Volume 12, Issue 4 (December 2025)
J. Food Qual. Hazards Control 2025, 12(4): 310-316 |
Back to browse issues page
Ethics code: IR.SSU.REC.1402.333
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yavari M, Khalili Sadrabad E, Vakili M, Barzegar K, Fattahi Bafghi A. Investigation of Intestinal Parasites of Ready-to-Eat Fresh Raw Vegetables from Delis in the City of Yazd, Iran. J. Food Qual. Hazards Control 2025; 12 (4) :310-316
URL: http://jfqhc.ssu.ac.ir/article-1-1238-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1238-en.html
Department of Parasitology and Mycology, Infectious Diseases Research Center, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran , a.fattahi@ssu.ac.ir
Full-Text [PDF 852 kb]
(49 Downloads)
| Abstract (HTML) (77 Views)
Full-Text: (7 Views)
Investigation of Intestinal Parasites of Ready-to-Eat Fresh Raw Vegetables from Delis in the City of Yazd, Iran
M.R. Yavari 1, E. Khalili Sadrabad 2, M. Vakili 3, K. Barzegar 4, A. Fattahi Bafghi 5**
1. Department of Maaref, School of Medicine, Yazd Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2. Department of Health and Food Safety, School of Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3. Department of Community and Preventive Medicine, Health Monitoring Research Center, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4. PhD in TEFL and Faculty Member, Foreign Languages Department, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
5. Department of Parasitology and Mycology, Infectious Diseases Research Center, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
HIGHLIGHTS
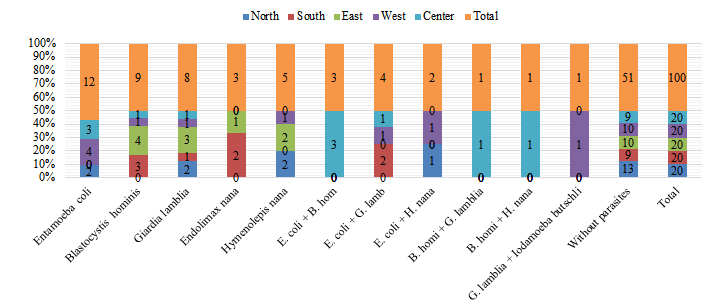
Figure 1: Incidence rate of enteric parasites in vegetables from delis in Yazd, by parasite species and sampling location
Pearson chi-square test, p=0.240
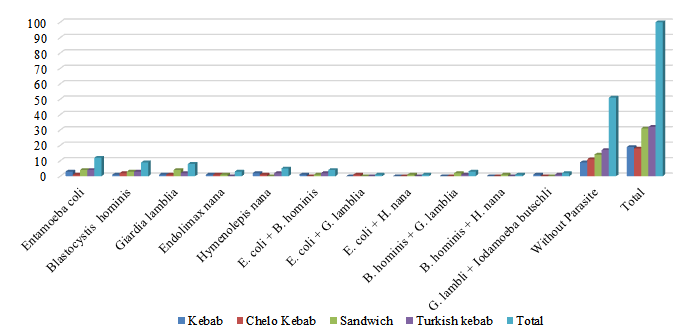
Figure 2: Prevalence of intestinal parasites (except coccidia) in vegetables from deli shops in Yazd, by establishment type
Pearson chi-square test, p=0.938
M.R. Yavari 1, E. Khalili Sadrabad 2, M. Vakili 3, K. Barzegar 4, A. Fattahi Bafghi 5**
1. Department of Maaref, School of Medicine, Yazd Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2. Department of Health and Food Safety, School of Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3. Department of Community and Preventive Medicine, Health Monitoring Research Center, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4. PhD in TEFL and Faculty Member, Foreign Languages Department, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
5. Department of Parasitology and Mycology, Infectious Diseases Research Center, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- The results showed that 51% of vegetables were free of intestinal parasitic contamination.
- Lettuce showed the highest incidence of contamination, with 32% of samples testing positive for intestinal parasites.
- White cabbage exhibited the lowest contamination level, with only 9% of samples affected.
| Article type Original article |
ABSTRACT Background: One of the common dietary practices in Iran is the consumption of raw vegetables, providing a significant range of vitamins and essential substances for the body. However, many human intestinal parasites are transmitted through water, soil, and vegetables including Cryptosporidium species. This study investigated the intestinal parasites of ready-to-eat fresh raw vegetables collected from Delis in Yazd, Iran, during 2023-2024. Methods: Following collection from food sales centers, the vegetables were transported to the laboratory. Samples were immersed in 1.5 L of a detergent solution containing 0.1% sodium dodecyl sulfate and 0.1% Tween 100 for 15 min under agitation. The samples were then centrifuged for 15 min at 1500 revolutions per min (rpm), after which the vegetable material was removed. Subsequently, 4% formaldehyde was added to the supernatant and centrifuged at 1500 revolutions per min (rpm) for 10 min. The obtained sediment was tested with Lugol dye and without Lugol under a microscope. Data analysis was performed using SPSS version 26. Results: The results indicated that lettuce was the most frequently sampled vegetable, comprising 32% of the total, while white cabbage had the lowest (9%). Furthermore, 51% of the vegetables were free from contamination with intestinal parasites, and 49% were contaminated. Based on Pearson's chi-square test, the average level of intestinal parasite infection was not significantly different among the vegetable types (p=0.382). Conclusion: Given the year-round consumption of vegetables, it is essential to implement strict hygienic controls and provide necessary public training on proper methods for consumption, cultivation, and harvesting. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Vegetables Intestinal Diseases Parasites Iran |
||
| Article history Received: 19 Jun 2024 Revised: 30 Nov 2025 Accepted: 02 Jul 2024 |
||
| Abbreviations RTE=Ready-To-Eat |
To cite: Yavari M.R., Khalili Sadrabad E., Vakili M., Barzegar K., Fattahi Bafghi A. (2025). Investigation of intestinal parasites of ready-to-eat fresh raw vegetables from delis in the city of Yazd, Iran. Journal of Food Quality and Hazards Control. 12: 310-316.
