Volume 11, Issue 3 (September 2024)
J. Food Qual. Hazards Control 2024, 11(3): 166-176 |
Back to browse issues page
Ethics code: not applicable
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Seki H. Developing Flowcharts for Hazard Analysis in Seafood Retail: Critical Control Point Verification. J. Food Qual. Hazards Control 2024; 11 (3) :166-176
URL: http://jfqhc.ssu.ac.ir/article-1-1218-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1218-en.html
Tokyo University of Technology , sekihrk@stf.teu.ac.jp
Keywords: Microbial Viability, Escherichia coli, Seafood, Hazard Analysis and Critical Control Points, Food Safety
Full-Text [PDF 584 kb]
(679 Downloads)
| Abstract (HTML) (1261 Views)
Full-Text: (407 Views)
Developing Flowcharts for Hazard Analysis in Seafood Retail: Critical Control Point Verification
H. Seki [*]*
Tokyo University of Technology
HIGHLIGHTS
To cite: Seki H. (2024). Developing flowcharts for hazard analysis in seafood retail: critical control point verification. Journal of Food Quality and Hazards Control. 11: 166-176.
Introduction
Figure 1: Flowchart of seafood sales at retail stores
CCPs=Critical Control Points
th alcohol before display, storage temperature 0-5 °C
Table 1: Temporal changes in the number of viable bacteria in each marine product (log Colony Forming Unit (CFU)/g)
Measurements were obtained using n=4 samples; data are expressed as mean±Standard Deviation (SD).
CCP=Critical Control Point.
Table 2: Temporal changes in the number of Vibrio parahaemolyticus in each marine product
The results are presented as number per 100 g of sample calculated from the Most Probable Number (MPN) table.
CCP=Critical Control Point; ND=Not Detected.
V. parahaemolyticus was detected in CCP-deviant and CCP-compliant frozen scallops on Day 0. However, this bacterium was not detected in either test plot on storage Days 1 or 2.
The V. parahaemolyticus count in CCP-deviant frozen shrimp increased on Day 2 compared to that on Day 0. In contrast, the V. parahaemolyticus count in CCP-compliant frozen shrimp decreased on Day 1 compared to that on Day 0 but increased on Day 2. Higher bacterial counts were observed in CCP-deviant samples than those in CCP-compliant samples on storage Days 1 and 2.
V. parahaemolyticus is abundant in seawater (Shimohata et al., 2022) and is consistently detected on the surfaces of marine products immediately after harvest. Bacteria invade the fish body through the skin because the regulatory mechanisms. That prevent invasion of the tissues by bacteria cease to function after death (Fraser and Sumar, 1998), V. parahaemolyticus contamination can be prevented by washing and sanitizing the fish well after catching. This may also account for why V. parahaemolyticus counts did not increase in this study.
V. parahaemolyticus has a high potential for growth in marine products as its optimal salt concentration for growth is 3%, and its optimum temperature is 37 °C (Liu et al., 2016). Hence, the CCP-deviant test plot represented an environment in which V. parahaemolyticus could easily proliferate. Additionally, all test plots in this study showed high bacterial counts on storage Day 0, with the number of bacteria decreasing over time. The pH of scallops decreases from approximately 7 to 6 over time (Seki, 2021), and the optimal pH for V. parahaemolyticus growth is 7.6-8.0. Therefore, a large amount of V. parahaemolyticus was detected on Day 0, when the pH was relatively high in the present study. Conversely, V. parahaemolyticus was detected on storage Day 0 in both CCP-deviant and CCP-compliant frozen scallops; however, the counts were lower than those of refrigerated scallops. In clams, the V. parahaemolyticus count slightly decreased at 4 and 15 °C in 96 h (Lopez-Joven et al., 2018); consistently, fewer bacteria were detected in frozen scallops in the present study.
The occurrence of Vibrio spp. is commonly associated with temperature, and these species are especially found in temperate climates, and Vibrio population variation is low in tropical and subtropical waters (Di et al., 2016), and 3.87-4.97 log MPN/g of V. parahaemolyticus was detected in shrimp from traditional markets in Hanoi, Vietnam (Vu et al., 2022). The samples used in the present study also included shrimp imported from tropical regions (India and Pakistan), with high values (93-240 MPN/g) on storage Day 1. It was reported that shrimp have to be stored and distributed in ice (rapid onboard icing and keeping fish iced until marketing or consumption) (Parlapani et al., 2020). Therefore, hygiene control during the processing stage is an issue for shrimp. Moreover, V. parahaemolyticus was likely attached to frozen shrimp during the processing stage in this study. Hence, the number of V. parahaemolyticus decreased from storage Days 0 to 1 in both test plots for shrimp that deviated from or complied with CCP. At 4 and 7 °C, V. parahaemolyticus growth was prevented, but at temperatures above 15 °C, V. parahaemolyticus growth was promoted (Wu et al., 2023). In the present study, the number of bacteria decreased from storage Day 1. The increase in bacterial count on storage Day 2 in the CCP-compliant plot was attributed to the potential presence of V. parahaemolyticus on the cutting boards, knives, and other cooking utensils used to collect the samples, which could have increased the detection rate.
K-value
Figure 2 shows the temporal changes in the K-values of refrigerated and frozen mackerel that deviated from or complied with CCP. The K-value of CCP-deviant refrigerated mackerel samples on Day 2 increased significantly compared to that on Day 0 (p<0.05). However, the K-value of CCP-compliant refrigerated mackerel samples increased slightly on Day 2 compared to that on Day 0 (p>0.05). Higher K-values were obtained for CCP-deviant mackerel than CCP-compliant mackerel on storage Days 0 to 2 (p<0.05). The K-values of CCP-deviant and CCP-compliant frozen mackerel samples increased significantly on Day 2 compared to that on Day 0 (p<0.05). The K-value of CCP-deviant frozen mackerel was higher than that of CCP-compliant frozen mackerel on Day 0 (p<0.05). Similarly, the K-value of CCP-deviant frozen mackerel was slightly higher than that of CCP-compliant frozen mackerel on Day 1, but the difference was not significant (p>0.05). The K-values of CCP-deviant and CCP-compliant mackerels were relatively the same (p>0.05) on storage Day 2.
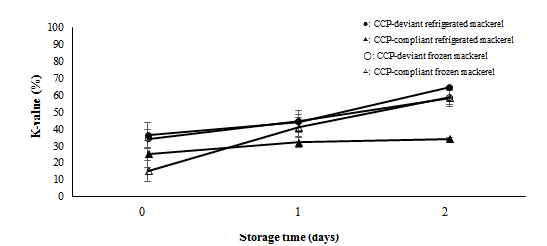
Figure 2: Temporal changes in the K-values of refrigerated and frozen mackerel that deviated and complied with the Critical Control Point (CCP). Measurements were performed using n=4 samples; the bars indicate Standard Deviation (SD)


Figure 4: Temporal changes in the K-values of frozen shrimp that deviated from or complied with the Critical Control Point (CCP)
H. Seki [*]*
Tokyo University of Technology
- The quality of marine products can be preserved by setting Critical Control Points.
- Storage conditions and management of displays and sales are Critical Control Points in Hazard Analysis and Critical Control Point.
- Ensuring proper temperature control during storage and sales preserves the quality of marine products.
| Article type Original article |
ABSTRACT Background: Implementing Hazard Analysis and Critical Control Point (HACCP) management across the supply chain is promoted globally to ensure the safety of marine food products due to their rapid quality deterioration. However, many seafood retail stores deviate from adopting these practices. Therefore, in this study, we aimed to create a flowchart based on HACCP and validate it in retail stores. Methods: Chub mackerel (Scomber japonicus), scallops (Patinopecten yessoensis), and whiteleg shrimp (Litopenaeus vannamei) specimens were purchased from a supermarket from August to December 2020. The handling information of these products from receipt to sales was obtained to prepare an HACCP plan for retail stores. Groups adhering to and deviating from flowchart conditions were categorized as Critical Control Point (CCP)-compliant and CCP-deviant, respectively. Four samples of each product for each condition were analyzed. Bacterial viability was evaluated using the flat agar culture method. Escherichia coli and Vibrio parahaemolyticus were detected using the Brilliant Green-Lactose-Bile and material point methods, respectively. Product freshness was assessed by determining K-values using High-Performance Liquid Chromatography. Results were compared using Student’s t-test. Results: At elevated storage temperatures, bacterial growth rates were higher in chub mackerel and whiteleg shrimp than those in scallops. E. coli was not detected in any sample, whereas V. parahaemolyticus was detected in scallops and whiteleg shrimp but not in chub mackerel. CCP-deviant refrigerated scallops had increased V. parahaemolyticus counts; however, it did not differ between the frozen scallop and whiteleg shrimp. K-values increased more rapidly in CCP-deviant chub mackerel, whiteleg shrimp, and refrigerated scallops, but not in frozen scallops. Inadequate temperature control during display and sale markedly deteriorated the quality of marine products. Conclusion: Setting CCPs for marine food product display and sale while controlling temperature can preserve product quality. The flowchart created in this study can be broadly used for marine retail stores. © 2024, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Microbial Viability Escherichia coli Seafood Hazard Analysis and Critical Control Points Food Safety |
||
| Article history Received: 12 Apr 2024 Revised: 30 Jul 2024 Accept: 02 Aug 2024 |
||
| Acronyms and abbreviations CCP=Critical Control Point CFU=Colony Forming Unit HACCP=Hazard Analysis and Critical Control Point MPN=Most Probable Number |
Introduction
The global consumption of seafood has considerably increased in recent years, with global per capita annual consumption rising from 9.1 kg in 1961 to 20.7 kg in 2022 (FAO, 2024). Thus, a substantial supply of marine products is needed to meet the increasing demand. Furthermore, owing to the highly perishable nature of these products, supplier attention to safety is paramount.
Hazard Analysis and Critical Control Point (HACCP) is a process control system that identifies potential hazards in all processes, from receiving raw materials to delivering the final product. This system identifies potential hazards and their corresponding preventative measures. Hazard analysis, which was developed by the National Aeronautics and Space Administration (Casal-Wardle, 2015), helps identify critical processes to prevent the occurrence of hazards (Critical Control Points (CCPs)) and continuously monitor these variables (Yamamoto, 2014). To ensure food safety, HACCP management must be implemented at all stages of the product development lifecycle, from acceptance of raw materials to shipment and delivery of the final product to consumers (Matsumoto, 2022).
Seafood is generally shipped to the market after harvest and then prepared at processing plants before being sold at retail outlets, such as supermarkets. The implementation of HACCP at these stages is necessary to ensure food safety throughout the distribution chain. In the United States, the implementation of HACCP has been made mandatory throughout the supply chain since its introduction in 1997 (Kobayashi et al., 2021). In the European :union:, the implementation of HACCP is mandatory in each member country; however, the management of HACCP is almost entirely the responsibility of the government and business organizations. Therefore, several retailers already have their preferred sanitation systems in place, and HACCP is often misconstrued as a specialized system that cannot be implemented (Kudo, 2016). In Taiwan, the implementation of HACCP has been progressively mandated to plants that process seafood, meat, and dairy products since 2003 (Yamamoto, 2014). Although some restaurants have introduced HACCP, retail stores have not followed suit (Ko, 2013). In Japan, the Food Sanitation Law was partially amended in June 2018 (Onozawa, 2021). Since June 2021, all food businesses comprising the food supply chain have been mandated to implement sanitation management in line with HACCP. However, while HACCP implementation throughout the supply chain is being promoted globally, the process is currently lagging in retail stores (Matsumoto, 2022).
The quality of marine products is primarily evaluated based on the number of microorganisms and K-value, a parameter used to indicate the degree of spoilage. Additionally, bacterial viability and the number of Escherichia coli, known as contaminating bacteria, are commonly used in general food quality evaluations. Vibrio parahaemolyticus grows well in seawater (NaCl; 0.5-3%) (Gao et al., 2022), representing a microorganism that may reduce marine product quality. Hence, the quality of several species of fish (Wong et al., 2015), including spear squid, sea robin (Kaneda et al., 2015), mackerel, and Akoya pearl oyster (Chung et al., 2021), is evaluated by measuring the bacterial viability, as well as E. coli and V. parahaemolyticus counts.
