| Tetracycline | sensible |
| Trimethoprim-sulfamethoxazole | sensible |
Table 3 presents the phylogenetic grouping of the E. coli strains found in pasta filata cheeses. Approximately half of the strains were classified in phylogroup A, followed by groups D and B1. Four strains isolated from telita cheese could not be distributed. The E. coli strains of phylogroup A were observed in all studied cheeses, while those of group D were discovered in mozzarella and telita cheeses, and group B1 in telita and guayanés cheeses. Phylogroups B2, C, E, F, and I clade were not detected.
Table 3: Distribution of phylogenetic groups of Escherichia coli according to the type of pasta filata cheese
| Type of artisanal cheese Escherichia coli strains |
E. coli phylogenetic group |
n (%) | ||
Mozzarella |
||||
| A | 10 (58.8) | |||
| D | 7 (41.2) | |||
Telita |
||||
| A | 7 (43.7) | |||
| B1 | 1 (6.3) | |||
| D | 4 (25.0) | |||
| UC* | 4 (25.0) | |||
Guayanés |
||||
| A | 2 (66.7) | |||
| B1 | 1 (33.3) |
The relationship between phylogenetic group and virulence gene profile of E. coli strains is illustrated in Table 4. All strains had at least one virulence gene, with fimH being the most abundant. Regardless of the phylogenetic group, most of the strains unveiled various virulence genes associations. The majority of profiles were formed by the combination of two or three virulence genes. Only one strain from phylogroup A presented a profile consisting of four virulence genes (fimH, fyuA, kpsMT II, and papAH). Table 5 reveals the distribution of phylogroups and virulence factors according to the type of cheese analyzed. Although most E. coli strains were isolated from mozzarella cheese, two phylogroups, A (10) and D (7), were exclusively identified. The strains isolated from telita cheese uncovered the greatest diversity of phylogroups, including A, B1, and D, and the four unclassified strains. The B1 phylogroup was detected in strains isolated from telita and guayanés cheeses, whereas D group was observed in strains from mozzarella and telita cheeses. Regardless of the type of cheese analyzed, 91.7% of the strains carried the virulence gene fimH, which was the only statistically significant virulence factor (p=0.008). The frequency of fyuA was greater than 50% in the strains isolated from these cheeses. kpsMT II was not detected in any of the strains isolated from guayanés cheese, nor in group D strains from mozzarella cheese or in E. coli phylogroups B1 and D isolated from telita cheese. Furthermore, the PAI was absent in all the studied strains.
Table 4: Distribution of the number and profile of virulence genes according to phylogenetic group of Escherichia coli from pasta filata cheeses
| Escherichia coli phylogenetic group n (%) |
No. virulence factors | Profile of virulence genes |
n (%) |
| A=19 (52.8) | 1 | fimH | 6 (31.5) |
| 2 | fimH; papAH | 3 (15.8) | |
| fimH; usp | 2 (10.5) | ||
| fimH; fyuA | 1 (5.3) | ||
| fyuA; usp | 1 (5.3) | ||
| 3 | fyuA;usp; kpsMT II | 1 (5.3) | |
| fimH; kpsMT II; papAH | 1 (5.3) | ||
| fimH; fyuA; papAH | 1 (5.3) | ||
| fimH; papAH; kpsMT II | 1 (5.3) | ||
| fimH; fyuA; kpsMT II | 1 (5.3) | ||
| 4 | fimH; fyuA; kpsMT II; papAH | 1 (5.3) | |
| B1=2 (5.6) | 1 | fimH | 1 (50.0) |
| 2 | fimH; fyuA | 1 (50.0) | |
| D=11 (30.5) | 1 | fimH | 1 (9.1) |
| usp | 1 (9.1) | ||
| 2 | fimH; fyuA | 2 (18.2) | |
| fimH; usp | 1 (9.1) | ||
| 3 | fimH; fyuA; papAH | 3 (27.3) | |
| fimH; fyuA;usp | 2 (18.2) | ||
| fimH; papAH;usp | 1 (9.1) | ||
| UC*=4 (11.1) | 2 | fimH; fyuA | 3 (75.0) |
| 3 | fimH; fyuA; kpsMT II | 1 (25.0) |
Table 5: Distribution of phylogenetic groups and presence of virulence genes of Escherichia coli according to the type of pasta filata cheese
| Escherichia coli | Virulence genes | |||||
| Phylogenetic group | n (%) | |||||
| fimH | fyuA | kpsMT II | papAH | usp | PAI | |
| Mozzarella cheese n=17 |
14 (82.4) |
6 (35.3) |
4 (23.5) |
8 (47.0) |
5 (29.4) |
0 |
| A=10 | 8 (80) | 3 (30) | 4 (40) | 4 (40) | 2 (20) | 0 |
| D=7 | 6 (85.7) | 3 (42.9) | 0 | 4 (57.1) | 3 (42.9) | 0 |
| Telita cheese n=16 |
16 (100) |
11 (68.8) |
2 (12.5) |
2 (12.5) |
3 (24.0) |
0 |
| A=7 | 7 (100) | 2 (28.6) | 1 (14.3) | 2 (28.6) | 1 (14.3) | 0 |
| B1=1 | 1 (100) | 1 (100) | 0 | 1 (100) | 0 | 0 |
| D=4 | 4 (100) | 4 (100) | 0 | 0 | 2 (50) | 0 |
| UC*=4 | 4 (100) | 4 (100) | 1 (25) | 0 | 0 | 0 |
| Guayanés cheese n=3 |
3 (100) |
2 (66.6) |
0 |
1 (33.3) |
1 (33.3) |
0 |
| A=2 | 2 (100) | 1 (50) | 0 | 1 (50) | 1 (50) | 0 |
| B1=1 | 1 (100) | 1 (100) | 0 | 0 | 0 | 0 |
| Total n=36 |
33 (91.7) | 19 (52.8) | 6 (16.7) | 12 (33.3) | 9 (25.0) | 0 |
| p-value | 0.008 | 0.138 | 0.502 | 0.226 | 0.733 | - |
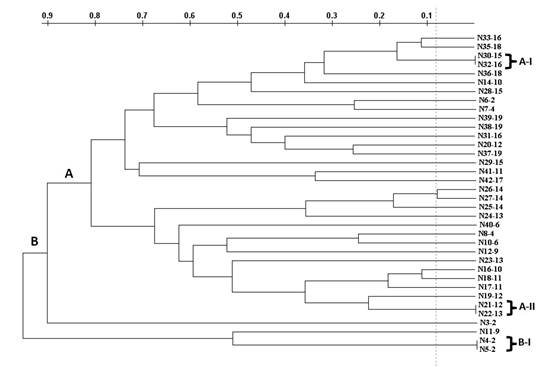