Introduction
Introduction
In recent years, concerns about the production of hygienic and safe food for the population have increased. Food-borne diseases are a major cause of mortality, particularly among immune compromised individuals, infants, and the elderly (Tosin et al., 2017). The food-borne parasites infect approximately 3.5 billion people worldwide with 200,000 deaths annually (Asfaw et al., 2023). The outbreak of food-borne parasitic pathogens is a serious health problem in developing countries. The intestinal parasites could contaminate the water and food consumed by human beings (Faria et al., 2023). The poor sanitation service as well as the improper agricultural process would predispose to pollution of fruits and vegetables (Amoah et al., 2023). Vegetables with high content of minerals, fibers, and vitamins (A, C, and K) could be considered as a healthy source of nutrition in human diets (Al-Sanabani et al., 2016; Said, 2012). As World Health Organization (WHO) reports, the consumption of 400 g vegetables each day is essential to prevent the chronic diseases (Bahramian et al., 2021). As mentioned, vegetables parasitic contamination could occur during growth in contaminated soils, i.e., by contaminated water, human or animal feces, improper washing, or handling process (Bahramian et al., 2021; Faria et al., 2023). The ingestion of fresh and raw vegetables could facilitate the transfer of different infectious parasitic conditions to human body (Al-Sanabani et al., 2016; Said, 2012). Therefore, there is a probable risk for ingestion of infectious cysts, Helminthes eggs, oocytes, and larvae during uncooked vegetable consumption (Bahramian et al., 2021). The parasitic infections with high incidence by vegetable consumption are reported to be Entamoeba, Giardia, Ascaris, Fasciola, Taenia, and Toxocara (Bahramian et al., 2021). Several complications including malnutrition, nutritional deficiency, anemia, growth retardation, immune system defect, physical and mental problems, and diarrhea have been observed following the intestinal parasitic contamination (Asfaw et al., 2023; Bahramian et al., 2021). The variety of intestinal parasitic infections are related to geographical condition such as environmental conditions (weather), sociocultural conditions, sampling season, adaption to certain definitive and mediating hosts (Asfaw et al., 2023; Tosin et al., 2017; Yavari et al., 2019). In Iran, the ingestion of raw and fresh vegetables along with meals becomes a regular habit (Bahramian et al., 2021). Furthermore, in recent years changes in life style and consumption of Ready-To-Eat Foods (RTE) as along with wanderer food vendors has increased the danger of parasite-induced food-borne contamination (Said, 2012). Therefore, in the present work, we examined the parasitic infection of commonly consumed vegetables in delis (Sandwich, kebab, chelo kebab, and Turkish kebab shops) from Yazd, Iran.
Materials and methods
Sample collection
The present cross-sectional research was carried out in 2023 for 10 months (from May to February). In this study, sampling was carried out in three stages. First, Yazd city was divided into 5 regions including North, South, East, West and Center of Yazd and classified. Then, 10 food stores were selected from each cluster sampling table and from each cluster by simple random sampling. Finally, 20 samples of RTE vegetables were collected from each region, and a total of 100 samples were selected. The vegetables available in delis in Yazd included: lettuce, white cabbage, red cabbage, basil, parsley, and chives.
Intestinal parasitic detection
In the first step, each sample was immersed in 1.5 L of water that containing 0.1% sodium dodecyl sulphate and 0.1% Tween 100 for 15 min under agitation. The samples were then centrifuged at 1500 revolutions per min (rpm) for 15 min, and the vegetables were taken out. Subsequently, 4% formaldehyde was added to the supernatant, and the solution was centrifuged at 1500 revolutions per min (rpm) for 10 min (Abougrain et al., 2010; Bafghi et al., 2020; Bier, 1991; Yavari et al., 2019). The resulting precipitate was examined under a microscope with and without Lugol's stain.
Statistical analysis
Data were analyzed using a Pearson Chi-square test in SPSS version 26, with a statistical significance level set at p<0.05.
Results and discussion
Based on our findings, lettuce was the most frequent vegetable with 32 items (32%), followed by basil with 21 items (21%), parsley with 16 items (16%), chives and parsley each with 11 items (11%), and the least frequent vegetable was green cabbage with 9 (9%) items (Table 1). Based on the results, there was insignificant difference in infection rate of intestinal parasites of vegetables (p=0.382).