Flowcharting and establishment of CCPs are important in developing an HACCP plan. This study sought to create a comprehensive flowchart, establish CCPs, and propose a HACCP-based flowchart for marine products in retail stores. In particular, data were compared between retail stores that exhibited deviations from and compliance with the CCP. The study focused on chub mackerel (Scomber japonicus) and scallops (Patinopecten yessoensis), distributed in refrigerated or frozen states, and whiteleg shrimp (Litopenaeus vannamei), exclusively distributed as frozen products. This study used bacterial viability, E. coli and V. parahaemolyticus counts, and K-values as the quality assessment criteria.
Materials and methods
Materials
This study was conducted from August to December 2020. One frozen and four refrigerated bags of chub mackerels (acquired from Ishikawa, Japan, and Denmark) and one bag of frozen whiteleg shrimp (acquired from India or Pakistan) were purchased from a supermarket near Tamagawa University (Tokyo, Japan). The samples were transported to the laboratory and cooled using refrigerants. Additionally, one bag of frozen and refrigerated scallops were purchased online (from Hokkaido) and transported to the laboratory. Following a HACCP-based flowchart, test plots were created for compliance with (5 °C) and deviation from (25 °C) CCP conditions. The temperatures were maintained for 5 h and initial samples were collected (Day 0). Afterward, both test plots were maintained at 5 °C for 48 h, with the samples collected at 24 (Day 1) and 48 h (Day 2).
Flowchart creation based on HACCP for retail stores
To prepare an HACCP plan for retail stores, an assessment of the handling of marine products from receipt to sales was conducted. This involved surveying the current practices at Odakyu OX seafood retail outlets (supermarkets) and Inageya Co., Ltd. food processing plant. The staff members were interviewed to gather detailed information. Specifically, the following questions were posed during personal interviews with four staff members:
1. What are the conditions under which fish are received at the store?
2. What procedures are followed for selling the fish after they have been received?
A flowchart from store acceptance of materials to sales of products was created, and CCPs were established.
Bacterial viability test
The bacterial viability test was conducted as described by Ichinohe et al. (2015). Briefly, to determine viable bacterial counts in the samples, 2.5 g of each sample was finely chopped and added into a 50 ml centrifuge tube. The sample was then diluted to 25 ml in 0.90% brine and mixed thoroughly. The supernatant was diluted by 10−1 to 10−5 times, and 100 µl of the diluted solution was smeared on a standard agar medium (Eiken Chemical Co., Ltd., Tokyo, Japan).
E. coli test
E. coli was detected using a previously described method (Ichinohe et al., 2015). The supernatant from the sample obtained in the bacterial viability test was extracted; 500 μl of its 10- and 100-fold dilutions was added to 5.0 ml of Brilliant Green-Lactose-Bile broth (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The samples were incubated at 44 °C for 24 h to confirm gas generation.
V. parahaemolyticus test
V. parahaemolyticus was detected using the test previously described by Ichinohe et al. (2015). Briefly, 2.5 g of each sample was finely chopped, added to a 50 ml centrifuge tube, and fixed in 25 ml of alkaline peptone water (Eiken Chemical Co., Ltd., Tokyo, Japan). Next, 500 µl of the 1-, 10-, and 100-fold dilutions of the sample supernatants were added to 5 ml of alkaline peptone water and incubated at 37 °C for 20 h. One inoculating loop full of samples from the upper layer of each test tube was smeared on thiosulfate citrate bile saccharose agar medium (Shimadzu Diagnostics Corporation, Tokyo, Japan). A test tube in which colonies were identified after 24 h of incubation at 37 °C was considered positive, and the Most Probable Number (MPN) was calculated using the MPN table.
K-value determination
The K-value indicates the ratio of inosine (HxR) and hypoxanthine (Hx) to total ATP-related compounds (nucleic acid-related substances). Given that ATP degradation reactions are enzymatic and K-values increase over time in most marine products after organismal death, K-values are used as an indicator of product freshness (Kato et al., 2009). The lower the K-value, the fresher the fish. The K-value was calculated using the following equation:
K-value (%)=((Hx+HxR)/(ATP+ADP+AMP+IMP+HxR+Hx))×100
Where, ATP represents adenosine triphosphate, ADP is adenosine diphosphate, ADM is adenosine monophosphate, and IMP is inosine monophosphate.
The K-value was determined as described by Seki et al. (2016). Briefly, 4.0 ml of 10% perchloric acid (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was added to 2.5 g of the sample to extract ATP-related compounds. The solids were removed via centrifugation (11,000×g for 10 min at 5 °C), and the supernatant was diluted to 10 ml with 10% perchloric acid. The mixture was neutralized using potassium hydroxide (KOH; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), the precipitate was removed by centrifugation (12,000×g for 5 min at 5 °C), and the supernatant was diluted to 5 ml using purified water. The solution was passed through a 0.22 µm filter and subjected to High-Performance Liquid Chromatography (HPLC; Shimadzu Corporation, Kyoto, Japan) (column: COSMOSIL Packed Column 5C18-PAQ, mobile phase: 20 mM potassium dihydrogen phosphate (KH2PO4) solution, flow rate: 1.0 ml/min, temperature: 40 °C, detector: UV, wavelength: 260 nm, injection volume; 20 µl) to determine the ATP-related compound content.
Statistical analysis
Data were evaluated using a one-way analysis of variance (ANOVA) based on Fisher's three principles. Differences in the mean values were evaluated using the Student’s t-test, and significance was set at p<0.05. Analyses were performed using Microsoft Excel. Bacterial measurements were performed on four samples. Additionally, owing to its tendency to fluctuate, the K-value measurements were performed with five samples.
Results and discussion
Flowchart creation based on HACCP for retail stores
The findings of the personal interviews are summarized as follows:
1. Most fish in supermarkets are accepted in packaged form and are not processed in the store.
2. Packaged products are displayed and sold immediately upon acceptance, with minimal storage.
Based on these findings, the flowchart in Figure 1 was developed to illustrate the process for pre-processed marine products from store acceptance to sale. The flowchart identifies product display and sale as CCPs, with a control temperature set at 5 °C.
Hazard Analysis and Critical Control Point (HACCP) is a process control system that identifies potential hazards in all processes, from receiving raw materials to delivering the final product. This system identifies potential hazards and their corresponding preventative measures. Hazard analysis, which was developed by the National Aeronautics and Space Administration (Casal-Wardle, 2015), helps identify critical processes to prevent the occurrence of hazards (Critical Control Points (CCPs)) and continuously monitor these variables (Yamamoto, 2014). To ensure food safety, HACCP management must be implemented at all stages of the product development lifecycle, from acceptance of raw materials to shipment and delivery of the final product to consumers (Matsumoto, 2022).
Seafood is generally shipped to the market after harvest and then prepared at processing plants before being sold at retail outlets, such as supermarkets. The implementation of HACCP at these stages is necessary to ensure food safety throughout the distribution chain. In the United States, the implementation of HACCP has been made mandatory throughout the supply chain since its introduction in 1997 (Kobayashi et al., 2021). In the European :union:, the implementation of HACCP is mandatory in each member country; however, the management of HACCP is almost entirely the responsibility of the government and business organizations. Therefore, several retailers already have their preferred sanitation systems in place, and HACCP is often misconstrued as a specialized system that cannot be implemented (Kudo, 2016). In Taiwan, the implementation of HACCP has been progressively mandated to plants that process seafood, meat, and dairy products since 2003 (Yamamoto, 2014). Although some restaurants have introduced HACCP, retail stores have not followed suit (Ko, 2013). In Japan, the Food Sanitation Law was partially amended in June 2018 (Onozawa, 2021). Since June 2021, all food businesses comprising the food supply chain have been mandated to implement sanitation management in line with HACCP. However, while HACCP implementation throughout the supply chain is being promoted globally, the process is currently lagging in retail stores (Matsumoto, 2022).
The quality of marine products is primarily evaluated based on the number of microorganisms and K-value, a parameter used to indicate the degree of spoilage. Additionally, bacterial viability and the number of Escherichia coli, known as contaminating bacteria, are commonly used in general food quality evaluations. Vibrio parahaemolyticus grows well in seawater (NaCl; 0.5-3%) (Gao et al., 2022), representing a microorganism that may reduce marine product quality. Hence, the quality of several species of fish (Wong et al., 2015), including spear squid, sea robin (Kaneda et al., 2015), mackerel, and Akoya pearl oyster (Chung et al., 2021), is evaluated by measuring the bacterial viability, as well as E. coli and V. parahaemolyticus counts.
Flowcharting and establishment of CCPs are important in developing an HACCP plan. This study sought to create a comprehensive flowchart, establish CCPs, and propose a HACCP-based flowchart for marine products in retail stores. In particular, data were compared between retail stores that exhibited deviations from and compliance with the CCP. The study focused on chub mackerel (Scomber japonicus) and scallops (Patinopecten yessoensis), distributed in refrigerated or frozen states, and whiteleg shrimp (Litopenaeus vannamei), exclusively distributed as frozen products. This study used bacterial viability, E. coli and V. parahaemolyticus counts, and K-values as the quality assessment criteria.
Materials and methods
Materials
This study was conducted from August to December 2020. One frozen and four refrigerated bags of chub mackerels (acquired from Ishikawa, Japan, and Denmark) and one bag of frozen whiteleg shrimp (acquired from India or Pakistan) were purchased from a supermarket near Tamagawa University (Tokyo, Japan). The samples were transported to the laboratory and cooled using refrigerants. Additionally, one bag of frozen and refrigerated scallops were purchased online (from Hokkaido) and transported to the laboratory. Following a HACCP-based flowchart, test plots were created for compliance with (5 °C) and deviation from (25 °C) CCP conditions. The temperatures were maintained for 5 h and initial samples were collected (Day 0). Afterward, both test plots were maintained at 5 °C for 48 h, with the samples collected at 24 (Day 1) and 48 h (Day 2).
Flowchart creation based on HACCP for retail stores
To prepare an HACCP plan for retail stores, an assessment of the handling of marine products from receipt to sales was conducted. This involved surveying the current practices at Odakyu OX seafood retail outlets (supermarkets) and Inageya Co., Ltd. food processing plant. The staff members were interviewed to gather detailed information. Specifically, the following questions were posed during personal interviews with four staff members:
1. What are the conditions under which fish are received at the store?
2. What procedures are followed for selling the fish after they have been received?
A flowchart from store acceptance of materials to sales of products was created, and CCPs were established.
Bacterial viability test
The bacterial viability test was conducted as described by Ichinohe et al. (2015). Briefly, to determine viable bacterial counts in the samples, 2.5 g of each sample was finely chopped and added into a 50 ml centrifuge tube. The sample was then diluted to 25 ml in 0.90% brine and mixed thoroughly. The supernatant was diluted by 10−1 to 10−5 times, and 100 µl of the diluted solution was smeared on a standard agar medium (Eiken Chemical Co., Ltd., Tokyo, Japan).
E. coli test
E. coli was detected using a previously described method (Ichinohe et al., 2015). The supernatant from the sample obtained in the bacterial viability test was extracted; 500 μl of its 10- and 100-fold dilutions was added to 5.0 ml of Brilliant Green-Lactose-Bile broth (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The samples were incubated at 44 °C for 24 h to confirm gas generation.
V. parahaemolyticus test
V. parahaemolyticus was detected using the test previously described by Ichinohe et al. (2015). Briefly, 2.5 g of each sample was finely chopped, added to a 50 ml centrifuge tube, and fixed in 25 ml of alkaline peptone water (Eiken Chemical Co., Ltd., Tokyo, Japan). Next, 500 µl of the 1-, 10-, and 100-fold dilutions of the sample supernatants were added to 5 ml of alkaline peptone water and incubated at 37 °C for 20 h. One inoculating loop full of samples from the upper layer of each test tube was smeared on thiosulfate citrate bile saccharose agar medium (Shimadzu Diagnostics Corporation, Tokyo, Japan). A test tube in which colonies were identified after 24 h of incubation at 37 °C was considered positive, and the Most Probable Number (MPN) was calculated using the MPN table.