A total of 30 Rep-PCR profiles were detected among the 36 E. coli strains analyzed (Figure 1). Two major clusters A and B were observed. A was the dominant cluster, concentrating most of the E. coli (33/36; 91.7%), while the B cluster consisted of three strains. Out of the analyzed E. coli strains, 6 (16.7%) showed a genetic relatedness of ≥95% and were grouped into three sub-clusters (A-I, A-II, and B-I), containing 2 strains each. The remaining profiles were represented by a single isolate. The phenotypic and genetic features of the six clonally related E. coli strains are displayed in Table 6. Two clonally related strains were identified for each type of analyzed cheese. Sub-cluster A-I involved two E. coli strains belonging to group D, which were isolated from guayanés cheese. Both strains harbored two and three virulence genes, respectively, with the presence of fimH and fyuA in both strains. Sub-cluster A-II comprises two groups A E. coli strains isolated from mozzarella cheese. These two strains had associations of three and four virulence genes, respectively, with fimH, fyuA, and kpsMT II being common to both. Sub-cluster B-I was formed by two strains of E. coli isolated from telita cheese. One of these strains, belonging to group A, had a single virulence gene (fimH), whereas the other, from phylogroup B1, had two virulence genes (fimH and fyuA).
Table 6: Genetic characteristics of the six clonally related strains of Escherichia coli (A-I, A-II and B-I)
| Rep-PCR Cluster |
Nº Strain Escherichia coli |
Cheese type |
Phylogenetic group |
Virulence genes |
| A-I | N30-15 | Guayanés | D | fimH, fyuA |
| N32-16 | Guayanés | D | fimH, fyuA, usp | |
| A-II | N21-12 | Mozzarella | A | fimH, kpsMT II, fyuA |
| N22-13 | Mozzarella | A | fimH, fyuA, kpsMT II, papAH | |
| B-I | N4-2 | Telita | A | fimH, |
| N5-2 | Telita | B1 | fimH, fyuA |

Figure 1: Genetic diversity of Escherichia coli strains isolated from fresh pasta filata cheeses based on similarity coefficients calculated from Repetitive element sequence-based Polymerase Chain Reaction (Rep-PCR) analysis data. Clusters with similarity of ≥95% were designed as: A-I (2 strains), AII (2 strains), and B-I (2 strains)
Discussion
One of the most appreciated fresh white cheeses in Venezuela is pasta filata. In this investigation, a collection of 36 E. coli strains was analyzed from three types of artisanal pasta filata cheeses, mozzarella, telita, and guayanés, purchased from different local popular markets and informal vendors in the urban area of the Caroní municipality of the city of Puerto Ordaz, Bolívar State, Venezuela, were analyzed. According to previous studies (Bagel and Sergentet, 2022; D’Amico, 2014) dairy products that have not undergone sanitization processes may contain an acceptable levels of E. coli without necessarily posing a risk to the consumers or degrading the quality of these products. However, levels above the recommended limits for E. coli may indicate fecal contamination, inappropriate handling, and poor hygiene practices (Pineda et al., 2021). Although it is frequently assumed that the production and marketing of pasta filata cheeses imply deficiencies in hygienic practices, the 36 E. coli strains isolated were susceptible to the tested antibiotics. These results may be connected to the healthy dairy animals that provided the milk. The use of antibiotics in dairy cows, which exerts selective pressure, is directly associated with bacterial resistance. However, if E. coli strains in dairy products carry virulence genes, they could potentially harm consumers.
Carlos et al. (2010) recommend that the distribution of E. coli phylogenetic groups can assist to identify sources of fecal contamination and the virulence potential of these strains in animal products. In this regard, A and B1 E. coli strains appear in a wide range of herbivorous and carnivorous mammals, whereas B2 and D have a narrow and specialized host range; B2 is an appropriate indicator of human fecal contamination. In this study, group A was the dominant phylogroup, corresponding to commensal E. coli strains probably present in a bovine host, while the absence of group B2 could indicate that the source of fecal contamination of artisanal cheeses was not of human origin. The obtained results are in agreement with those of Bujnáková et al. (2021), De Campos et al. (2018), and Ombarak et al. (2016). They reported that E. coli isolated from unpasteurized ovine cheeses in Slovakia, Minas cheeses in Brazil, and raw milk cheeses in Egypt, respectively, were predominantly from the A phylogenetic group. On the other hand, Beghain et al. (2018) reported that only 1% of E. coli strains cannot be assigned to one of the eight recognized phylogroups. However, in this work, the number of unclassified strains was ten times higher than what might normally be expected. It is possible that these 4 (11.1%) unclassified strains are the result of recombination between various phylogroups.