Table 1: Incidence of intestinal parasites in vegetables of delis in Yazd in terms of type of vegetables
Materials and methods
Sample collection
The present cross-sectional research was carried out in 2023 for 10 months (from May to February). In this study, sampling was carried out in three stages. First, Yazd city was divided into 5 regions including North, South, East, West and Center of Yazd and classified. Then, 10 food stores were selected from each cluster sampling table and from each cluster by simple random sampling. Finally, 20 samples of RTE vegetables were collected from each region, and a total of 100 samples were selected. The vegetables available in delis in Yazd included: lettuce, white cabbage, red cabbage, basil, parsley, and chives.
Intestinal parasitic detection
In the first step, each sample was immersed in 1.5 L of water that containing 0.1% sodium dodecyl sulphate and 0.1% Tween 100 for 15 min under agitation. The samples were then centrifuged at 1500 revolutions per min (rpm) for 15 min, and the vegetables were taken out. Subsequently, 4% formaldehyde was added to the supernatant, and the solution was centrifuged at 1500 revolutions per min (rpm) for 10 min (Abougrain et al., 2010; Bafghi et al., 2020; Bier, 1991; Yavari et al., 2019). The resulting precipitate was examined under a microscope with and without Lugol's stain.
Statistical analysis
Data were analyzed using a Pearson Chi-square test in SPSS version 26, with a statistical significance level set at p<0.05.
Results and discussion
Based on our findings, lettuce was the most frequent vegetable with 32 items (32%), followed by basil with 21 items (21%), parsley with 16 items (16%), chives and parsley each with 11 items (11%), and the least frequent vegetable was green cabbage with 9 (9%) items (Table 1). Based on the results, there was insignificant difference in infection rate of intestinal parasites of vegetables (p=0.382).
Table 1: Incidence of intestinal parasites in vegetables of delis in Yazd in terms of type of vegetables
| Type of vegetable | Contaminated Number |
Uncontaminated Number |
Total Number |
| Lettuce | 14 | 18 | 32 |
| White cabbage | 5 | 4 | 9 |
| Red cabbage | 7 | 4 | 11 |
| Basil | 8 | 13 | 21 |
| Parsley | 8 | 8 | 16 |
| Chives | 7 | 4 | 11 |
| Total | 49 | 51 | 100 |
Pearson chi-square test, p=0.382
Out of all 100 vegetable samples, 49 vegetables (49%) were contaminated with intestinal parasites, except coccidia, and 51 vegetables (51%) were uncontaminated (Table 1). In lettuce, out of a total of 32 samples, 14 vegetables (43.8%) were infected with intestinal stigmas and 18 vegetables (56.2%) were free of infection. Furthermore, the most frequent infection were related to Entamoeba coli with 4 samples (12.5%), followed by Blastocystis hominis with 3 samples (9.6%), and Giardia lamblia with 2 samples (6.2). The least frequent infection was related to double infections (E. coli + B. hominis), (E. coli + G. lamblia), and (B. hominis + G. lamblia) with one sample (3.1%). For white cabbage, out of a total of 9 samples, 5 vegetables (45.6%) were infected and 4 (44.4%) were not. In green cabbage samples, only infections with E. coli, Endolimax nana, Hymenolepis nana, double infections of (E. coli + B. hominis), (E. coli + G. lamblia), and (B. hominis + G. lamblia) were detected, each with a rate of 11.1 %. Out of a total of 9 white cabbage samples, 5 (55.6%) were contaminated, while 4 (44.6%) were not. In red cabbage vegetables, out of a total of 11 samples, 7 (63.6%) were infected with intestinal parasites and 4 (36.4%) were free of infection. Among the samples of green cabbage, only two types of parasites of E. coli had the highest rate of infection with 3 samples (27.3%), whereas G. lamblia with one (9.1%) had the lowest rate. Regarding basil vegetables, out of a total of 21 samples, 8 (38.1%) were infected and 13 (61.9%) were not. In addition, the greatest incidence of infection pertained to E. coli with 3 samples (14.3%), followed by the double infection (E. coli + G. Lamblia) with 2 (9.6%). The lowest amount of contamination pertained to G. Lamblia, E. Nana, and the double infection (G. Lamblia + Iodamoeba butschli), each with one sample (4.8%). As for Parsley, out of a total of 16 samples, 8 (50%) were infected and 8 (50%) were not. The most frequent infection was due to intestinal parasites related to B. hominis, G. lamblia, and H. nana, each with 2 samples (12.5%), whereas the lowest infection was due to double intestinal parasites (E. coli + B. hominis) and (B. hominis + G. lamblia), each with one case (6.2%). out of 11 chives samples, 7 (65.6%) were contaminated and 4 (36.4%) were not. the most frequent infection were G. lamblia (18.2%), while the least frequent was related to E. coli, B. hominis, H. nana, and double infections of (E. coli + H. nana) and (E. coli + G. lamblia) and (B. hominis + G. lamblia) (9.1%). According to results presented in Table 1, the infection with intestinal parasites (except coccidia) did not suggest any significant difference in any of the vegetables (p=0.382).