K-value determination
The K-value indicates the ratio of inosine (HxR) and hypoxanthine (Hx) to total ATP-related compounds (nucleic acid-related substances). Given that ATP degradation reactions are enzymatic and K-values increase over time in most marine products after organismal death, K-values are used as an indicator of product freshness (Kato et al., 2009). The lower the K-value, the fresher the fish. The K-value was calculated using the following equation:
K-value (%)=((Hx+HxR)/(ATP+ADP+AMP+IMP+HxR+Hx))×100
Where, ATP represents adenosine triphosphate, ADP is adenosine diphosphate, ADM is adenosine monophosphate, and IMP is inosine monophosphate.
The K-value was determined as described by Seki et al. (2016). Briefly, 4.0 ml of 10% perchloric acid (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was added to 2.5 g of the sample to extract ATP-related compounds. The solids were removed via centrifugation (11,000×g for 10 min at 5 °C), and the supernatant was diluted to 10 ml with 10% perchloric acid. The mixture was neutralized using potassium hydroxide (KOH; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), the precipitate was removed by centrifugation (12,000×g for 5 min at 5 °C), and the supernatant was diluted to 5 ml using purified water. The solution was passed through a 0.22 µm filter and subjected to High-Performance Liquid Chromatography (HPLC; Shimadzu Corporation, Kyoto, Japan) (column: COSMOSIL Packed Column 5C18-PAQ, mobile phase: 20 mM potassium dihydrogen phosphate (KH2PO4) solution, flow rate: 1.0 ml/min, temperature: 40 °C, detector: UV, wavelength: 260 nm, injection volume; 20 µl) to determine the ATP-related compound content.
Statistical analysis
Data were evaluated using a one-way analysis of variance (ANOVA) based on Fisher's three principles. Differences in the mean values were evaluated using the Student’s t-test, and significance was set at p<0.05. Analyses were performed using Microsoft Excel. Bacterial measurements were performed on four samples. Additionally, owing to its tendency to fluctuate, the K-value measurements were performed with five samples.
Results and discussion
Flowchart creation based on HACCP for retail stores
The findings of the personal interviews are summarized as follows:
1. Most fish in supermarkets are accepted in packaged form and are not processed in the store.
2. Packaged products are displayed and sold immediately upon acceptance, with minimal storage.
Based on these findings, the flowchart in Figure 1 was developed to illustrate the process for pre-processed marine products from store acceptance to sale. The flowchart identifies product display and sale as CCPs, with a control temperature set at 5 °C.

Figure 1: Flowchart of seafood sales at retail stores
CCPs=Critical Control Points
th alcohol before display, storage temperature 0-5 °C
The survey revealed that approximately 70% of marine products received by stores were pre-processed, whereas 30% were processed in-store. However, it is anticipated that the proportion of preprocessed products will increase, thereby reducing the need for in-store processing.
Viable bacterial counts
Table 1 shows the temporal changes in the number of viable bacteria in the CCP-compliant and CCP-deviant samples. The bacterial count of CCP-deviant and -compliant refrigerated and CCP-compliant frozen mackerels increased slightly on Day 2 from that on Day 0 (p>0.05), whereas that of CCP-deviant frozen mackerels increased significantly on Day 2 from that on Day 0 (p<0.05). Furthermore, CCP-deviant products showed slightly higher bacterial counts than CCP-compliant products on Days 1 and 2 of storage (p>0.05).
Viable bacterial counts
Table 1 shows the temporal changes in the number of viable bacteria in the CCP-compliant and CCP-deviant samples. The bacterial count of CCP-deviant and -compliant refrigerated and CCP-compliant frozen mackerels increased slightly on Day 2 from that on Day 0 (p>0.05), whereas that of CCP-deviant frozen mackerels increased significantly on Day 2 from that on Day 0 (p<0.05). Furthermore, CCP-deviant products showed slightly higher bacterial counts than CCP-compliant products on Days 1 and 2 of storage (p>0.05).
Table 1: Temporal changes in the number of viable bacteria in each marine product (log Colony Forming Unit (CFU)/g)
| Sample condition | Deviation or compliance with CCP | Number of viable bacteria (log CFU/g) | ||
| Day 0 | Day 1 | Day 2 | ||
| Refrigerated Mackerel | CCP-deviant | 0±0 | 1.8±0.67 | 2.9±0.63 |
| CCP-compliant | 0±0 | 0.65±0.92 | 1.5±0.34 | |
| Frozen Mackerel | CCP-deviant | 0±0 | 1.8±0.76 | 4.4±0.08 |
| CCP-compliant | 0.65±0.92 | 1.1±1.5 | 3.1±0.23 | |
| Refrigerated Scallops | CCP-deviant | 0±0 | 1.0±1.4 | 0±0 |
| CCP-compliant | 0±0 | 0±0 | 0±0 | |
| Frozen Scallops | CCP-deviant | 2.5±0.088 | 0±0 | 0±0 |
| CCP-compliant | 0±0 | 0±0 | 1.0±1.4 | |
| Frozen Shrimp | CCP-deviant | 4.6±0.031 | 4.9±0.11 | 5.4±0.075 |
| CCP-compliant | 4.1±0.069 | 4.6±0.040 | 4.9±0.051 | |
CCP=Critical Control Point.
No viable bacteria were detected in refrigerated scallops on storage Days 0 and 2, regardless of CCP deviation or compliance, while the bacterial count on Day 1 was positive. In contrast, the bacterial count in CCP-deviant frozen scallops on Day 0 was positive. However, no bacteria were detected on storage Days 1 and 2. On Day 2 viable bacteria were detected in CCP-compliant scallops, whereas no bacteria were detected on storage Days 0 and 1.
The bacterial counts of both CCP-deviant and CCP-compliant frozen shrimp significantly increased on Day 2 from that on Day 0 (p<0.05). On storage Days 0 and 1, the bacterial counts in CCP-deviant shrimp were slightly higher than those in CCP-compliant frozen shrimp (p>0.05). However, on storage Day 2 the bacterial counts in CCP-deviant shrimp were significantly higher than those in CCP-compliant shrimp (p<0.05).
In the present study, the CCP-deviant test plots showed higher viable bacterial counts, indicating that storage temperature affected the bacterial counts. A previous study reported that the viable bacterial count increased significantly when modified atmosphere packaged horse mackerel fillets was stored at 10 °C compared to when they were stored at 2 °C (Alfaro et al., 2013). Another study showed lower viable bacterial count in Japanese horse mackerel stored at 4 °C for approximately two days than that in those stored at 20 °C for the same duration (Kyoui et al., 2022). The bacterial counts of scallops stored at 5 °C on Day 0 and after 1-2 days of storage in the refrigerator (Narita et al., 2018) were higher than those observed in the present study. The presence of viable bacteria is mainly caused by contamination during processing (Lucas, 2015). In the present study, the low bacterial count was attributed to the in-store processing of products and thorough disinfection of the utensils used during de-hulling.
In previous studies, viable bacteria were detected in CCP-deviant and CCP-compliant frozen shrimp samples on all storage days. For instance, the bacterial count of frozen shrimp stored at 4 °C (Guo et al., 2013) was higher and that at 2 °C (López-Caballero et al., 2007) was lower than that of those in the present study. Frozen shrimp production areas range widely from cold regions near the Arctic Circle to tropical regions. The frozen shrimp used in the present study were obtained from tropical areas in India or Pakistan (as indicated on the purchased pack). A high prevalence of Salmonella spp. has been also observed in Asian countries, particularly in tropical regions. Salmonella spp. contamination has been found in 24.5% of shrimp samples in Vietnam, and this is a major threat for seafood products like shrimp and prawns (Ali et al., 2020). Therefore, it is considered that those collected in temperate or tropical regions had higher levels and frequencies of bacterial contamination. This study revealed general bacterial contamination, with approximately half of the samples collected from tropical regions showing a bacterial count of 5.0 log Colony Forming Unit (CFU)/g. As shrimp are primarily farmed and processed in Thailand and Vietnam (Yamao and Amano, 2018) and Japan imports approximately 60% of its shrimp from Southeast and South Asian regions such as Vietnam and India (JIRCAS, 2022), the viable bacterial counts were also relatively high in the present study.
Similarly, the bacterial counts of frozen shrimp reportedly increased from storage Days 0 to 3 at 4 and 12 °C (Gornik et al., 2011). These results indicated that microbial growth was faster at higher storage temperatures.
E. coli test
E. coli was not detected in the refrigerated mackerel, scallops, frozen scallops, or frozen shrimp during the three storage days. Additionally, E. coli could not be detected in any of the test plots examined in this study. Consistent with the current study, E. coli was not detected in shrimp and oysters purchased from open markets or supermarkets (Minami et al., 2010). Similarly, Narita et al. (2018) detected no E. coli in scallop adductor muscle separated using an automated scallop sheller. In contrast, E. coli was detected in mollusks cultured in a contaminated water environment (Souza et al., 2012), which differed from the culture conditions in the present study. Tayel et al. (2020) reported approximately 2.5 log CFU/g E. coli in shrimp stored at 4 °C over two storage days, which was higher than the levels observed in the present study. E. coli contamination typically occurs during food processing (Medina-Rodríguez et al., 2020) and can be present in water (Holvoet et al., 2012). As E. coli contamination was not found in slaughterhouses with sanitary environments (Morita et al., 2010), it can be hypothesized that the shrimp used in this study were processed in a sanitary environment.
V. parahaemolyticus test
Table 2 shows the temporal changes in V. parahaemolyticus counts in CCP-deviant and CCP-compliant products. This bacterium was not detected in refrigerated or frozen mackerel after three days of storage. In contrast, the V. parahaemolyticus count in CCP-deviant refrigerated scallops on Day 0 was detected, which significantly decreased on Days 1 and 2. However, V. parahaemolyticus was not detected in CCP-compliant refrigerated scallops throughout the study period.
The bacterial counts of both CCP-deviant and CCP-compliant frozen shrimp significantly increased on Day 2 from that on Day 0 (p<0.05). On storage Days 0 and 1, the bacterial counts in CCP-deviant shrimp were slightly higher than those in CCP-compliant frozen shrimp (p>0.05). However, on storage Day 2 the bacterial counts in CCP-deviant shrimp were significantly higher than those in CCP-compliant shrimp (p<0.05).
In the present study, the CCP-deviant test plots showed higher viable bacterial counts, indicating that storage temperature affected the bacterial counts. A previous study reported that the viable bacterial count increased significantly when modified atmosphere packaged horse mackerel fillets was stored at 10 °C compared to when they were stored at 2 °C (Alfaro et al., 2013). Another study showed lower viable bacterial count in Japanese horse mackerel stored at 4 °C for approximately two days than that in those stored at 20 °C for the same duration (Kyoui et al., 2022). The bacterial counts of scallops stored at 5 °C on Day 0 and after 1-2 days of storage in the refrigerator (Narita et al., 2018) were higher than those observed in the present study. The presence of viable bacteria is mainly caused by contamination during processing (Lucas, 2015). In the present study, the low bacterial count was attributed to the in-store processing of products and thorough disinfection of the utensils used during de-hulling.
In previous studies, viable bacteria were detected in CCP-deviant and CCP-compliant frozen shrimp samples on all storage days. For instance, the bacterial count of frozen shrimp stored at 4 °C (Guo et al., 2013) was higher and that at 2 °C (López-Caballero et al., 2007) was lower than that of those in the present study. Frozen shrimp production areas range widely from cold regions near the Arctic Circle to tropical regions. The frozen shrimp used in the present study were obtained from tropical areas in India or Pakistan (as indicated on the purchased pack). A high prevalence of Salmonella spp. has been also observed in Asian countries, particularly in tropical regions. Salmonella spp. contamination has been found in 24.5% of shrimp samples in Vietnam, and this is a major threat for seafood products like shrimp and prawns (Ali et al., 2020). Therefore, it is considered that those collected in temperate or tropical regions had higher levels and frequencies of bacterial contamination. This study revealed general bacterial contamination, with approximately half of the samples collected from tropical regions showing a bacterial count of 5.0 log Colony Forming Unit (CFU)/g. As shrimp are primarily farmed and processed in Thailand and Vietnam (Yamao and Amano, 2018) and Japan imports approximately 60% of its shrimp from Southeast and South Asian regions such as Vietnam and India (JIRCAS, 2022), the viable bacterial counts were also relatively high in the present study.
Similarly, the bacterial counts of frozen shrimp reportedly increased from storage Days 0 to 3 at 4 and 12 °C (Gornik et al., 2011). These results indicated that microbial growth was faster at higher storage temperatures.