Although E. coli strains classified into phylogroups A and B1 are typically considered to be of low virulence, in this study it was recognized that most of the strains carry at least two virulence genes encoding adhesion (fimH) and iron uptake (fyuA) mechanisms. It is crucial to highlight that the only strain carrying four (fimH, fyuA, kpsMT II, and papAH) of the six virulence genes studied, belonged to phylogroup A. In addition, four strains that couldn't be classified according to the Clermont's scheme also contained resistance genes (fimH, fyuA, kpsMT II) (Clermont et al., 2013). These results demonstrate the heterogeneity in the distribution of virulence factors among E. coli strains isolated from pasta filata cheeses, highlighting the multifactorial and complex nature of their pathogenic potential. In bacterial pathogens which infect mucosal tissues, such as E. coli, the expression of a single adhesin is necessary and sufficient for pathogenesis. However, if this adhesin is linked to genes for iron uptake, a critical factor for survival, it may allow the bacterium to establish itself in a competitive environment (Sarowska et al., 2019).
In recent years, researchers have postulated that food, including unpasteurized dairy products, can serve as a reservoir for numerous virulence factors responsible for extra intestinal infections and play a significant role in the transmission of ExPEC strains (Bujnáková et al., 2021, Ovi et al., 2023). The E. coli isolated in this study showed a virulence factor load identical to that reported by Millán et al. 2020 and Quijada-Martínez et al. in Uropathogenic E. coli (UPEC) isolated from hospitalized patients in Mérida, Venezuela. Sarowska et al. (2019) describe Food-borne Urinary Tract Infection (FUTI) as UTIs caused by contaminated food. The specific pathotypes of E. coli that are responsible for FUTI have not yet to been precisely defined. However, Jakobsen et al. (2010) evaluated the presence of ExPEC-associated virulence genes in E. coli isolated from UTI patients, in dairy derivatives and meat. Similarly, De Campos et al. (2018) isolated E. coli strains in unpasteurized cheeses in Brazil with virulence patterns comparable to the ones detected in E. coli causing Meningitis Neonatal E. coli (MNEC). Presumably, this phenomenon is based on the observation that the virulence genes of E. coli are located in transmissible genetic elements, including genomic islands, bacteriophages, Insertion Sequences (ISs), integrons, plasmids, and transposons; therefore, these elements can be readily transferred among various bacterial species and induce genetic rearrangements. As a result, the horizontal transfer between different E. coli strains facilitates the emergence of novel pathogenic strains, designated as Hybrid Pathogenic E. coli. (HyPEC) (Braz et al., 2020). Although, in this work, approximately 80% of the E. coli strains isolated in pasta filata cheeses were assigned to the low virulence phylogroups (A and B1), their virulence factor content was remarkably comparable to the profile of UPEC pathotypes of clinical origin recorded in the region (fimH, fyuA, kpsMT II, papAH, and usp), making these HyPEC strains represent a highly serious threat that requires further study.
Rep-PCR typing of the 36 E. coli strains isolated from the three pasta filata cheeses revealed a heterogeneous population structure, which was clustered into 30 profiles. The strains were distributed in two main clusters (A and B) with similarity indices not exceeding 10%; however, three sub-clusters (A-I, A-II, and B-I) with a genetically distant relationship emerged with internal similarity ranges of >95%, without any association with phenotypic or genetic characteristics. Each subcluster contained two strains from the identical type of pasta filata cheese, implying a frequent source of contamination, presumably raw milk. The genetic diversity and polyclonal distribution of the E. coli strains observed in this research may have been influenced by various uncontrolled factors, including the origin of the raw milk, the manufacturing process, the preservation conditions of the final product, the randomness in the selection of the strains and the geographical area where the study was implemented.
Conclusion
The results of the study confirm the hypothesis that the E. coli strains isolated from the three types of fresh pasta filata cheeses share characteristics and virulence factors compatible with ExPEC strains from animals and humans, and therefore pose a health risk. However, the presence of E. coli in these cheeses not only indicates contamination of fecal origin, which could potentially be associated with other enteropathogens, but also suggests that these cheeses may serve as a means of transmission and selection of virulent clones causing intestinal and extra intestinal diseases. This study highlights the need to strengthen hygienic and sanitary controls at all stages of cheese production and to implement measures for epidemiological surveillance of potentially pathogenic bacterial strains found in fresh, artisanal pasta filata cheeses sold in the state of Bolívar, Venezuela. Although this study was limited to a specific geographical area, the obtained results may be representative of a common issue detected in other regions of the world with similar socio-economic and cultural characteristics.
Author contributions
Y.Y.V.-R. and L.G. investigated, analyzed data, and participated in the experimental development of the study; C.C.-S. investigated, analyzed data, and conducted the experimental work; M.A. investigated, analyzed data, conducted the experimental work, corrections, and critical review of the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest
We declare that there is no conflict of interest.
Funding
This study was partially supported by the Council of Scientific, Humanistic, technological and Arts of University of The Andes (CDCHTA-ULA), Mérida, Venezuela (grant CVI-ADG-FA-02-97)
Ethical consideration
No ethical approval is required. No human or animal was used for the study.