The prevalence of enteric parasites (except coccidia) in vegetables from delis in Yazd
Out of 100 vegetable samples, 20 (20%) were obtained in the North, 20 (20%) in the south, 20 (20%) in the East, 20 (20%) in the West, and 20 (20%) in the Center of Yazd city (Figure 2). In the northern region of Yazd, out of a total of 20 samples, 7 (35%) showed to be contaminated with intestinal parasites except intestinal coccidia, and 13 (65%) were without infection. The greatest incidence of infection belonged to E. coli, G. lamblia, and H. nana (10%), and the lowest rate of infection was related to the double infection (E. coli + H. nana) with one case (5%). According to the results presented in Figure 1, the rate of infection with intestinal parasites (except for coccidia) was not significantly different in all vegetables (p=0.240).
Out of all 100 vegetable samples, 49 vegetables (49%) were contaminated with intestinal parasites, except coccidia, and 51 vegetables (51%) were uncontaminated (Table 1). In lettuce, out of a total of 32 samples, 14 vegetables (43.8%) were infected with intestinal stigmas and 18 vegetables (56.2%) were free of infection. Furthermore, the most frequent infection were related to Entamoeba coli with 4 samples (12.5%), followed by Blastocystis hominis with 3 samples (9.6%), and Giardia lamblia with 2 samples (6.2). The least frequent infection was related to double infections (E. coli + B. hominis), (E. coli + G. lamblia), and (B. hominis + G. lamblia) with one sample (3.1%). For white cabbage, out of a total of 9 samples, 5 vegetables (45.6%) were infected and 4 (44.4%) were not. In green cabbage samples, only infections with E. coli, Endolimax nana, Hymenolepis nana, double infections of (E. coli + B. hominis), (E. coli + G. lamblia), and (B. hominis + G. lamblia) were detected, each with a rate of 11.1 %. Out of a total of 9 white cabbage samples, 5 (55.6%) were contaminated, while 4 (44.6%) were not. In red cabbage vegetables, out of a total of 11 samples, 7 (63.6%) were infected with intestinal parasites and 4 (36.4%) were free of infection. Among the samples of green cabbage, only two types of parasites of E. coli had the highest rate of infection with 3 samples (27.3%), whereas G. lamblia with one (9.1%) had the lowest rate. Regarding basil vegetables, out of a total of 21 samples, 8 (38.1%) were infected and 13 (61.9%) were not. In addition, the greatest incidence of infection pertained to E. coli with 3 samples (14.3%), followed by the double infection (E. coli + G. Lamblia) with 2 (9.6%). The lowest amount of contamination pertained to G. Lamblia, E. Nana, and the double infection (G. Lamblia + Iodamoeba butschli), each with one sample (4.8%). As for Parsley, out of a total of 16 samples, 8 (50%) were infected and 8 (50%) were not. The most frequent infection was due to intestinal parasites related to B. hominis, G. lamblia, and H. nana, each with 2 samples (12.5%), whereas the lowest infection was due to double intestinal parasites (E. coli + B. hominis) and (B. hominis + G. lamblia), each with one case (6.2%). out of 11 chives samples, 7 (65.6%) were contaminated and 4 (36.4%) were not. the most frequent infection were G. lamblia (18.2%), while the least frequent was related to E. coli, B. hominis, H. nana, and double infections of (E. coli + H. nana) and (E. coli + G. lamblia) and (B. hominis + G. lamblia) (9.1%). According to results presented in Table 1, the infection with intestinal parasites (except coccidia) did not suggest any significant difference in any of the vegetables (p=0.382).
The prevalence of enteric parasites (except coccidia) in vegetables from delis in Yazd
Out of 100 vegetable samples, 20 (20%) were obtained in the North, 20 (20%) in the south, 20 (20%) in the East, 20 (20%) in the West, and 20 (20%) in the Center of Yazd city (Figure 2). In the northern region of Yazd, out of a total of 20 samples, 7 (35%) showed to be contaminated with intestinal parasites except intestinal coccidia, and 13 (65%) were without infection. The greatest incidence of infection belonged to E. coli, G. lamblia, and H. nana (10%), and the lowest rate of infection was related to the double infection (E. coli + H. nana) with one case (5%). According to the results presented in Figure 1, the rate of infection with intestinal parasites (except for coccidia) was not significantly different in all vegetables (p=0.240).