E. coli test
E. coli was not detected in the refrigerated mackerel, scallops, frozen scallops, or frozen shrimp during the three storage days. Additionally, E. coli could not be detected in any of the test plots examined in this study. Consistent with the current study, E. coli was not detected in shrimp and oysters purchased from open markets or supermarkets (Minami et al., 2010). Similarly, Narita et al. (2018) detected no E. coli in scallop adductor muscle separated using an automated scallop sheller. In contrast, E. coli was detected in mollusks cultured in a contaminated water environment (Souza et al., 2012), which differed from the culture conditions in the present study. Tayel et al. (2020) reported approximately 2.5 log CFU/g E. coli in shrimp stored at 4 °C over two storage days, which was higher than the levels observed in the present study. E. coli contamination typically occurs during food processing (Medina-Rodríguez et al., 2020) and can be present in water (Holvoet et al., 2012). As E. coli contamination was not found in slaughterhouses with sanitary environments (Morita et al., 2010), it can be hypothesized that the shrimp used in this study were processed in a sanitary environment.
V. parahaemolyticus test
Table 2 shows the temporal changes in V. parahaemolyticus counts in CCP-deviant and CCP-compliant products. This bacterium was not detected in refrigerated or frozen mackerel after three days of storage. In contrast, the V. parahaemolyticus count in CCP-deviant refrigerated scallops on Day 0 was detected, which significantly decreased on Days 1 and 2. However, V. parahaemolyticus was not detected in CCP-compliant refrigerated scallops throughout the study period.
Table 2: Temporal changes in the number of Vibrio parahaemolyticus in each marine product
| Sample condition | Deviation or compliance with CCP | Number of Vibrio parahaemolyticus (MPN/100 g) | ||
| Day 0 | Day 1 | Day 2 | ||
| Refrigerated Mackerel | CCP-deviant | ND | ND | ND |
| CCP-compliant | ND | ND | ND | |
| Frozen Mackerel | CCP-deviant | ND | ND | ND |
| CCP-compliant | ND | ND | ND | |
| Refrigerated Scallops | CCP-deviant | 4300 | 400 | 400 |
| CCP-compliant | ND | ND | ND | |
| Frozen Scallops | CCP-deviant | 400 | 0 | 0 |
| CCP-compliant | 300 | 0 | 0 | |
| Frozen Shrimp | CCP-deviant | 400 | 24,000 | 46,000 |
| CCP-compliant | 24,000 | 9,300 | 12,000 | |
CCP=Critical Control Point; ND=Not Detected.
V. parahaemolyticus was detected in CCP-deviant and CCP-compliant frozen scallops on Day 0. However, this bacterium was not detected in either test plot on storage Days 1 or 2.
The V. parahaemolyticus count in CCP-deviant frozen shrimp increased on Day 2 compared to that on Day 0. In contrast, the V. parahaemolyticus count in CCP-compliant frozen shrimp decreased on Day 1 compared to that on Day 0 but increased on Day 2. Higher bacterial counts were observed in CCP-deviant samples than those in CCP-compliant samples on storage Days 1 and 2.
V. parahaemolyticus is abundant in seawater (Shimohata et al., 2022) and is consistently detected on the surfaces of marine products immediately after harvest. Bacteria invade the fish body through the skin because the regulatory mechanisms. That prevent invasion of the tissues by bacteria cease to function after death (Fraser and Sumar, 1998), V. parahaemolyticus contamination can be prevented by washing and sanitizing the fish well after catching. This may also account for why V. parahaemolyticus counts did not increase in this study.
V. parahaemolyticus has a high potential for growth in marine products as its optimal salt concentration for growth is 3%, and its optimum temperature is 37 °C (Liu et al., 2016). Hence, the CCP-deviant test plot represented an environment in which V. parahaemolyticus could easily proliferate. Additionally, all test plots in this study showed high bacterial counts on storage Day 0, with the number of bacteria decreasing over time. The pH of scallops decreases from approximately 7 to 6 over time (Seki, 2021), and the optimal pH for V. parahaemolyticus growth is 7.6-8.0. Therefore, a large amount of V. parahaemolyticus was detected on Day 0, when the pH was relatively high in the present study. Conversely, V. parahaemolyticus was detected on storage Day 0 in both CCP-deviant and CCP-compliant frozen scallops; however, the counts were lower than those of refrigerated scallops. In clams, the V. parahaemolyticus count slightly decreased at 4 and 15 °C in 96 h (Lopez-Joven et al., 2018); consistently, fewer bacteria were detected in frozen scallops in the present study.
The occurrence of Vibrio spp. is commonly associated with temperature, and these species are especially found in temperate climates, and Vibrio population variation is low in tropical and subtropical waters (Di et al., 2016), and 3.87-4.97 log MPN/g of V. parahaemolyticus was detected in shrimp from traditional markets in Hanoi, Vietnam (Vu et al., 2022). The samples used in the present study also included shrimp imported from tropical regions (India and Pakistan), with high values (93-240 MPN/g) on storage Day 1. It was reported that shrimp have to be stored and distributed in ice (rapid onboard icing and keeping fish iced until marketing or consumption) (Parlapani et al., 2020). Therefore, hygiene control during the processing stage is an issue for shrimp. Moreover, V. parahaemolyticus was likely attached to frozen shrimp during the processing stage in this study. Hence, the number of V. parahaemolyticus decreased from storage Days 0 to 1 in both test plots for shrimp that deviated from or complied with CCP. At 4 and 7 °C, V. parahaemolyticus growth was prevented, but at temperatures above 15 °C, V. parahaemolyticus growth was promoted (Wu et al., 2023). In the present study, the number of bacteria decreased from storage Day 1. The increase in bacterial count on storage Day 2 in the CCP-compliant plot was attributed to the potential presence of V. parahaemolyticus on the cutting boards, knives, and other cooking utensils used to collect the samples, which could have increased the detection rate.
K-value
Figure 2 shows the temporal changes in the K-values of refrigerated and frozen mackerel that deviated from or complied with CCP. The K-value of CCP-deviant refrigerated mackerel samples on Day 2 increased significantly compared to that on Day 0 (p<0.05). However, the K-value of CCP-compliant refrigerated mackerel samples increased slightly on Day 2 compared to that on Day 0 (p>0.05). Higher K-values were obtained for CCP-deviant mackerel than CCP-compliant mackerel on storage Days 0 to 2 (p<0.05). The K-values of CCP-deviant and CCP-compliant frozen mackerel samples increased significantly on Day 2 compared to that on Day 0 (p<0.05). The K-value of CCP-deviant frozen mackerel was higher than that of CCP-compliant frozen mackerel on Day 0 (p<0.05). Similarly, the K-value of CCP-deviant frozen mackerel was slightly higher than that of CCP-compliant frozen mackerel on Day 1, but the difference was not significant (p>0.05). The K-values of CCP-deviant and CCP-compliant mackerels were relatively the same (p>0.05) on storage Day 2.

Figure 2: Temporal changes in the K-values of refrigerated and frozen mackerel that deviated and complied with the Critical Control Point (CCP). Measurements were performed using n=4 samples; the bars indicate Standard Deviation (SD)
Figure 3 presents the temporal changes in the K-values of CCP-deviant and CCP-compliant refrigerated and frozen scallops. The K-values of CCP-deviant and CCP-compliant refrigerated scallops increased significantly on Day 2 compared to those on Day 0 (p<0.05). Higher K-values were observed for CCP-deviant than that for CCP-compliant refrigerated scallops during the study period (p<0.05). The K-value of CCP-deviant frozen scallops showed no increasing or decreasing trend (p>0.05). On the contrary, the K-value of CCP-compliant frozen scallops increased significantly on Day 2 compared to that on Day 2 (p<0.05). The K-values were higher for CCP-deviant than CCP-compliant scallops (p<0.05) throughout the study period.

Figure 3: Temporal changes in the K-values of refrigerated and frozen scallops that deviated from and complied with the Critical Control Point (CCP)
Figure 4 presents the temporal changes in the K-values of CCP-deviant and CCP-compliant shrimp. The K-values of CCP-deviant and CCP-compliant shrimp increased slightly on Day 2 compared to that on Day 0 (p>0.05). Higher K-values were observed for CCP-deviant shrimp than for CCP-compliant shrimp on all storage days (p<0.05).

Figure 4: Temporal changes in the K-values of frozen shrimp that deviated from or complied with the Critical Control Point (CCP)
The K-value of mackerel at 2 °C was 20% on Day 0 and increased to approximately 40% on Day 5 (El Sheikha et al., 2022). In this study, although the experimental period was only two days, the results were close to the values reported by El Sheikha et al. (2022). In contrast, the K-value of mackerel stored at 20 °C increased to 90% within two days in a previous study (Ohashi, 2002), which was higher than that in this study. The K-value at 5 °C in the present study was higher than that reported by El Sheikha et al. (2022). However, they reported a higher K-value at 20 °C than that observed in the present study. In the CCP-deviant test plot of the present study, the sample was placed at 25 °C for 5 h and then refrigerated, which may have resulted in lower K-values than those of samples stored at 20 °C. Ismail et al. (2023) reported that the K-value of mackerel that was frozen and thawed in a refrigerator increased by approximately 5 and 7% on storage Days 1 and 3, respectively, at 5 °C. In contrast, K-values of the CCP-compliant products increased to approximately 60% on Day 2 in the present study. Additionally, Nakazawa et al. (2015) reported a significant variation in the K-value of frozen mackerel, owing to differences in freshness between fishing vessels and fishing tanks where mackerel are stored after harvest. This finding suggests that the discrepancies in these results may be due to differences among individual mackerel or in post-harvest handling.
In this study, the K value increased on Day 2 from that on Day 0 for the chilled scallops, while the frozen scallops showed a high K value on Day 0. The K-values of scallops tend to increase rapidly after two or three days of refrigeration (Seki, 2021). The CCP-compliant zone of refrigerated scallops in the present study showed a similar trend, with a gradual increase on the second day. However, the K-value increased over time by Day 2 in the CCP-compliant zone. Similarly, 15 day storage of Catarina scallops at 0 °C increased the K-value over time (Ocaño-Higuera et al., 2006). These reports suggest that the K-value of scallops also increases with time, depending on myriad conditions. However, the elucidation of these conditions warrants further study.
In general, freezing stops the enzymatic activity of the muscle and suppresses the increase in K-values. However, Kimura et al. (1997) observed rising K-values in frozen scallops, suggesting that they may have been high at approximately 80% at purchase.
In this study, K values increased from 0 to 2 days in shrimp. In a previous study, the K-values of shrimp stored in ice were approximately 10% on storage Day 2 (Koseki et al., 2006). However, higher values were obtained in the present study. The K-value of frozen shrimp was 15% on storage Day 1 at 15 °C (Koyama et al., 2008); a higher value was obtained in the present study. The K-value increased faster in the CCP-deviant test plot than in the compliant test plot. Crustacean muscle quickly produces and accumulates Hx as an end product when stored at “room temperature” (Koseki et al., 2006). Therefore, it can be inferred that the small amount of Hx that accumulated during storage at 25 °C in the present study contributed to the increase in K-values. Fishery products vary in quality depending on harvest location, environment, and age, and individual differences are significant. The inability to obtain the same sample from different sources was a limitation of this study. Moreover, as the tendency of increased K-values of marine products differs depending on the species, future studies should investigate additional marine species. The results of this study could facilitate the implementation of HACCP in retail seafood stores.
Conclusions
In this study, the validity of CCP was assessed by creating compliant and deviant test plots for refrigerated and frozen mackerel, scallops, and frozen shrimp. Comparison of bacterial viability, E. coli, and V. parahaemolyticus counts, along with K-values demonstrated that the CCP-deviant samples exhibited higher viable bacterial counts than the CCP-compliant samples. V. parahaemolyticus was absent in mackerel but present in CCP-deviant refrigerated and frozen scallops, with higher counts in CCP-deviant frozen shrimp than those in CCP-compliant ones on storage Days 1 and 2. These findings suggest that displays and sales should be managed as CCPs, and the proposed flowchart is applicable in practice.
Author contributions
Not applicable.
Conflicts of interest
There are no conflicts of interest to declare
Acknowledgments
I would like to thank Ms. Taira N. from the Department of Advanced Food Sciences, Tamagawa University for her support.