Acknowledgements
The authors would like to thank the Fundación Empresas Polar, Caracas, Venezuela (Grant No. 140275) for their support in carrying out this study
References
Bagel A., Sergentet D. (2022). Shiga toxin-producing Escherichia coli and milk fat globules. Microorganisms. 10: 496. [DOI: 10.3390/ microorganisms10030496]
Beghain J., Bridier-Nahmias A., Le Nagard H., Denamur E., Clermont O. (2018). Clermon typing: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microbial Genomics. 4: e000192. [DOI: 10.1099/mgen.0.000192]
Braz V.S., Melchior K., Moreira C.G. (2020). Escherichia coli as a multifaceted pathogenic and versatile bacterium. Frontiers in Cellular and Infection Microbiology. 10: 548492. [DOI: 10.3389/fcimb.2020.548492]
Bujnáková D., Karahutová L., Kmet V. (2021). Escherichia coli specific virulence-gene markers analysis for quality control of ovine cheese in Slovakia. Microorganisms. 9: 1808. [DOI: 10.3390/microorganisms9091808]
Carlos C., Pires M.M., Stoppe N.C., Hachich E.M., Sato M.I., Gomes T.A., Amaral L.A., Ottoboni L.M.M. (2010). Escherichia coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiology. 10: 161. [DOI: 10.1186/1471-2180-10-161]
Clermont O., Christenson J.K., Denamur E., Gordon D.M. (2013). The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylogroups. Environmental Microbiology Reports. 5: 58-65. [DOI: 10.1111/1758-2229.12019]
Clinical and Laboratory Standards Institute (CLSI). (2023). Performance standards for antimicrobial susceptibility testing. 33th edition. CLSI Supplement M100-S27, Wayne, Pennsylvaniya. URL: https://clsi.org/standards/products/ microbiology/ documents/m100/. Accessed 03 February 2024.
D’Amico D.J. (2014). Microbiological quality and safety issues in cheesemaking. Microbiology Spectrum. 2: CM-0011-2012. [DOI: 10.1128/microbiolspec.CM-0011-2012]
De Campos A.C.L.P., Puño-Sarmiento J.J., Medeiros L.P., Gazal L.E.S., Maluta R.P., Navarro A., Kobayashi R.K.T., Fagan E.P., Nakazato G. (2018). Virulence genes and antimicrobial resistance in Escherichia coli from cheese made from unpasteurized milk in Brazil. Foodborne Pathogens and Disease. 15: 94-100. [DOI: 10.1089/fpd.2017.2345]
Guillén L., Millán B., Araque M. (2014). Molecular characterization of Escherichia coli strains isolated from homemade dairy foods produced in Mérida, Venezuela. Infectio. 18: 100-108. [DOI: 10.1016/j.infect.2014.04.004]. [Spanish with English abstract]
Jakobsen L., Spangholm D.J., Pedersen K., Jensen L.B., Emborg H.D., Agerso Y., Aarestrup F.M., Hammerum A.M., Frimodt-Moller N. (2010). Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. International Journal of Food Microbiology. 142: 264-272. [DOI: 10.1016/j.ijfoodmicro.2010.06.025]
Johnson J.R., Stell A.L. (2000). Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. The Journal of Infectious Diseases. 181: 261-272. [DOI: 10.1086/315217]
Koski L., Kisselburgh H., Landsman L., Hulkower R., Howard-Williams M., Salah Z., Kim S., Bruce B.B., Bazaco M.C., Batz M.B., Parker C.C., Leonard C.L., et al. (2022). Foodborne illness outbreaks linked to unpasteurised milk and relationship to changes in state laws – United States, 1998–2018. Epidemiology and Infection. 150: e183. [DOI: 10.1017/S0950268822001649]
Maldonado Gómez R.J, Rodríguez M., Llanca Córdova L.R., Román Montilla Y.J., Isturiz Vásquez R., Giménez Alfaro O.J., Gámez Mendoza L.A., Meléndez B. (2011). General technological flow diagram and characterization of filata cheese telita type. Agronomía Tropical. 61: 177-188. [Spanish with English abstract]
Márquez J.G., García R C.E. (2007). Pathogenic microflora found in white "telita" cheese made in four states of Venezuela. Anales Venezolanos de Nutrición. 20: 17-21. [Spanish with English abstract]
Millán Y., Méndez A., Burguera M., Pimentel P., Araque M., Ramírez A. (2018). Determination of enterobacteria and detection of virulence genes in Escherichia coli isolated from raw milk. Revista de la Sociedad Venezolana de Microbiología. 38: 58-63. [Spanish with English abstract]
Millán Y., Araque M., Ramírez A. (2020). Distribution of phylogenetic groups, virulence factors and antimicrobial susceptibility in strains of uropathogenic Escherichia coli. Revista Chilena de Infectología. 37: 117-123. [DOI: 10.4067/s0716-10182020000200117]. [Spanish with English abstract] One of the most appreciated fresh white cheeses in Venezuela is pasta filata. In this investigation, a collection of 36 E. coli strains was analyzed from three types of artisanal pasta filata cheeses, mozzarella, telita, and guayanés, purchased from different local popular markets and informal vendors in the urban area of the Caroní municipality of the city of Puerto Ordaz, Bolívar State, Venezuela, were analyzed. According to previous studies (Bagel and Sergentet, 2022; D’Amico, 2014) dairy products that have not undergone sanitization processes may contain an acceptable levels of E. coli without necessarily posing a risk to the consumers or degrading the quality of these products. However, levels above the recommended limits for E. coli may indicate fecal contamination, inappropriate handling, and poor hygiene practices (Pineda et al., 2021). Although it is frequently assumed that the production and marketing of pasta filata cheeses imply deficiencies in hygienic practices, the 36 E. coli strains isolated were susceptible to the tested antibiotics. These results may be connected to the healthy dairy animals that provided the milk. The use of antibiotics in dairy cows, which exerts selective pressure, is directly associated with bacterial resistance. However, if E. coli strains in dairy products carry virulence genes, they could potentially harm consumers.