Figure 1: Incidence rate of enteric parasites in vegetables from delis in Yazd, by parasite species and sampling location
Pearson chi-square test, p=0.240
Frequency of intestinal parasites (except coccidia) in vegetables from delis in Yazd, by establishment type
Out of 100 vegetable samples taken from delis in Yazd, 19 (19%) were taken from kebab shops, 18 (18%) from Chelo kebab shops, 31 (31%) from sandwich bars, and 32 (32%) from Turkish kebab shops. In kebab shops, out of 19 samples, 10 (52.6%) were infected with intestinal parasites (except coccidia), and 9 (47.4%) were not. The highest infection rate pertained to B. hominis (11.1%), and the lowest infection rate belonged to E. coli, G. lamblia, E. nana, H. nana, and the double infection (E. coli + G. lamblia) with one case (5.6%). In Chelo kebab shops, out of 19 samples, 7 (38.9%) were infected, and 11 (61.1%) were not. The most frequent infection was related to E. coli (15.8%), followed by H. nana (10.5%) and the least frequent infection related to B. hominis, G. lamblia, E. Nana, and double infections of (E. bacilli + B. hominis) and (G. lamblia + Iodamoeba butschli), each with one case (5.3%). In sandwich bars, out of 31 samples, 16 (46.8%) were contaminated, and 14 (45.2%) were not. The highest infection rate was related to E. coli and G. lamblia with 4 cases (12.9%), followed by B. hominis with 3 (9.7%), and lastly, the double infection (B. hominis + G. lamblia) with 2 (6.4%). The lowest infection rate related to E. Nana and double infections of (E. coli + B. hominis), (B. hominis + G. lamblia), and (E. coli + H. nana), each with one case (5.3%). In the Turkish kebab shops, out of 32 samples, 15 (46.9%) were infected, while 17 (53.1%) were not. The highest infection rate pertained to E. coli with 4 cases (59%), followed by B. hominis with 3 (9.4%), and G. Lamblia, H. nana, and the double infection (B. hominis + G. lamblia), each with 2 cases (6.4%). The lowest rate of contamination belonged to E. nana and the double infection (E. coli + B. hominis) with 2 cases (6.2%). Furthermore, the lowest contamination rate was attributed to double infections (B. hominis + G. lamblia) and (G. lamblia + Iodamoeba butschli), each with one case (3.1%). Based on the results, the infection rate of intestinal parasites, except coccidia, did not show any significant difference among the vegetables (P=0.938). The observation of two or more parasite species indicated a high level of contamination in washed, RTE vegetables. Although at least one of these parasite species is non-pathogenic and all are protozoa, it is advisable to either avoid consuming these vegetables raw or to prevent contamination during planting, growth, and harvesting (Figure 2).
Out of 100 vegetable samples taken from delis in Yazd, 19 (19%) were taken from kebab shops, 18 (18%) from Chelo kebab shops, 31 (31%) from sandwich bars, and 32 (32%) from Turkish kebab shops. In kebab shops, out of 19 samples, 10 (52.6%) were infected with intestinal parasites (except coccidia), and 9 (47.4%) were not. The highest infection rate pertained to B. hominis (11.1%), and the lowest infection rate belonged to E. coli, G. lamblia, E. nana, H. nana, and the double infection (E. coli + G. lamblia) with one case (5.6%). In Chelo kebab shops, out of 19 samples, 7 (38.9%) were infected, and 11 (61.1%) were not. The most frequent infection was related to E. coli (15.8%), followed by H. nana (10.5%) and the least frequent infection related to B. hominis, G. lamblia, E. Nana, and double infections of (E. bacilli + B. hominis) and (G. lamblia + Iodamoeba butschli), each with one case (5.3%). In sandwich bars, out of 31 samples, 16 (46.8%) were contaminated, and 14 (45.2%) were not. The highest infection rate was related to E. coli and G. lamblia with 4 cases (12.9%), followed by B. hominis with 3 (9.7%), and lastly, the double infection (B. hominis + G. lamblia) with 2 (6.4%). The lowest infection rate related to E. Nana and double infections of (E. coli + B. hominis), (B. hominis + G. lamblia), and (E. coli + H. nana), each with one case (5.3%). In the Turkish kebab shops, out of 32 samples, 15 (46.9%) were infected, while 17 (53.1%) were not. The highest infection rate pertained to E. coli with 4 cases (59%), followed by B. hominis with 3 (9.4%), and G. Lamblia, H. nana, and the double infection (B. hominis + G. lamblia), each with 2 cases (6.4%). The lowest rate of contamination belonged to E. nana and the double infection (E. coli + B. hominis) with 2 cases (6.2%). Furthermore, the lowest contamination rate was attributed to double infections (B. hominis + G. lamblia) and (G. lamblia + Iodamoeba butschli), each with one case (3.1%). Based on the results, the infection rate of intestinal parasites, except coccidia, did not show any significant difference among the vegetables (P=0.938). The observation of two or more parasite species indicated a high level of contamination in washed, RTE vegetables. Although at least one of these parasite species is non-pathogenic and all are protozoa, it is advisable to either avoid consuming these vegetables raw or to prevent contamination during planting, growth, and harvesting (Figure 2).

Figure 2: Prevalence of intestinal parasites (except coccidia) in vegetables from deli shops in Yazd, by establishment type
Pearson chi-square test, p=0.938
RTE vegetables or salads have been introduced as an important route in human diet to transmit intestinal parasites. The parasitic contamination can occur during planting, harvesting, and processing in restaurants through cross-contamination (Said, 2012). People in developing countries are more prone to parasitic infections (Khan et al., 2021). Uncooked fresh vegetables are an important component of dishes in Yazd, Iran. Given that farmers' access to clean water, as opposed to wastewater, is a significant challenge for irrigation in cities like Yazd with limited water sources, identifying parasitic sources and their presence in vegetables is essential for prevention (Ezatpour et al., 2013).