Funding
This research was funded by the Tamagawa University Graduate Research Fund.
Ethical consideration
Not applicable.
References
Alfaro B., Hernandez I., Baliño-Zuazo L., Barranco A. (2013). Quality changes of Atlantic horse mackerel fillets (Trachurus trachurus) packed in a modified atmosphere at different storage temperatures. Journal of the Science of Food and Agriculture. 93: 2179-2187. [DOI: 10.1002/jsfa.6025]
Ali A., Parisi A., Conversano M.C., Iannacci A., D’Emilio F., Mercurio V., Normanno G. (2020). Food-borne bacteria associated with seafoods: a brief review. Journal of Food Quality and Hazards Control. 7: 4-10. [DOI: 10.18502/jfqhc.7.1.2446]
Casal-Wardle L. (2015). Global food safety: a journey developing a food safety culture within one's enterprise is an essential step in the food safety process. The Manufacturing Confectioner. 95: 55-66.
Chung W.H., Howieson J., Chaklader R. (2021). The ameliorative effects of low-temperature pasteurization on physicochemical and microbiological quality of raw Akoya pearl oyster (Pinctada fucata). Food Control. 129: 108241. [DOI: 10.1016/j.foodcont.2021.108241]
Di D.Y.W., Lee A., Jang J., Han D., Hur H.-G. (2016). Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Applied and Environmental Microbiology. 83: e02680-16. [DOI: 10.1128/AEM.02680-16]
El Sheikha A.F., Allam A.Y., Oz E., Khan M.R., Proestos C., Oz F. (2022). Edible xanthan/propolis coating and its effect on physicochemical, microbial, and sensory quality indices in mackerel tuna (Euthynnus affinis) fillets during chilled storage. Gels. 8: 405. [DOI: 10.3390/gels8070405]
Food and Agriculture Organization (FAO). (2024). FAO report: global fisheries and aquaculture production reaches a new record high. URL: https://www.fao.org/newsroom/detail/fao-report-global-fisheries-and-aquaculture-production-reaches-a-new-record-high/en. Accessed 11 October 2024.
Fraser O.P., Sumar S. (1998). Compositional changes and spoilage in fish (part II)‐ microbiological induced deterioration. Nutrition and Food Science. 98: 325-329. [DOI: 10.1108/00346659810235242]
Gao C., Yang X., Zhao C., Li C., Wang S., Zhang X., Xue B., Cao Z., Zhou H., Yang Y., Shen Z., Yu P., et al. (2022). Characterization of a novel Vibrio parahaemolyticus host-phage pair and antibacterial effect against the host. Archives of Virology. 167: 531-544. [DOI: 10.1007/s00705-021-05278-6]
Gornik S.G., Albalat A., Macpherson H., Brikbeck H., Neil D.M. (2011). The effect of temperature on the bacterial load and microbial composition in Norway lobster (Nephrops norvegicus) tail meat during storage. Journal of Applied Microbiology. 111: 582-592. [DOI: 10.1111/j.1365-2672.2011.05081.x]
Guo M., Jin T.Z., Yang R., Antenucci R., Mills B., Cassidy J., Scullen O.J., Sites J.E., Rajkowski K.T., Sommers C.H. (2013). Inactivation of natural microflora and inoculated Listeria innocua on whole raw shrimp by ozonated water, antimicrobial coatings, and cryogenic freezing. Food Control. 34: 24-30. [DOI: 10.1016/j.foodcont.2013.04.009]
Holvoet K., Jacxsens L., Sampers I., Uyttendaele M. (2012). Insight into the prevalence and distribution of microbial contamination to evaluate water management in the fresh produce processing industry. Journal of Food Protection. 75: 671-681. [DOI: 10.4315/0362-028X.JFP-11-175]
Ichinohe M., Nishijima M., Ishida H., Ibe A., Ota T., Okabe T., Chiba T., Murakami R., Watanabe A. (2015). Illustrated experiments in food hygiene. 3rd edition. Kodansha Scientific Ltd, Tokyo. pp: 29-44.
Ismail A., Ryu J., Yim D.-G., Kim G., Kim S.-S., Lee H.J., Jo C. (2023). Quality evaluation of mackerel fillets stored under different conditions by hyperspectral imaging analysis. Food Science of Animal Resources. 43: 840-858. [DOI: 10.5851/kosfa.2023.e39]
Japan International Research Center for Agricultural Sciences (JIRCAS). (2022). 520. Three major farming methods for shrimp imported to Japan. URL: https://www.jircas.go.jp/ en/program/proc/blog/20220418. Accessed 10 October 2024.
Kaneda T., Yamamoto J., Ueno Y., Satomi M. (2015). Evaluation method of the effects of hygiene management in fishing ports considering the bacteria count. Journal of Japan Society of Civil Engineers, Ser. B3 (Ocean Engineering). 71: I_868-I_873. [DOI: 10.2208/jscejoe.71.I_868]. [Japanese with English abstract]
Kato N., Kunimoto M., Koseki S., Kitakami S., Arai K.-I. (2009). Freshness and quality of fish and shellfish (supplementary edition). Journal of the School of Marine Science and Technology, Tokai University. 7: 87-99.
Kimura M., Narita M., Nomata H., Kaneko H., Yamanaka H. (1997). Effect of storage temperature on the rigor of scallop adductor muscle. Nippon Suisan Gakkaishi. 63: 621-626. [DOI: 10.2331/suisan.63.621]. [Japanese with English abstract]
Ko W.-H. (2013). The relationship among food safety knowledge, attitudes and self-reported HACCP practices in restaurant employees. Food Control. 29: 192-197. [DOI: 10.1016/j.foodcont.2012.05.076]
Kobayashi K., Ochiai Y., Sekiya J., Arai T., Ueda F. (2021). Relationship between the current status and problems of hygiene management based on HACCP, and the actual situation of meat contamination with Campylobacter spp. and Listeria spp. Studies in Animals. 3: 1-14. [Japanese with English abstract]
Koseki S., Kitakami S., Kato N., Arai K.-I. (2006). Rigor mortis of fish and shellfish and evaluation of freshness of their muscles as K value. Journal of the School of Marine Science and Technology, Tokai University. 4: 31-46. [Japanese with English abstract]
Koyama N., Matsukawa M., Shimada M., Sato R. (2008). The change of ATP and its related compounds of the Penaeus vannamei muscle and effect of the sense of taste. Nippon Suisan Gakkaishi. 74: 1068-1074. [DOI: 10.2331/suisan. 74.1068]. [Japanese with English abstract]
Kudo H. (2016). Investigation of the role of food business operators and central/local governments in ensuring food safety. Kakenhi. URL: https://kaken.nii.ac.jp/en/file/ KAKENHI-PROJECT-26450312/26450312seika.pdf. Accessed 8 March 2024. [Japanese with English abstract]
Kyoui D., Fukasawa Y., Miyanaga W., Nakamura Y., Yamane T., Sugita K., Yamadera S., Kai M., Shinoda K., Kawarai T., Ogihara H. (2022). Identification of changes in the microflora composition of Japanese horse mackerel (Trachurus japonicus) during storage to identify specific spoilage organisms. Current Research in Food Science. 5: 1216-1224. [DOI: 10.1016/j.crfs.2022.07.015]
Liu B., Liu H., Pan Y., Xie J., Zhao Y. (2016). Comparison of the effects of environmental parameters on the growth variability of Vibrio parahaemolyticus coupled with strain sources and genotypes analyses. Frontiers in Microbiology. 7: 994. [DOI: 10.3389/fmicb.2016.00994]
López-Caballero M.E., Martı´nez-Alvarez O., Go´mez-Guille´n M.D.C., Montero P. (2007). Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis-inhibiting formulations during chilled storage. International Journal of Food Science and Technology. 42: 1029-1038. [DOI: 10.1111/j.1365-2621.2006.01328.x]
Lopez-Joven C., De Blas I., Roque A. (2018). Temperature effects on the growth and survival of tdh positive Vibrio parahaemolyticus in tissues of postharvest Manila clam (Ruditapes philippinarum). Food Microbiology. 75: 61-64. [DOI: 10.1016/j.fm.2017.10.016]
Lucas J. (2015). Responding to an environmental positive microbial contamination of food from the processing environment presents a significant challenge. The Manufacturing Confectioner. 95: 39-41. URL: https://www. gomc.com/firstpage/201502039.pdf.
Matsumoto T. (2022). Fact-finding survey on quality assurance among food manufacturers. Nippon Shokuhin Kagaku Kogaku Kaishi. 69: 431-442. [DOI: 10.3136/nskkk.69.431]. [Japanese with English abstract]
Medina-Rodríguez A.C., Ávila-Sierra A., Ariza J.J., Guillamón E., Baños-Arjona A., Vicaria J.M., Jurado E. (2020). Clean-in-place disinfection of dual-species biofilm (Listeria and Pseudomonas) by a green antibacterial product made from citrus extract. Food Control. 118: 107422. [DOI: 10.1016/j.foodcont.2020.107422]
Minami A., Chaicumpa W., Chongsa-Nguan M., Samosornsuk S., Monden S., Takeshi K., Makino S.-I., Kawamoto K. (2010). Prevalence of foodborne pathogens in open markets and supermarkets in Thailand. Food Control. 21: 221-226. [DOI: 10.1016/j.foodcont.2009.05.011]
Morita Y., Komoda E., Shiwaku J., Hosomi T., Itagaki M., Nakata K., Nakai H., Watanabe S., Kozawa K., Yamamoto S., Kimura H. (2010). Contamination of cattle and pig carcasses in abattoir in Japan [in Japanese]. Japanese Journal of Food Microbiology. 27: 90-95. [DOI: 10.5803/jsfm.27.90]
Nakazawa N., Wada R., Tanaka R., Okano T., Fukushima H., Maeda T., Okazaki E., Fukuda Y. (2015). Influence of freshness on the quality of frozen mackerel products. Transactions of the Japan Society of Refrigerating and Air Conditioning Engineers. 32: 29-37. [Japanese with English abstract]
Narita M., Kumotsu K., Miyazaki A., Sato A., Shimizu S., Ebitani K. (2018). Characteristics of scallop adductor muscle separated using automated scallop sheller. Journal of Fisheries Technology. 10: 9-17. [Japanese with English abstract]
Ocaño-Higuera V.M., Maeda-Martínez A.N., Lugo-Sánchez M.E., Pacheco-Aguilar R. (2006). Postmortem biochemical and textural changes in the adductor muscle of Catarina scallop stored at 0°C. Journal of Food Biochemistry. 30: 373-389. [DOI: 10.1111/j.1745-4514.2006.00071.x]
Ohashi M. (2002). Determination of K-value and histamine of mackerel and tuna, by using the oxygen-sensor method. Journal of the Food Hygienic Society of Japan. 43: 39-43. [DOI: 10.3358/shokueishi.43.39]. [Japanese with English abstract]
Onozawa Y. (2021). Institutionalization of sanitation control in compliance with HACCP. Journal of the National Institute of Public Health. 70: 90-99. [DOI: 10.20683/jniph.70.2_90]. [Japanese with English abstract]
Parlapani F.F., Ferrocino I., Michailidou S., Argiriou A., Haroutounian S.A., Kokokiris L., Rantsiou K., Boziaris I.S. (2020). Microbiota and volatilome profile of fresh and chill-stored deepwater rose shrimp (Parapenaeus longirostris). Food Research International. 132: 109057. [DOI: 10.1016/j.foodres.2020.109057]
Seki H. (2021). Quality changes in the adductor muscle of Ezo giant scallop Mizuhopecten yessoensis (Jay, 1857) during refrigerated storage. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology. 16: 110-118. [DOI: 10.15578/squalen.585].