Carlos et al. (2010) recommend that the distribution of E. coli phylogenetic groups can assist to identify sources of fecal contamination and the virulence potential of these strains in animal products. In this regard, A and B1 E. coli strains appear in a wide range of herbivorous and carnivorous mammals, whereas B2 and D have a narrow and specialized host range; B2 is an appropriate indicator of human fecal contamination. In this study, group A was the dominant phylogroup, corresponding to commensal E. coli strains probably present in a bovine host, while the absence of group B2 could indicate that the source of fecal contamination of artisanal cheeses was not of human origin. The obtained results are in agreement with those of Bujnáková et al. (2021), De Campos et al. (2018), and Ombarak et al. (2016). They reported that E. coli isolated from unpasteurized ovine cheeses in Slovakia, Minas cheeses in Brazil, and raw milk cheeses in Egypt, respectively, were predominantly from the A phylogenetic group. On the other hand, Beghain et al. (2018) reported that only 1% of E. coli strains cannot be assigned to one of the eight recognized phylogroups. However, in this work, the number of unclassified strains was ten times higher than what might normally be expected. It is possible that these 4 (11.1%) unclassified strains are the result of recombination between various phylogroups.
Although E. coli strains classified into phylogroups A and B1 are typically considered to be of low virulence, in this study it was recognized that most of the strains carry at least two virulence genes encoding adhesion (fimH) and iron uptake (fyuA) mechanisms. It is crucial to highlight that the only strain carrying four (fimH, fyuA, kpsMT II, and papAH) of the six virulence genes studied, belonged to phylogroup A. In addition, four strains that couldn't be classified according to the Clermont's scheme also contained resistance genes (fimH, fyuA, kpsMT II) (Clermont et al., 2013). These results demonstrate the heterogeneity in the distribution of virulence factors among E. coli strains isolated from pasta filata cheeses, highlighting the multifactorial and complex nature of their pathogenic potential. In bacterial pathogens which infect mucosal tissues, such as E. coli, the expression of a single adhesin is necessary and sufficient for pathogenesis. However, if this adhesin is linked to genes for iron uptake, a critical factor for survival, it may allow the bacterium to establish itself in a competitive environment (Sarowska et al., 2019).
In recent years, researchers have postulated that food, including unpasteurized dairy products, can serve as a reservoir for numerous virulence factors responsible for extra intestinal infections and play a significant role in the transmission of ExPEC strains (Bujnáková et al., 2021, Ovi et al., 2023). The E. coli isolated in this study showed a virulence factor load identical to that reported by Millán et al. 2020 and Quijada-Martínez et al.
Rep-PCR typing of the 36 E. coli strains isolated from the three pasta filata cheeses revealed a heterogeneous population structure, which was clustered into 30 profiles. The strains were distributed in two main clusters (A and B) with similarity indices not exceeding 10%; however, three sub-clusters (A-I, A-II, and B-I) with a genetically distant relationship emerged with internal similarity ranges of >95%, without any association with phenotypic or genetic characteristics. Each subcluster contained two strains from the identical type of pasta filata cheese, implying a frequent source of contamination, presumably raw milk. The genetic diversity and polyclonal distribution of the E. coli strains observed in this research may have been influenced by various uncontrolled factors, including the origin of the raw milk, the manufacturing process, the preservation conditions of the final product, the randomness in the selection of the strains and the geographical area where the study was implemented.
Conclusion
The results of the study confirm the hypothesis that the E. coli strains isolated from the three types of fresh pasta filata cheeses share characteristics and virulence factors compatible with ExPEC strains from animals and humans, and therefore pose a health risk. However, the presence of E. coli in these cheeses not only indicates contamination of fecal origin, which could potentially be associated with other enteropathogens, but also suggests that these cheeses may serve as a means of transmission and selection of virulent clones causing intestinal and extra intestinal diseases. This study highlights the need to strengthen hygienic and sanitary controls at all stages of cheese production and to implement measures for epidemiological surveillance of potentially pathogenic bacterial strains found in fresh, artisanal pasta filata cheeses sold in the state of Bolívar, Venezuela. Although this study was limited to a specific geographical area, the obtained results may be representative of a common issue detected in other regions of the world with similar socio-economic and cultural characteristics.
Author contributions
Y.Y.V.-R. and L.G. investigated, analyzed data, and participated in the experimental development of the study; C.C.-S. investigated, analyzed data, and conducted the experimental work; M.A. investigated, analyzed data, conducted the experimental work, corrections, and critical review of the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest
We declare that there is no conflict of interest.
Funding
This study was partially supported by the Council of Scientific, Humanistic, technological and Arts of University of The Andes (CDCHTA-ULA), Mérida, Venezuela (grant CVI-ADG-FA-02-97)
Ethical consideration
No ethical approval is required. No human or animal was used for the study.