According to the results, 49% of vegetable samples were contaminated, with lettuce showing the highest rate of contamination (14%) and white cabbage the lowest (5%). The pattern of parasitic contamination of samples was shown as lettuce > parsley = basil > chives = red cabbage > white cabbage. The infection rate of the present work was higher than studies in Egypt (31.7%) (Said, 2012), Gezira state in Sudan (27.7%) (Alnor and Younis, 2020), Lower Dir and Peshawar districts in Pakistan (19.7%) (Khan et al., 2021), Accra metropolis of Ghana (32%) (Amissah-Reynolds et al., 2019), Ashanti region of Ghana (22.7%) (Amoah et al., 2023), Northeast of Addis Ababa (41.7%) (Asfaw et al., 2023), UAE (15.1%) (El Bakri et al., 2020), and Tehran, Iran (8.5%) (Nouroozi, 2015). Nonetheless, the parasitic contamination rates in Caborca region of Northwest Mexico (45%) (Morales-Figueroa et al., 2021), Tabriz (76%) (Garedaghi et al., 2011), south-western Nigeria (69.7%) (Tosin et al., 2017), Khorramabad (52.8% in spring), and Asante-Mampong Municipal of Ashanti Region, Ghana (48.7%) (Amissah-Reynolds et al., 2020) were estimated higher than those in the current study. These differences in intestinal parasitic infections are related to geographical condition and environmental situations (Amoah et al., 2023; Asfaw et al., 2023). As could be seen, the lettuce showed the highest parasitic contamination rate. The higher incidence of parasites in lettuce could be related to its rough surface and open shoot architecture, which facilitates parasite attachment (Marček et al., 2018; Morales-Figueroa et al., 2021). Furthermore, the soft, tender structure of lettuce makes it more susceptible to damage during washing and enhances cyst adherence compared to cabbage. (Marček et al., 2018). The surface of leafy vegetables (lettuce and parsley) is in the direct contact of soil, increasing the odds of parasitic infection (Bahramian et al., 2021). Moreover, the higher contamination rate of lettuce could be related to higher irrigation process to keep plant fresh (Marček et al., 2018). These results are consistent with those of Morales-Figueroa et al. (2021), El Bakri et al. (2020), Mohemeed et al. (2021), Marček et al. (2018), Mufida et al. (2022), and Rodrigues et al. (2020). The lower infection rate of cabbage could be related to the closed-shoot structure of cabbage, which has limited the parasitic infections during improper transportation and handling (Marček et al., 2018). Furthermore, the smooth surface of chives makes the attachment of parasites harder and slipping easier during washing (Asfaw et al., 2023). In addition, the outer leaves of cabbage, which may be contaminated by parasites, are usually peeled off after harvest (Amissah-Reynolds et al., 2020). Hence, proper vegetable washing could reduce the risk of food-borne parasitic infections (Amoah et al., 2023). In a research by Ezatpour et al. (2013) leek (80%) followed by garden cress (54.5 %) were the highest contaminated vegetables. The highest and least contaminated samples in a study by Said (2012) were rocket or arugula (46.7%) and green onion (13.3 %), respectively.
In the current study, the parasites observed in raw legumes were E. coli (12%), B. hominis (9%), G. lamblia (8%), H. nana (5%), and E. nana (3%). In the study by El Bakri et al. (2020), the most prevalent parasites were reported as Entamoeba complex (30.3%), Strongyloides (12.1%), Trichuris trichiura (12.1%), Ascaris lumbricoides eggs (9.1%), Enterobius vermicularis egg (6.1%), E. nana cyst (6.1%), H. nana (3%), and G. lamblia (3%). According to the results, the most frequent parasitic contamination in all samples except for chives and white cabbage belonged to E. coli. The presence of E. coli in vegetables is due to human fecal contamination of waste waters or organic fertilizers used for vegetable irrigation (Gharavi et al., 2002). Moreover, the ability to survive in cold or humid storage condition would increase the incidence rate (Bahramian et al., 2021). The existence of pseudopods for motility as well as Trophozoite and cyst stages in life cycle resulted in its higher prevalence (Agbalaka et al., 2018). B. hominis, a fecal parasite with good resistance to environmental condition and disinfectants, was the second most frequent parasites in the current study, which is consistent with the results of Barua et al. (2023). In the current research, G. lamblia incidence ranged from 0 to 18.2% in white cabbage and chives, respectively. The global prevalence of 5 to 20% were observed for G. lamblia (Bahramian et al., 2021). The most prevalent parasitic pathogens in vegetables of Iraq was G. lamblia (21.9%) followed by E. histolytica (21.4%) (Mohemeed et al., 2021). G. lamblia cyst was the most prevalent parasites in studies by Alnor and Younis (2020) (48% of total parasites), Amissah-Reynolds et al. (2019), and Gharavi et al. (2002) (18.6%), which was attributed to the poor sanitary condition and probable surface or water contamination by fecal sources (Amissah-Reynolds et al., 2019; Bahramian et al., 2021) as well as higher sensivity of iodine wet mount method to detect them (Asfaw et al., 2023). Furthermore, G. cysts are resistant to hard environmental situations including acidic condition of stomach, chlorinated or UV treated water, and cold storage conditions (Agbalaka et al., 2018). The A. lumbricoides eggs (12.3%) in a study by Khan et al. (2021), E. histolytica (26.9%) as reported by Al-Sanabani et al. (2016), and Cystoisospora belli (38%) by Barua et al. (2023), were the most frequent parasites. According to a study by Morales-Figueroa (2021), the most frequent parasites were Cryptosporidium spp. (11.7%), Cyclospora spp., (11.0%), and B. hominis (9.2%). Hymenolepis is a common cestodes infection that belongs to Helminthes spp., generally found in tropical or subtropical countries (Morales-Figueroa et al., 2021); therefore, compatible with the climate of Yazd, Iran. The results of the current study, reported 5% prevalence of H. nana in vegetables, which was lower than that in Barua et al. (2023) (6%), and Bekele and Shumbej (2019) (11.9%) and higher than that in the study by Lawal et al. (2015) (3.05 %). The simultaneous presence of E. nana and E. coli confirms the vegetables contamination with human waste (El Bakri et al., 2020).