Seki H., Nakazato K., Kobayashi K., Lee T.S., Sakurada M., Hamada-Sato N. (2016). Effect of freezing and thawing on the quality of northern bluefin tuna Thunnus Thynnus (Linnaeus 1758). Asian Fisheries Science. 29: 232-244. [DOI: 10.33997/j.afs.2016.29.4.006]
Shimohata T., Uebanso T., Mawatari K., Takahashi A. (2022). Metabolic analysis of Vibrio parahaemolyticus in different salinity. The Salt Science Research Foundation Annual Research Report. 2020: 203-214. URL: https://www. saltscience.or.jp/images/2023/07/202044.pdf. [Japanese with English abstract]
Souza D.S.M., Ramos A.P.D., Nunes F.F., Moresco V., Taniguchi S., Leal D.A.G., Sasaki S.T., Bícego M.C., Montone R.C., Durigan M., Teixeira A.L., Pilotto M.R., et al. (2012). Evaluation of tropical water sources and mollusks in southern Brazil using microbiological, biochemical, and chemical parameters. Ecotoxicology and Environmental Safety. 76: 153-161. [DOI: 10.1016/j.ecoenv.2011.09.018]
Tayel A.A., Elzahy A.F., Moussa S.H., Al-Saggaf M.S., Diab A.M. (2020). Biopreservation of shrimps using composed edible coatings from chitosan nanoparticles and cloves extract. Journal of Food Quality. 2020. [DOI: 10.1155/2020/8878452]
Vu T.T.T., Hoang T.T.H., Fleischmann S., Pham H.N., Lai T.L.H., Cam T.T.H., Truong L.O., Phung V.P.L.D.C., Alter T. (2022). Quantification and antimicrobial resistance of Vibrio parahaemolyticus in retail seafood in Hanoi, Vietnam. Journal of Food Protection. 85: 786-791. [DOI: 10.4315/JFP-21-444]
Wong H.-C., Jiang H.-Y., Lin H.-Y., Wang Y.-T. (2015) Microbiological quality of seafood marketed in Taiwan. Journal of Food Protection. 78: 1973-1979. [DOI: 10.4315/0362-028X.JFP-15-152]
Wu Q., Liu J., Malakar P.K., Pan Y., Zhao Y., Zhang Z. (2023). Modeling naturally-occurring Vibrio parahaemolyticus in post-harvest raw shrimps. Food Research International. 173: 113462. [DOI: 10.1016/j.foodres.2023.113462]
Yamamoto S. (2014). Hazard analysis and critical control (HACCP) system will be an imposition near future. Journal of Veterinary Epidemiology. 18: 144-147. [DOI: 10.2743/jve.18.144]. [Japanese with English abstract]
Yamao M., Amano M. (2018). A study on development of GAP in Thai aquaculture: focus on shrimp farming. Journal of Regional Fisheries. 58: 89-98. [DOI: 10.34510/jrfs.58.2_89]. [Japanese with English abstract]
In this study, the K value increased on Day 2 from that on Day 0 for the chilled scallops, while the frozen scallops showed a high K value on Day 0. The K-values of scallops tend to increase rapidly after two or three days of refrigeration (Seki, 2021). The CCP-compliant zone of refrigerated scallops in the present study showed a similar trend, with a gradual increase on the second day. However, the K-value increased over time by Day 2 in the CCP-compliant zone. Similarly, 15 day storage of Catarina scallops at 0 °C increased the K-value over time (Ocaño-Higuera et al., 2006). These reports suggest that the K-value of scallops also increases with time, depending on myriad conditions. However, the elucidation of these conditions warrants further study.
In general, freezing stops the enzymatic activity of the muscle and suppresses the increase in K-values. However, Kimura et al. (1997) observed rising K-values in frozen scallops, suggesting that they may have been high at approximately 80% at purchase.
In this study, K values increased from 0 to 2 days in shrimp. In a previous study, the K-values of shrimp stored in ice were approximately 10% on storage Day 2 (Koseki et al., 2006). However, higher values were obtained in the present study. The K-value of frozen shrimp was 15% on storage Day 1 at 15 °C (Koyama et al., 2008); a higher value was obtained in the present study. The K-value increased faster in the CCP-deviant test plot than in the compliant test plot. Crustacean muscle quickly produces and accumulates Hx as an end product when stored at “room temperature” (Koseki et al., 2006). Therefore, it can be inferred that the small amount of Hx that accumulated during storage at 25 °C in the present study contributed to the increase in K-values. Fishery products vary in quality depending on harvest location, environment, and age, and individual differences are significant. The inability to obtain the same sample from different sources was a limitation of this study. Moreover, as the tendency of increased K-values of marine products differs depending on the species, future studies should investigate additional marine species. The results of this study could facilitate the implementation of HACCP in retail seafood stores.
Conclusions
In this study, the validity of CCP was assessed by creating compliant and deviant test plots for refrigerated and frozen mackerel, scallops, and frozen shrimp. Comparison of bacterial viability, E. coli, and V. parahaemolyticus counts, along with K-values demonstrated that the CCP-deviant samples exhibited higher viable bacterial counts than the CCP-compliant samples. V. parahaemolyticus was absent in mackerel but present in CCP-deviant refrigerated and frozen scallops, with higher counts in CCP-deviant frozen shrimp than those in CCP-compliant ones on storage Days 1 and 2. These findings suggest that displays and sales should be managed as CCPs, and the proposed flowchart is applicable in practice.
Author contributions
Not applicable.
Conflicts of interest
There are no conflicts of interest to declare
Acknowledgments
I would like to thank Ms. Taira N. from the Department of Advanced Food Sciences, Tamagawa University for her support.
Funding
This research was funded by the Tamagawa University Graduate Research Fund.
Ethical consideration
Not applicable.
References
Alfaro B., Hernandez I., Baliño-Zuazo L., Barranco A. (2013). Quality changes of Atlantic horse mackerel fillets (Trachurus trachurus) packed in a modified atmosphere at different storage temperatures. Journal of the Science of Food and Agriculture. 93: 2179-2187. [DOI: 10.1002/jsfa.6025]
Ali A., Parisi A., Conversano M.C., Iannacci A., D’Emilio F., Mercurio V., Normanno G. (2020). Food-borne bacteria associated with seafoods: a brief review. Journal of Food Quality and Hazards Control. 7: 4-10. [DOI: 10.18502/jfqhc.7.1.2446]
Casal-Wardle L. (2015). Global food safety: a journey developing a food safety culture within one's enterprise is an essential step in the food safety process. The Manufacturing Confectioner. 95: 55-66.
Chung W.H., Howieson J., Chaklader R. (2021). The ameliorative effects of low-temperature pasteurization on physicochemical and microbiological quality of raw Akoya pearl oyster (Pinctada fucata). Food Control. 129: 108241. [DOI: 10.1016/j.foodcont.2021.108241]
Di D.Y.W., Lee A., Jang J., Han D., Hur H.-G. (2016). Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Applied and Environmental Microbiology. 83: e02680-16. [DOI: 10.1128/AEM.02680-16]
El Sheikha A.F., Allam A.Y., Oz E., Khan M.R., Proestos C., Oz F. (2022). Edible xanthan/propolis coating and its effect on physicochemical, microbial, and sensory quality indices in mackerel tuna (Euthynnus affinis) fillets during chilled storage. Gels. 8: 405. [DOI: 10.3390/gels8070405]
Food and Agriculture Organization (FAO). (2024). FAO report: global fisheries and aquaculture production reaches a new record high. URL: https://www.fao.org/newsroom/detail/fao-report-global-fisheries-and-aquaculture-production-reaches-a-new-record-high/en. Accessed 11 October 2024.
Fraser O.P., Sumar S. (1998). Compositional changes and spoilage in fish (part II)‐ microbiological induced deterioration. Nutrition and Food Science. 98: 325-329. [DOI: 10.1108/00346659810235242]
Gao C., Yang X., Zhao C., Li C., Wang S., Zhang X., Xue B., Cao Z., Zhou H., Yang Y., Shen Z., Yu P., et al. (2022). Characterization of a novel Vibrio parahaemolyticus host-phage pair and antibacterial effect against the host. Archives of Virology. 167: 531-544. [DOI: 10.1007/s00705-021-05278-6]
Gornik S.G., Albalat A., Macpherson H., Brikbeck H., Neil D.M. (2011). The effect of temperature on the bacterial load and microbial composition in Norway lobster (Nephrops norvegicus) tail meat during storage. Journal of Applied Microbiology. 111: 582-592. [DOI: 10.1111/j.1365-2672.2011.05081.x]
Guo M., Jin T.Z., Yang R., Antenucci R., Mills B., Cassidy J., Scullen O.J., Sites J.E., Rajkowski K.T., Sommers C.H. (2013). Inactivation of natural microflora and inoculated Listeria innocua on whole raw shrimp by ozonated water, antimicrobial coatings, and cryogenic freezing. Food Control. 34: 24-30. [DOI: 10.1016/j.foodcont.2013.04.009]
Holvoet K., Jacxsens L., Sampers I., Uyttendaele M. (2012). Insight into the prevalence and distribution of microbial contamination to evaluate water management in the fresh produce processing industry. Journal of Food Protection. 75: 671-681. [DOI: 10.4315/0362-028X.JFP-11-175]
Ichinohe M., Nishijima M., Ishida H., Ibe A., Ota T., Okabe T., Chiba T., Murakami R., Watanabe A. (2015). Illustrated experiments in food hygiene. 3rd edition. Kodansha Scientific Ltd, Tokyo. pp: 29-44.
Ismail A., Ryu J., Yim D.-G., Kim G., Kim S.-S., Lee H.J., Jo C. (2023). Quality evaluation of mackerel fillets stored under different conditions by hyperspectral imaging analysis. Food Science of Animal Resources. 43: 840-858. [DOI: 10.5851/kosfa.2023.e39]
Japan International Research Center for Agricultural Sciences (JIRCAS). (2022). 520. Three major farming methods for shrimp imported to Japan. URL: https://www.jircas.go.jp/ en/program/proc/blog/20220418. Accessed 10 October 2024.
Kaneda T., Yamamoto J., Ueno Y., Satomi M. (2015). Evaluation method of the effects of hygiene management in fishing ports considering the bacteria count. Journal of Japan Society of Civil Engineers, Ser. B3 (Ocean Engineering). 71: I_868-I_873. [DOI: 10.2208/jscejoe.71.I_868]. [Japanese with English abstract]
Kato N., Kunimoto M., Koseki S., Kitakami S., Arai K.-I. (2009). Freshness and quality of fish and shellfish (supplementary edition). Journal of the School of Marine Science and Technology, Tokai University. 7: 87-99.
Kimura M., Narita M., Nomata H., Kaneko H., Yamanaka H. (1997). Effect of storage temperature on the rigor of scallop adductor muscle. Nippon Suisan Gakkaishi. 63: 621-626. [DOI: 10.2331/suisan.63.621]. [Japanese with English abstract]
Ko W.-H. (2013). The relationship among food safety knowledge, attitudes and self-reported HACCP practices in restaurant employees. Food Control. 29: 192-197. [DOI: 10.1016/j.foodcont.2012.05.076]
Kobayashi K., Ochiai Y., Sekiya J., Arai T., Ueda F. (2021). Relationship between the current status and problems of hygiene management based on HACCP, and the actual situation of meat contamination with Campylobacter spp. and Listeria spp. Studies in Animals. 3: 1-14. [Japanese with English abstract]
Koseki S., Kitakami S., Kato N., Arai K.-I. (2006). Rigor mortis of fish and shellfish and evaluation of freshness of their muscles as K value. Journal of the School of Marine Science and Technology, Tokai University. 4: 31-46. [Japanese with English abstract]
Koyama N., Matsukawa M., Shimada M., Sato R. (2008). The change of ATP and its related compounds of the Penaeus vannamei muscle and effect of the sense of taste. Nippon Suisan Gakkaishi. 74: 1068-1074. [DOI: 10.2331/suisan. 74.1068]. [Japanese with English abstract]
Kudo H. (2016). Investigation of the role of food business operators and central/local governments in ensuring food safety. Kakenhi. URL: https://kaken.nii.ac.jp/en/file/ KAKENHI-PROJECT-26450312/26450312seika.pdf. Accessed 8 March 2024. [Japanese with English abstract]
Kyoui D., Fukasawa Y., Miyanaga W., Nakamura Y., Yamane T., Sugita K., Yamadera S., Kai M., Shinoda K., Kawarai T., Ogihara H. (2022). Identification of changes in the microflora composition of Japanese horse mackerel (Trachurus japonicus) during storage to identify specific spoilage organisms. Current Research in Food Science. 5: 1216-1224. [DOI: 10.1016/j.crfs.2022.07.015]
Liu B., Liu H., Pan Y., Xie J., Zhao Y. (2016). Comparison of the effects of environmental parameters on the growth variability of Vibrio parahaemolyticus coupled with strain sources and genotypes analyses. Frontiers in Microbiology. 7: 994. [DOI: 10.3389/fmicb.2016.00994]
López-Caballero M.E., Martı´nez-Alvarez O., Go´mez-Guille´n M.D.C., Montero P. (2007). Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis-inhibiting formulations during chilled storage. International Journal of Food Science and Technology. 42: 1029-1038. [DOI: 10.1111/j.1365-2621.2006.01328.x]
Lopez-Joven C., De Blas I., Roque A. (2018). Temperature effects on the growth and survival of tdh positive Vibrio parahaemolyticus in tissues of postharvest Manila clam (Ruditapes philippinarum). Food Microbiology. 75: 61-64. [DOI: 10.1016/j.fm.2017.10.016]
Lucas J. (2015). Responding to an environmental positive microbial contamination of food from the processing environment presents a significant challenge. The Manufacturing Confectioner. 95: 39-41. URL: https://www. gomc.com/firstpage/201502039.pdf.