Acknowledgements
The authors would like to thank the Fundación Empresas Polar, Caracas, Venezuela (Grant No. 140275) for their support in carrying out this study
References
Bagel A., Sergentet D. (2022). Shiga toxin-producing Escherichia coli and milk fat globules. Microorganisms. 10: 496. [DOI: 10.3390/ microorganisms10030496]
Beghain J., Bridier-Nahmias A., Le Nagard H., Denamur E., Clermont O. (2018). Clermon typing: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microbial Genomics. 4: e000192. [DOI: 10.1099/mgen.0.000192]
Braz V.S., Melchior K., Moreira C.G. (2020). Escherichia coli as a multifaceted pathogenic and versatile bacterium. Frontiers in Cellular and Infection Microbiology. 10: 548492. [DOI: 10.3389/fcimb.2020.548492]
Bujnáková D., Karahutová L., Kmet V. (2021). Escherichia coli specific virulence-gene markers analysis for quality control of ovine cheese in Slovakia. Microorganisms. 9: 1808. [DOI: 10.3390/microorganisms9091808]
Carlos C., Pires M.M., Stoppe N.C., Hachich E.M., Sato M.I., Gomes T.A., Amaral L.A., Ottoboni L.M.M. (2010). Escherichia coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiology. 10: 161. [DOI: 10.1186/1471-2180-10-161]
Clermont O., Christenson J.K., Denamur E., Gordon D.M. (2013). The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylogroups. Environmental Microbiology Reports. 5: 58-65. [DOI: 10.1111/1758-2229.12019]
Clinical and Laboratory Standards Institute (CLSI). (2023). Performance standards for antimicrobial susceptibility testing. 33th edition. CLSI Supplement M100-S27, Wayne, Pennsylvaniya. URL: https://clsi.org/standards/products/ microbiology/ documents/m100/. Accessed 03 February 2024.
D’Amico D.J. (2014). Microbiological quality and safety issues in cheesemaking. Microbiology Spectrum. 2: CM-0011-2012. [DOI: 10.1128/microbiolspec.CM-0011-2012]
De Campos A.C.L.P., Puño-Sarmiento J.J., Medeiros L.P., Gazal L.E.S., Maluta R.P., Navarro A., Kobayashi R.K.T., Fagan E.P., Nakazato G. (2018). Virulence genes and antimicrobial resistance in Escherichia coli from cheese made from unpasteurized milk in Brazil. Foodborne Pathogens and Disease. 15: 94-100. [DOI: 10.1089/fpd.2017.2345]
Guillén L., Millán B., Araque M. (2014). Molecular characterization of Escherichia coli strains isolated from homemade dairy foods produced in Mérida, Venezuela. Infectio. 18: 100-108. [DOI: 10.1016/j.infect.2014.04.004]. [Spanish with English abstract]
Jakobsen L., Spangholm D.J., Pedersen K., Jensen L.B., Emborg H.D., Agerso Y., Aarestrup F.M., Hammerum A.M., Frimodt-Moller N. (2010). Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. International Journal of Food Microbiology. 142: 264-272. [DOI: 10.1016/j.ijfoodmicro.2010.06.025]
Johnson J.R., Stell A.L. (2000). Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. The Journal of Infectious Diseases. 181: 261-272. [DOI: 10.1086/315217]
Koski L., Kisselburgh H., Landsman L., Hulkower R., Howard-Williams M., Salah Z., Kim S., Bruce B.B., Bazaco M.C., Batz M.B., Parker C.C., Leonard C.L., et al. (2022). Foodborne illness outbreaks linked to unpasteurised milk and relationship to changes in state laws – United States, 1998–2018. Epidemiology and Infection. 150: e183. [DOI: 10.1017/S0950268822001649]
Maldonado Gómez R.J, Rodríguez M., Llanca Córdova L.R., Román Montilla Y.J., Isturiz Vásquez R., Giménez Alfaro O.J., Gámez Mendoza L.A., Meléndez B. (2011). General technological flow diagram and characterization of filata cheese telita type. Agronomía Tropical. 61: 177-188. [Spanish with English abstract]
Márquez J.G., García R C.E. (2007). Pathogenic microflora found in white "telita" cheese made in four states of Venezuela. Anales Venezolanos de Nutrición. 20: 17-21. [Spanish with English abstract]
Millán Y., Méndez A., Burguera M., Pimentel P., Araque M., Ramírez A. (2018). Determination of enterobacteria and detection of virulence genes in Escherichia coli isolated from raw milk. Revista de la Sociedad Venezolana de Microbiología. 38: 58-63. [Spanish with English abstract]
Ombarak R.A., Hinenoya A., Awasthi S.P., Iguchi A., Shima A., Elbagory A.R.M., Yamasaki S. (2016). Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt. International Journal of Food Microbiology. 221: 69-76. [DOI: 10/1016/j. ijfoodmicro.2016.01.009]
Ovi F., Zhang L., Nabors H., Jia L., Adhikari P. (2023). A compilation of virulence-associated genes that are frequently reported in avian pathogenic Escherichia coli (APEC) compared to other E.coli. Journal of Applied Microbiology. 134: 1-20. [DOI: 10.1093/jambio/lxad014]
Perdomo C., Gutiérrez F., García O., Acevedo I., Bastidas Z., Kowalski A. (2015). Physicochemical and bacteriological characterization of white artisan cheese in Buria parish, Lara state, Venezuela. Gaceta de Ciencias Veterinarias. 20: 35-44. [Spanish with English abstract]
Pineda A.P.A, Campos G.Z., Pimentel-Filho N.J., Franco B.D.G.d.M., Pinto U.M. (2021). Brazilian artisanal cheeses: diversity, microbiological safety, and challenges for the sector. Frontiers in Microbiology. 12: 666922. [DOI: 10.3389/fmicb.2021.666922]
Quijada-Martínez P., Flores-Carrero A., Labrador I., Millán Y., Araque M. (2017). Microbiological profile and molecular characterization of multidrug-resistant gram-negative bacilli producing catheter–associated urinary tract infections in the internal medicine services of a Venezuelan university hospital. Austin Journal of Infectious Diseases. 4: 1030.