The isolation of multiple parasite species from vegetable samples suggests a high likelihood of fecal contamination (Tosin et al., 2017). Based on our findings, the incidence of multiple parasitic contaminations of E. coli- B. hominis and B. hominis- G. lamblia were higher than other parasites. The multiple parasitic contamination of E. coli- B. hominis was highest in chives (9.6%), followed by lettuce (9.1%), and basil (3.1%). The multiple parasitic contaminations of E. coli- G. lamblia and E. coli- H. nana were only reported in lettuce (3.1%) and parsley (6.2%) samples, respectively. Furthermore, the highest incidence of multiple parasitic contaminations of B. hominis -G. lamblia and B. hominis- H. nana were shown in white cabbage. According to a study by El Bakri (2020), the multiple parasitic contamination were reported in all of vegetable samples except for chard and tomato.
In the present study, the samples gathered from North of Yazd had lower contamination rate of E. coli, B. hominis, and E. nana. The vegetable samples gathered from East of Yazd showed higher contamination rate with B. hominis, G. lamblia, and H. nana. E. nana was only detected in samples gathered from the South of Yazd following by East samples. The prevalence of multiple parasitic contaminations of E. coli- B. hominis, B. hominis, G. lamblia, B. hominis, and H. nana was only detected in samples gathered from Central part of Yazd. E. coli- H. nana was found in samples gathered from North and West of Yazd. Such contaminations could be attributed to improper storage procedures, inadequate packaging, as well as the poor hygienic conditions of agricultural farms where the samples were prepared (Rodrigues et al., 2020). Furthermore, the agricultural practices of different locations and the environmental conditions could affect the type of parasitic contamination (Amoah et al., 2023).
Regarding sandwich bars, the highest prevalence was reported by E. coli and G. lamblia (12.9%) followed by multiple parasitic contaminations of B. hominis, and G. lamblia. The differences in the type of parasitic prevalence in sandwich bars are related to type of used vegetables, improper handling, presence of dust, low quality of raw materials, cross-contamination, and storage conditions (Morales-Figueroa et al., 2021).
Generally, the difference in parasitic contamination could be attributed to poor hygienic procedure, improper handling and transportation, geographical situation, intestinal parasites detection methods, sampling size, sampling season, the type of water used in irrigation, type of vegetables, and contamination of soil with human or animal feces (Al-Sanabani et al., 2016; Bahramian et al., 2021; Ezatpour et al., 2013; Morales-Figueroa et al., 2021).
Although common vegetable washing methods—using pure water, salt water, or vinegar water—are widely practiced, none are sufficient to ensure the safety and absence of contaminants.
Conclusion
The results of this study highlight the necessity of proper preparation, processing, and handling of vegetables in delicatessens to ensure their safety and reduce infections from both pathogenic and non-pathogenic enteric parasites. Given the high year-round consumption of vegetables in the community, it is essential to implement strict hygienic controls, alongside public education on safe consumption, cultivation, and harvesting practices.
Author contributions
A.F.B. and M.R.Y. collaborated on the study design, literature review, and data collection; A.F.B. and E.K.S. contributed to writing the manuscript; M.V. supervised the data analysis; K.B. prepared the English version of the manuscript. All authors read and approved the final version.
Acknowledgments
We appreciate Mrs. Modarres Sanavi M. and Mrs. Gholami. M., technicians of the Department of Parasitology and Mycology, Shahid Sadoughi Medical School, Yazd for their technical during this research.
Conflicts of interest
The authors declared that there is no conflict of interest.
Funding
This study project was bestowed a specific grant based on a thesis submitted (No. 1202) in the Infectious Diseases Research Center, School of Medicine, Yazd Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Ethical considerations
The experiments were confirmed by the Ethics Committee at Vice-Chancellor of Research, with ethics code: No: IR.SSU.REC.1402.333.
References
Abougrain A.K., Nahaisi M.H., Madi N.S., Saied M.M., Ghenghesh K.S. (2010). Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control. 21: 760-762. [DOI: 10.1016/j.foodcont.2009.11.005]
Agbalaka P., Obeta M., Daniel K. (2018). Food-Safety regarding intestinal parasites on edible fruits and vegetables. The Diagnostics. 1: 13-24.
Alnor A., Younis M.S. (2020). Prevalence of pathogenic intestinal parasites in common raw edible vegetables in gezira state, Sudan: A cross-sectional study. Prevalence. 4: 1-8.
Al-Sanabani A.-W., AbdAlgalil F.M., Radman B.A., Al-Manusori R.T. (2016). Prevalence of intestinal parasites in fresh leafy vegetables in some farms at Dhamar city, Yemen. Prevalence. 1.