Matsumoto T. (2022). Fact-finding survey on quality assurance among food manufacturers. Nippon Shokuhin Kagaku Kogaku Kaishi. 69: 431-442. [DOI: 10.3136/nskkk.69.431]. [Japanese with English abstract]
Medina-Rodríguez A.C., Ávila-Sierra A., Ariza J.J., Guillamón E., Baños-Arjona A., Vicaria J.M., Jurado E. (2020). Clean-in-place disinfection of dual-species biofilm (Listeria and Pseudomonas) by a green antibacterial product made from citrus extract. Food Control. 118: 107422. [DOI: 10.1016/j.foodcont.2020.107422]
Minami A., Chaicumpa W., Chongsa-Nguan M., Samosornsuk S., Monden S., Takeshi K., Makino S.-I., Kawamoto K. (2010). Prevalence of foodborne pathogens in open markets and supermarkets in Thailand. Food Control. 21: 221-226. [DOI: 10.1016/j.foodcont.2009.05.011]
Morita Y., Komoda E., Shiwaku J., Hosomi T., Itagaki M., Nakata K., Nakai H., Watanabe S., Kozawa K., Yamamoto S., Kimura H. (2010). Contamination of cattle and pig carcasses in abattoir in Japan [in Japanese]. Japanese Journal of Food Microbiology. 27: 90-95. [DOI: 10.5803/jsfm.27.90]
Nakazawa N., Wada R., Tanaka R., Okano T., Fukushima H., Maeda T., Okazaki E., Fukuda Y. (2015). Influence of freshness on the quality of frozen mackerel products. Transactions of the Japan Society of Refrigerating and Air Conditioning Engineers. 32: 29-37. [Japanese with English abstract]
Narita M., Kumotsu K., Miyazaki A., Sato A., Shimizu S., Ebitani K. (2018). Characteristics of scallop adductor muscle separated using automated scallop sheller. Journal of Fisheries Technology. 10: 9-17. [Japanese with English abstract]
Ocaño-Higuera V.M., Maeda-Martínez A.N., Lugo-Sánchez M.E., Pacheco-Aguilar R. (2006). Postmortem biochemical and textural changes in the adductor muscle of Catarina scallop stored at 0°C. Journal of Food Biochemistry. 30: 373-389. [DOI: 10.1111/j.1745-4514.2006.00071.x]
Ohashi M. (2002). Determination of K-value and histamine of mackerel and tuna, by using the oxygen-sensor method. Journal of the Food Hygienic Society of Japan. 43: 39-43. [DOI: 10.3358/shokueishi.43.39]. [Japanese with English abstract]
Onozawa Y. (2021). Institutionalization of sanitation control in compliance with HACCP. Journal of the National Institute of Public Health. 70: 90-99. [DOI: 10.20683/jniph.70.2_90]. [Japanese with English abstract]
Parlapani F.F., Ferrocino I., Michailidou S., Argiriou A., Haroutounian S.A., Kokokiris L., Rantsiou K., Boziaris I.S. (2020). Microbiota and volatilome profile of fresh and chill-stored deepwater rose shrimp (Parapenaeus longirostris). Food Research International. 132: 109057. [DOI: 10.1016/j.foodres.2020.109057]
Seki H. (2021). Quality changes in the adductor muscle of Ezo giant scallop Mizuhopecten yessoensis (Jay, 1857) during refrigerated storage. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology. 16: 110-118. [DOI: 10.15578/squalen.585].
Seki H., Nakazato K., Kobayashi K., Lee T.S., Sakurada M., Hamada-Sato N. (2016). Effect of freezing and thawing on the quality of northern bluefin tuna Thunnus Thynnus (Linnaeus 1758). Asian Fisheries Science. 29: 232-244. [DOI: 10.33997/j.afs.2016.29.4.006]
Shimohata T., Uebanso T., Mawatari K., Takahashi A. (2022). Metabolic analysis of Vibrio parahaemolyticus in different salinity. The Salt Science Research Foundation Annual Research Report. 2020: 203-214. URL: https://www. saltscience.or.jp/images/2023/07/202044.pdf. [Japanese with English abstract]
Souza D.S.M., Ramos A.P.D., Nunes F.F., Moresco V., Taniguchi S., Leal D.A.G., Sasaki S.T., Bícego M.C., Montone R.C., Durigan M., Teixeira A.L., Pilotto M.R., et al. (2012). Evaluation of tropical water sources and mollusks in southern Brazil using microbiological, biochemical, and chemical parameters. Ecotoxicology and Environmental Safety. 76: 153-161. [DOI: 10.1016/j.ecoenv.2011.09.018]
Tayel A.A., Elzahy A.F., Moussa S.H., Al-Saggaf M.S., Diab A.M. (2020). Biopreservation of shrimps using composed edible coatings from chitosan nanoparticles and cloves extract. Journal of Food Quality. 2020. [DOI: 10.1155/2020/8878452]
Vu T.T.T., Hoang T.T.H., Fleischmann S., Pham H.N., Lai T.L.H., Cam T.T.H., Truong L.O., Phung V.P.L.D.C., Alter T. (2022). Quantification and antimicrobial resistance of Vibrio parahaemolyticus in retail seafood in Hanoi, Vietnam. Journal of Food Protection. 85: 786-791. [DOI: 10.4315/JFP-21-444]
Wong H.-C., Jiang H.-Y., Lin H.-Y., Wang Y.-T. (2015) Microbiological quality of seafood marketed in Taiwan. Journal of Food Protection. 78: 1973-1979. [DOI: 10.4315/0362-028X.JFP-15-152]
Wu Q., Liu J., Malakar P.K., Pan Y., Zhao Y., Zhang Z. (2023). Modeling naturally-occurring Vibrio parahaemolyticus in post-harvest raw shrimps. Food Research International. 173: 113462. [DOI: 10.1016/j.foodres.2023.113462]
Yamamoto S. (2014). Hazard analysis and critical control (HACCP) system will be an imposition near future. Journal of Veterinary Epidemiology. 18: 144-147. [DOI: 10.2743/jve.18.144]. [Japanese with English abstract]
Yamao M., Amano M. (2018). A study on development of GAP in Thai aquaculture: focus on shrimp farming. Journal of Regional Fisheries. 58: 89-98. [DOI: 10.34510/jrfs.58.2_89]. [Japanese with English abstract]
* Corresponding author (H. Seki)
* E-mail: sekihrk@stf.teu.ac.jp
ORCID ID: https://orcid.org/0000-0003-2426-7554
* E-mail: sekihrk@stf.teu.ac.jp
ORCID ID: https://orcid.org/0000-0003-2426-7554
Type of Study: Original article |
Subject:
Special
Received: 24/04/12 | Accepted: 24/08/02 | Published: 24/09/30
Received: 24/04/12 | Accepted: 24/08/02 | Published: 24/09/30
References
1. Alfaro B., Hernandez I., Baliño-Zuazo L., Barranco A. (2013). Quality changes of Atlantic horse mackerel fillets (Trachurus trachurus) packed in a modified atmosphere at different storage temperatures. Journal of the Science of Food and Agriculture. 93: 2179-2187. [DOI: 10.1002/jsfa.6025] [DOI:10.1002/jsfa.6025] [PMID]
2. Ali A., Parisi A., Conversano M.C., Iannacci A., D'Emilio F., Mercurio V., Normanno G. (2020). Food-borne bacteria associated with seafoods: a brief review. Journal of Food Quality and Hazards Control. 7: 4-10. [DOI: 10.18502/jfqhc.7.1.2446] [DOI:10.18502/jfqhc.7.1.2446]
3. Casal-Wardle L. (2015). Global food safety: a journey developing a food safety culture within one's enterprise is an essential step in the food safety process. The Manufacturing Confectioner. 95: 55-66.
4. Chung W.H., Howieson J., Chaklader R. (2021). The ameliorative effects of low-temperature pasteurization on physicochemical and microbiological quality of raw Akoya pearl oyster (Pinctada fucata). Food Control. 129: 108241. [DOI: 10.1016/j.foodcont.2021.108241] [DOI:10.1016/j.foodcont.2021.108241]
5. Di D.Y.W., Lee A., Jang J., Han D., Hur H.-G. (2016). Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Applied and Environmental Microbiology. 83: e02680-16. [DOI: 10.1128/AEM.02680-16] [DOI:10.1128/AEM.02680-16] [PMID] [PMCID]
6. El Sheikha A.F., Allam A.Y., Oz E., Khan M.R., Proestos C., Oz F. (2022). Edible xanthan/propolis coating and its effect on physicochemical, microbial, and sensory quality indices in mackerel tuna (Euthynnus affinis) fillets during chilled storage. Gels. 8: 405. [DOI: 10.3390/gels8070405] [DOI:10.3390/gels8070405] [PMID] [PMCID]
7. Food and Agriculture Organization (FAO). (2024). FAO report: global fisheries and aquaculture production reaches a new record high. URL: https://www.fao.org/newsroom/detail/fao-report-global-fisheries-and-aquaculture-production-reaches-a-new-record-high/en. Accessed 11 October 2024.
8. Fraser O.P., Sumar S. (1998). Compositional changes and spoilage in fish (part II)‐ microbiological induced deterioration. Nutrition and Food Science. 98: 325-329. [DOI: 10.1108/00346659810235242] [DOI:10.1108/00346659810235242]
9. Gao C., Yang X., Zhao C., Li C., Wang S., Zhang X., Xue B., Cao Z., Zhou H., Yang Y., Shen Z., Yu P., et al. (2022). Characterization of a novel Vibrio parahaemolyticus host-phage pair and antibacterial effect against the host. Archives of Virology. 167: 531-544. [DOI: 10.1007/s00705-021-05278-6] [DOI:10.1007/s00705-021-05278-6] [PMID]
10. Gornik S.G., Albalat A., Macpherson H., Brikbeck H., Neil D.M. (2011). The effect of temperature on the bacterial load and microbial composition in Norway lobster (Nephrops norvegicus) tail meat during storage. Journal of Applied Microbiology. 111: 582-592. [DOI: 10.1111/j.1365-2672.2011.05081.x] [DOI:10.1111/j.1365-2672.2011.05081.x] [PMID]
11. Guo M., Jin T.Z., Yang R., Antenucci R., Mills B., Cassidy J., Scullen O.J., Sites J.E., Rajkowski K.T., Sommers C.H. (2013). Inactivation of natural microflora and inoculated Listeria innocua on whole raw shrimp by ozonated water, antimicrobial coatings, and cryogenic freezing. Food Control. 34: 24-30. [DOI: 10.1016/j.foodcont.2013.04.009] [DOI:10.1016/j.foodcont.2013.04.009]
12. Holvoet K., Jacxsens L., Sampers I., Uyttendaele M. (2012). Insight into the prevalence and distribution of microbial contamination to evaluate water management in the fresh produce processing industry. Journal of Food Protection. 75: 671-681. [DOI: 10.4315/0362-028X.JFP-11-175] [DOI:10.4315/0362-028X.JFP-11-175] [PMID]
13. Ichinohe M., Nishijima M., Ishida H., Ibe A., Ota T., Okabe T., Chiba T., Murakami R., Watanabe A. (2015). Illustrated experiments in food hygiene. 3rd edition. Kodansha Scientific Ltd, Tokyo. pp: 29-44.