Rodríguez C., Caldas L., Ogeerally P. (2009). Sanitary conditions of hand-made “telita” type cheese in Upata, Bolivar State, Venezuela. Revista de la Sociedad Venezolana de Microbiología. 29: 98-102. [Spanish with English abstract]
Sarowska J., Futoma-Koloch B., Jama-Kmiecik A., Frej-Madrzak M., Ksiazczyk M., Bugla-Ploskonska G., Choroszy-Krol I. (2019). Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathogens. 11: 10. [DOI: 10.1186/s13099-019-0290-0]
Sebastianski M., Bridger N.A., Featherstone R.M., Robinson J.L. (2022). Diseases outbreaks linked to pasteurized and unpasteurized dairy products in Canada and the United States: a systematic review. Canadian Journal of Public Health. 113: 569-578. [DOI: 10.17269/s41997-022-00614-y]
Venezuelan Industrial Standards Commission (COVENIN). (1989). Foodstuffs. Identification and sample preparation for microbiological analysis. Standards Nº 1126-89. URL: https://www.scribd.com/doc/50579844/1126-89. Accessed 10 January 2024.
Versalovic J., Koeuth T., Lupski J.R. (1991). Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Research. 19: 6823-6831. [DOI: 10.1093/nar/19.24.6823]
* Corresponding author (M. Araque)
* E-mail: araquemc@ula.ve
ORCID ID: https://orcid.org/0000-0001-6517-953X
* E-mail: araquemc@ula.ve
ORCID ID: https://orcid.org/0000-0001-6517-953X
Type of Study: Original article |
Subject:
Special
Received: 24/03/13 | Accepted: 24/09/05 | Published: 24/09/30
Received: 24/03/13 | Accepted: 24/09/05 | Published: 24/09/30
References
1. Bagel A., Sergentet D. (2022). Shiga toxin-producing Escherichia coli and milk fat globules. Microorganisms. 10: 496. [DOI: 10.3390/ microorganisms10030496] [DOI:10.3390/microorganisms10030496] [PMID] [PMCID]
2. Beghain J., Bridier-Nahmias A., Le Nagard H., Denamur E., Clermont O. (2018). Clermon typing: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microbial Genomics. 4: e000192. [DOI: 10.1099/mgen.0.000192] [DOI:10.1099/mgen.0.000192] [PMID] [PMCID]
3. Braz V.S., Melchior K., Moreira C.G. (2020). Escherichia coli as a multifaceted pathogenic and versatile bacterium. Frontiers in Cellular and Infection Microbiology. 10: 548492. [DOI: 10.3389/fcimb.2020.548492] [DOI:10.3389/fcimb.2020.548492] [PMID] [PMCID]
4. Bujnáková D., Karahutová L., Kmet V. (2021). Escherichia coli specific virulence-gene markers analysis for quality control of ovine cheese in Slovakia. Microorganisms. 9: 1808. [DOI: 10.3390/microorganisms9091808] [DOI:10.3390/microorganisms9091808] [PMID] [PMCID]
5. Carlos C., Pires M.M., Stoppe N.C., Hachich E.M., Sato M.I., Gomes T.A., Amaral L.A., Ottoboni L.M.M. (2010). Escherichia coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiology. 10: 161. [DOI: 10.1186/1471-2180-10-161] [DOI:10.1186/1471-2180-10-161] [PMID] [PMCID]
6. Clermont O., Christenson J.K., Denamur E., Gordon D.M. (2013). The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylogroups. Environmental Microbiology Reports. 5: 58-65. [DOI: 10.1111/1758-2229.12019] [DOI:10.1111/1758-2229.12019] [PMID]
7. Clinical and Laboratory Standards Institute (CLSI). (2023). Performance standards for antimicrobial susceptibility testing. 33th edition. CLSI Supplement M100-S27, Wayne, Pennsylvaniya. URL: https://clsi.org/standards/products/ microbiology/ documents/m100/. Accessed 03 February 2024.