14. Ismail A., Ryu J., Yim D.-G., Kim G., Kim S.-S., Lee H.J., Jo C. (2023). Quality evaluation of mackerel fillets stored under different conditions by hyperspectral imaging analysis. Food Science of Animal Resources. 43: 840-858. [DOI: 10.5851/kosfa.2023.e39] [DOI:10.5851/kosfa.2023.e39] [PMID] [PMCID]
15. Japan International Research Center for Agricultural Sciences (JIRCAS). (2022). 520. Three major farming methods for shrimp imported to Japan. URL: https://www.jircas.go.jp/ en/program/proc/blog/20220418. Accessed 10 October 2024.
16. Kaneda T., Yamamoto J., Ueno Y., Satomi M. (2015). Evaluation method of the effects of hygiene management in fishing ports considering the bacteria count. Journal of Japan Society of Civil Engineers, Ser. B3 (Ocean Engineering). 71: I_868-I_873. [DOI: 10.2208/jscejoe.71.I_868]. [Japanese with English abstract] [DOI:10.2208/jscejoe.71.I_868]
17. Kato N., Kunimoto M., Koseki S., Kitakami S., Arai K.-I. (2009). Freshness and quality of fish and shellfish (supplementary edition). Journal of the School of Marine Science and Technology, Tokai University. 7: 87-99.
18. Kimura M., Narita M., Nomata H., Kaneko H., Yamanaka H. (1997). Effect of storage temperature on the rigor of scallop adductor muscle. Nippon Suisan Gakkaishi. 63: 621-626. [DOI: 10.2331/suisan.63.621]. [Japanese with English abstract] [DOI:10.2331/suisan.63.621]
19. Ko W.-H. (2013). The relationship among food safety knowledge, attitudes and self-reported HACCP practices in restaurant employees. Food Control. 29: 192-197. [DOI: 10.1016/j.foodcont.2012.05.076] [DOI:10.1016/j.foodcont.2012.05.076]
20. Kobayashi K., Ochiai Y., Sekiya J., Arai T., Ueda F. (2021). Relationship between the current status and problems of hygiene management based on HACCP, and the actual situation of meat contamination with Campylobacter spp. and Listeria spp. Studies in Animals. 3: 1-14. [Japanese with English abstract]
21. Koseki S., Kitakami S., Kato N., Arai K.-I. (2006). Rigor mortis of fish and shellfish and evaluation of freshness of their muscles as K value. Journal of the School of Marine Science and Technology, Tokai University. 4: 31-46. [Japanese with English abstract]
22. Koyama N., Matsukawa M., Shimada M., Sato R. (2008). The change of ATP and its related compounds of the Penaeus vannamei muscle and effect of the sense of taste. Nippon Suisan Gakkaishi. 74: 1068-1074. [DOI: 10.2331/suisan. 74.1068]. [Japanese with English abstract] [DOI:10.2331/suisan.74.1068]
23. Kudo H. (2016). Investigation of the role of food business operators and central/local governments in ensuring food safety. Kakenhi. URL: https://kaken.nii.ac.jp/en/file/ KAKENHI-PROJECT-26450312/26450312seika.pdf. Accessed 8 March 2024. [Japanese with English abstract]
24. Kyoui D., Fukasawa Y., Miyanaga W., Nakamura Y., Yamane T., Sugita K., Yamadera S., Kai M., Shinoda K., Kawarai T., Ogihara H. (2022). Identification of changes in the microflora composition of Japanese horse mackerel (Trachurus japonicus) during storage to identify specific spoilage organisms. Current Research in Food Science. 5: 1216-1224. [DOI: 10.1016/j.crfs.2022.07.015] [DOI:10.1016/j.crfs.2022.07.015] [PMID] [PMCID]
25. Liu B., Liu H., Pan Y., Xie J., Zhao Y. (2016). Comparison of the effects of environmental parameters on the growth variability of Vibrio parahaemolyticus coupled with strain sources and genotypes analyses. Frontiers in Microbiology. 7: 994. [DOI: 10.3389/fmicb.2016.00994] [DOI:10.3389/fmicb.2016.00994]
26. López-Caballero M.E., Martı'nez-Alvarez O., Go'mez-Guille'n M.D.C., Montero P. (2007). Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis-inhibiting formulations during chilled storage. International Journal of Food Science and Technology. 42: 1029-1038. [DOI: 10.1111/j.1365-2621.2006.01328.x] [DOI:10.1111/j.1365-2621.2006.01328.x]
27. Lopez-Joven C., De Blas I., Roque A. (2018). Temperature effects on the growth and survival of tdh positive Vibrio parahaemolyticus in tissues of postharvest Manila clam (Ruditapes philippinarum). Food Microbiology. 75: 61-64. [DOI: 10.1016/j.fm.2017.10.016] [DOI:10.1016/j.fm.2017.10.016] [PMID]
28. Lucas J. (2015). Responding to an environmental positive microbial contamination of food from the processing environment presents a significant challenge. The Manufacturing Confectioner. 95: 39-41. URL: https://www. gomc.com/firstpage/201502039.pdf.
29. Matsumoto T. (2022). Fact-finding survey on quality assurance among food manufacturers. Nippon Shokuhin Kagaku Kogaku Kaishi. 69: 431-442. [DOI: 10.3136/nskkk.69.431]. [Japanese with English abstract] [DOI:10.3136/nskkk.69.431]
30. Medina-Rodríguez A.C., Ávila-Sierra A., Ariza J.J., Guillamón E., Baños-Arjona A., Vicaria J.M., Jurado E. (2020). Clean-in-place disinfection of dual-species biofilm (Listeria and Pseudomonas) by a green antibacterial product made from citrus extract. Food Control. 118: 107422. [DOI: 10.1016/j.foodcont.2020.107422] [DOI:10.1016/j.foodcont.2020.107422]
31. Minami A., Chaicumpa W., Chongsa-Nguan M., Samosornsuk S., Monden S., Takeshi K., Makino S.-I., Kawamoto K. (2010). Prevalence of foodborne pathogens in open markets and supermarkets in Thailand. Food Control. 21: 221-226. [DOI: 10.1016/j.foodcont.2009.05.011] [DOI:10.1016/j.foodcont.2009.05.011]
32. Morita Y., Komoda E., Shiwaku J., Hosomi T., Itagaki M., Nakata K., Nakai H., Watanabe S., Kozawa K., Yamamoto S., Kimura H. (2010). Contamination of cattle and pig carcasses in abattoir in Japan [in Japanese]. Japanese Journal of Food Microbiology. 27: 90-95. [DOI: 10.5803/jsfm.27.90] [DOI:10.5803/jsfm.27.90]
33. Nakazawa N., Wada R., Tanaka R., Okano T., Fukushima H., Maeda T., Okazaki E., Fukuda Y. (2015). Influence of freshness on the quality of frozen mackerel products. Transactions of the Japan Society of Refrigerating and Air Conditioning Engineers. 32: 29-37. [Japanese with English abstract]
34. Narita M., Kumotsu K., Miyazaki A., Sato A., Shimizu S., Ebitani K. (2018). Characteristics of scallop adductor muscle separated using automated scallop sheller. Journal of Fisheries Technology. 10: 9-17. [Japanese with English abstract]
35. Ocaño-Higuera V.M., Maeda-Martínez A.N., Lugo-Sánchez M.E., Pacheco-Aguilar R. (2006). Postmortem biochemical and textural changes in the adductor muscle of Catarina scallop stored at 0°C. Journal of Food Biochemistry. 30: 373-389. [DOI: 10.1111/j.1745-4514.2006.00071.x] [DOI:10.1111/j.1745-4514.2006.00071.x]
36. Ohashi M. (2002). Determination of K-value and histamine of mackerel and tuna, by using the oxygen-sensor method. Journal of the Food Hygienic Society of Japan. 43: 39-43. [DOI: 10.3358/shokueishi.43.39]. [Japanese with English abstract] [DOI:10.3358/shokueishi.43.39] [PMID]
37. Onozawa Y. (2021). Institutionalization of sanitation control in compliance with HACCP. Journal of the National Institute of Public Health. 70: 90-99. [DOI: 10.20683/jniph.70.2_90]. [Japanese with English abstract]
38. Parlapani F.F., Ferrocino I., Michailidou S., Argiriou A., Haroutounian S.A., Kokokiris L., Rantsiou K., Boziaris I.S. (2020). Microbiota and volatilome profile of fresh and chill-stored deepwater rose shrimp (Parapenaeus longirostris). Food Research International. 132: 109057. [DOI: 10.1016/j.foodres.2020.109057] [DOI:10.1016/j.foodres.2020.109057] [PMID]
39. Seki H. (2021). Quality changes in the adductor muscle of Ezo giant scallop Mizuhopecten yessoensis (Jay, 1857) during refrigerated storage. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology. 16: 110-118. [DOI: 10.15578/squalen.585]. [DOI:10.15578/squalen.585]
40. Seki H., Nakazato K., Kobayashi K., Lee T.S., Sakurada M., Hamada-Sato N. (2016). Effect of freezing and thawing on the quality of northern bluefin tuna Thunnus Thynnus (Linnaeus 1758). Asian Fisheries Science. 29: 232-244. [DOI: 10.33997/j.afs.2016.29.4.006] [DOI:10.33997/j.afs.2016.29.4.006]
41. Shimohata T., Uebanso T., Mawatari K., Takahashi A. (2022). Metabolic analysis of Vibrio parahaemolyticus in different salinity. The Salt Science Research Foundation Annual Research Report. 2020: 203-214. URL: https://www. saltscience.or.jp/images/2023/07/202044.pdf. [Japanese with English abstract]
42. Souza D.S.M., Ramos A.P.D., Nunes F.F., Moresco V., Taniguchi S., Leal D.A.G., Sasaki S.T., Bícego M.C., Montone R.C., Durigan M., Teixeira A.L., Pilotto M.R., et al. (2012). Evaluation of tropical water sources and mollusks in southern Brazil using microbiological, biochemical, and chemical parameters. Ecotoxicology and Environmental Safety. 76: 153-161. [DOI: 10.1016/j.ecoenv.2011.09.018] [DOI:10.1016/j.ecoenv.2011.09.018] [PMID]
43. Tayel A.A., Elzahy A.F., Moussa S.H., Al-Saggaf M.S., Diab A.M. (2020). Biopreservation of shrimps using composed edible coatings from chitosan nanoparticles and cloves extract. Journal of Food Quality. 2020. [DOI: 10.1155/2020/8878452] [DOI:10.1155/2020/8878452]
44. Vu T.T.T., Hoang T.T.H., Fleischmann S., Pham H.N., Lai T.L.H., Cam T.T.H., Truong L.O., Phung V.P.L.D.C., Alter T. (2022). Quantification and antimicrobial resistance of Vibrio parahaemolyticus in retail seafood in Hanoi, Vietnam. Journal of Food Protection. 85: 786-791. [DOI: 10.4315/JFP-21-444] [DOI:10.4315/JFP-21-444] [PMID]
45. Wong H.-C., Jiang H.-Y., Lin H.-Y., Wang Y.-T. (2015) Microbiological quality of seafood marketed in Taiwan. Journal of Food Protection. 78: 1973-1979. [DOI: 10.4315/0362-028X.JFP-15-152] [DOI:10.4315/0362-028X.JFP-15-152] [PMID]
46. Wu Q., Liu J., Malakar P.K., Pan Y., Zhao Y., Zhang Z. (2023). Modeling naturally-occurring Vibrio parahaemolyticus in post-harvest raw shrimps. Food Research International. 173: 113462. [DOI: 10.1016/j.foodres.2023.113462] [DOI:10.1016/j.foodres.2023.113462] [PMID]
47. Yamamoto S. (2014). Hazard analysis and critical control (HACCP) system will be an imposition near future. Journal of Veterinary Epidemiology. 18: 144-147. [DOI: 10.2743/jve.18.144]. [Japanese with English abstract] [DOI:10.2743/jve.18.144]
48. Yamao M., Amano M. (2018). A study on development of GAP in Thai aquaculture: focus on shrimp farming. Journal of Regional Fisheries. 58: 89-98. [DOI: 10.34510/jrfs.58.2_89]. [Japanese with English abstract]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