8. D'Amico D.J. (2014). Microbiological quality and safety issues in cheesemaking. Microbiology Spectrum. 2: CM-0011-2012. [DOI: 10.1128/microbiolspec.CM-0011-2012] [DOI:10.1128/microbiolspec.CM-0011-2012] [PMID]
9. De Campos A.C.L.P., Puño-Sarmiento J.J., Medeiros L.P., Gazal L.E.S., Maluta R.P., Navarro A., Kobayashi R.K.T., Fagan E.P., Nakazato G. (2018). Virulence genes and antimicrobial resistance in Escherichia coli from cheese made from unpasteurized milk in Brazil. Foodborne Pathogens and Disease. 15: 94-100. [DOI: 10.1089/fpd.2017.2345] [DOI:10.1089/fpd.2017.2345] [PMID]
10. Guillén L., Millán B., Araque M. (2014). Molecular characterization of Escherichia coli strains isolated from homemade dairy foods produced in Mérida, Venezuela. Infectio. 18: 100-108. [DOI: 10.1016/j.infect.2014.04.004]. [Spanish with English abstract] [DOI:10.1016/j.infect.2014.04.004]
11. Jakobsen L., Spangholm D.J., Pedersen K., Jensen L.B., Emborg H.D., Agerso Y., Aarestrup F.M., Hammerum A.M., Frimodt-Moller N. (2010). Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. International Journal of Food Microbiology. 142: 264-272. [DOI: 10.1016/j.ijfoodmicro.2010.06.025] [DOI:10.1016/j.ijfoodmicro.2010.06.025] [PMID]
12. Johnson J.R., Stell A.L. (2000). Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. The Journal of Infectious Diseases. 181: 261-272. [DOI: 10.1086/315217] [DOI:10.1086/315217] [PMID]
13. Koski L., Kisselburgh H., Landsman L., Hulkower R., Howard-Williams M., Salah Z., Kim S., Bruce B.B., Bazaco M.C., Batz M.B., Parker C.C., Leonard C.L., et al. (2022). Foodborne illness outbreaks linked to unpasteurised milk and relationship to changes in state laws - United States, 1998-2018. Epidemiology and Infection. 150: e183. [DOI: 10.1017/S0950268822001649] [DOI:10.1017/S0950268822001649] [PMID] [PMCID]
14. Maldonado Gómez R.J, Rodríguez M., Llanca Córdova L.R., Román Montilla Y.J., Isturiz Vásquez R., Giménez Alfaro O.J., Gámez Mendoza L.A., Meléndez B. (2011). General technological flow diagram and characterization of filata cheese telita type. Agronomía Tropical. 61: 177-188. [Spanish with English abstract]
15. Márquez J.G., García R C.E. (2007). Pathogenic microflora found in white "telita" cheese made in four states of Venezuela. Anales Venezolanos de Nutrición. 20: 17-21. [Spanish with English abstract]
16. Millán Y., Méndez A., Burguera M., Pimentel P., Araque M., Ramírez A. (2018). Determination of enterobacteria and detection of virulence genes in Escherichia coli isolated from raw milk. Revista de la Sociedad Venezolana de Microbiología. 38: 58-63. [Spanish with English abstract]
17. Millán Y., Araque M., Ramírez A. (2020). Distribution of phylogenetic groups, virulence factors and antimicrobial susceptibility in strains of uropathogenic Escherichia coli. Revista Chilena de Infectología. 37: 117-123. [DOI: 10.4067/s0716-10182020000200117]. [Spanish with English abstract] [DOI:10.4067/s0716-10182020000200117] [PMID]
18. Ombarak R.A., Hinenoya A., Awasthi S.P., Iguchi A., Shima A., Elbagory A.R.M., Yamasaki S. (2016). Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt. International Journal of Food Microbiology. 221: 69-76. [DOI: 10/1016/j. ijfoodmicro.2016.01.009] [DOI:10.1016/j.ijfoodmicro.2016.01.009] [PMID]
19. Ovi F., Zhang L., Nabors H., Jia L., Adhikari P. (2023). A compilation of virulence-associated genes that are frequently reported in avian pathogenic Escherichia coli (APEC) compared to other E.coli. Journal of Applied Microbiology. 134: 1-20. [DOI: 10.1093/jambio/lxad014] [DOI:10.1093/jambio/lxad014] [PMID]
20. Perdomo C., Gutiérrez F., García O., Acevedo I., Bastidas Z., Kowalski A. (2015). Physicochemical and bacteriological characterization of white artisan cheese in Buria parish, Lara state, Venezuela. Gaceta de Ciencias Veterinarias. 20: 35-44. [Spanish with English abstract]
21. Pineda A.P.A, Campos G.Z., Pimentel-Filho N.J., Franco B.D.G.d.M., Pinto U.M. (2021). Brazilian artisanal cheeses: diversity, microbiological safety, and challenges for the sector. Frontiers in Microbiology. 12: 666922. [DOI: 10.3389/fmicb.2021.666922] [DOI:10.3389/fmicb.2021.666922] [PMID] [PMCID]
22. Quijada-Martínez P., Flores-Carrero A., Labrador I., Millán Y., Araque M. (2017). Microbiological profile and molecular characterization of multidrug-resistant gram-negative bacilli producing catheter-associated urinary tract infections in the internal medicine services of a Venezuelan university hospital. Austin Journal of Infectious Diseases. 4: 1030.
23. Rodríguez C., Caldas L., Ogeerally P. (2009). Sanitary conditions of hand-made "telita" type cheese in Upata, Bolivar State, Venezuela. Revista de la Sociedad Venezolana de Microbiología. 29: 98-102. [Spanish with English abstract]
24. Sarowska J., Futoma-Koloch B., Jama-Kmiecik A., Frej-Madrzak M., Ksiazczyk M., Bugla-Ploskonska G., Choroszy-Krol I. (2019). Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathogens. 11: 10. [DOI: 10.1186/s13099-019-0290-0] [DOI:10.1186/s13099-019-0290-0] [PMID] [PMCID]
25. Sebastianski M., Bridger N.A., Featherstone R.M., Robinson J.L. (2022). Diseases outbreaks linked to pasteurized and unpasteurized dairy products in Canada and the United States: a systematic review. Canadian Journal of Public Health. 113: 569-578. [DOI: 10.17269/s41997-022-00614-y] [DOI:10.17269/s41997-022-00614-y] [PMID] [PMCID]
26. Venezuelan Industrial Standards Commission (COVENIN). (1989). Foodstuffs. Identification and sample preparation for microbiological analysis. Standards Nº 1126-89. URL: https://www.scribd.com/doc/50579844/1126-89. Accessed 10 January 2024.
27. Versalovic J., Koeuth T., Lupski J.R. (1991). Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Research. 19: 6823-6831. [DOI: 10.1093/nar/19.24.6823] [DOI:10.1093/nar/19.24.6823] [PMID] [PMCID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