Amissah-Reynolds P.K., Yar D.D., Aboagye V., Monney I., Nuamah F., Ndego E.A. (2019). Parasitic contamination in ready-to-eat salads in the Accra metropolis, Ghana. South Asian Journal of Parasitology. 3: 1-11. [DOI: 10.9734/ejnfs/2020/v12i930308]
Amissah-Reynolds P.K., Yar D.D., Gyamerah I., Apenteng O.Y., Sakyi S. (2020). Fresh vegetables and ready-to-eat salads: sources of parasitic zoonoses in mampong-Ashanti, Ghana. European Journal of Nutrition and Food Safety. 12: 47-55.
Amoah B.D., Effah-Yeboah E., Owusu-Asenso C.M., Aduhene E., Mensah A., Dzotefe G.B., Obeng B.C., Dumev C.Y., Arhin G.D., Asante C. (2023). Gastrointestinal parasite contamination of ready-to-eat vegetables sold in selected markets in Ashanti region, Ghana. South Asian Journal of Parasitology. 6: 161-171.
Asfaw T., Genetu D., Shenkute D., Shenkutie T.T., Amare Y.E., Yitayew B. (2023). Parasitic contamination and microbiological quality of commonly consumed fresh vegetables marketed in debreberhan town, Ethiopia. Environmental Health Insights. 17: 11786302231154755. [DOI: 10.1177/11786302231154755]
Bafghi A.F., Mirzaei F., Yavari M.R., Siyadatpanah A., Mitsuwan W., Nissapatorn V., De Lourdes Pereira M., Norouzi R., Hosseini S.A. (2020). Prevalence and risk factors associated with cryptosporidium infection in raw vegetables in Yazd District, Iran. World's Veterinary Journal. 10: 260-266. [DOI: 10.54203/scil.2020.wvj33]
Bahramian B., Afshari A., Kiani B., Sani M.A., Hashemi M. (2021). The prevalence of foodborne parasites in raw vegetables in Iran: a comprehensive systematic review and meta-analysis. Journal of Environmental Health Science and Engineering. 19: 2027-2045. [DOI: 10.1007/s40201-021-00748-0]
Barua P., Banik K.S., Saha S., Musa S. (2023). Parasitic contamination of street food samples from school-based food vendors of Dhaka city, Bangladesh. Bangladesh Journal of Zoology. 51: 217-229.
Bekele F., Shumbej T. (2019). Fruit and vegetable contamination with medically important helminths and protozoans in Tarcha town, Dawuro zone, South West Ethiopia. Research and Reports in Tropical Medicine. 10: 19-23. [DOI: 10.2147/RRTM.S205350]
Bier J.W. (1991).Isolation of parasites on fruits and vegetables. The Southeast Asian Journal of Tropical Medicine and Public Health. 22: 144-145.
El Bakri A., Hussein N.M., Ibrahim Z.A., Hasan H., AbuOdeh R. (2020). Intestinal parasite detection in assorted vegetables in the United Arab Emirates. Oman Medical Journal. 35: e128.
Ezatpour B., Chegeni A.S., Abdollahpour F., Aazami M., Alirezaei M. (2013). Prevalence of parasitic contamination of raw vegetables in Khorramabad, Iran. Food Control. 34: 92-95. [DOI: 10.1016/j.foodcont.2013.03.026]
Faria C.P., Pereira A., Almeida D., Pinto M., Lourenço Á., Do Céu Sousa M. (2023). Molecular investigation of ready-to-eat salads for Giardia duodenalis and Cryptosporidium spp. in Portugal. Food and Waterborne Parasitology. 30: e00190. [DOI: 10.1016/j.fawpar.2023.e00190]
Garedaghi Y., Farhang H.H., Pooryagoobi S. (2011). Parasitic contamination of fresh vegetables consumed in Tabriz, Iran. Researcher. 3: 76-79.
Gharavi M., Jahani M., Rokni M. (2002). Parasitic contamination of vegetables from farms and markets in Tehran. Iranian Journal of Public Health. 31: 83-86.
Khan W., Rafiq N., Nawaz M., Kabir M., Farooqi Z.U.R., Romman M., Parvez R., Alfarraj S., Noor A., Ujjan A. (2021). Parasitic contamination of fresh vegetables sold in open markets: a public health threat. Brazilian Journal of Biology. 82: e242614. [DOI: 10.1590/1519-6984.242614]
Lawal S., Wada Y., Ifraimu D. (2015). Parasitic contamination of commonly consumed fresh fruits and vegetables sold in open-air markets in Zaria Metropolis, Nigeria. Journal of Tropical Biosciences. 10: 68-75.
Marček T., Čorluka S., Gložinić M., Jažić E., Radman P., Sučić M., Ižaković M., Banjari I. (2018). A comparative survey on the prevalence of parasite elements in fresh vegetables and ready-to-eat salads. Hrana u Zdravlju i Bolesti: Znanstveno-StručniČasopiszaNutricionizam i Dijetetiku. 7: 26-30.
Mohemeed A.A., Alrawi Z.A.A., Mahdi N.K., Salih T.A. (2021). Immunological and molecular investigation of the level of parasite contamination of some vegetables sold in the local markets of Ramadi City–Iraq. Annals of the Romanian Society for Cell Biology. 25: 5964-5973.
Morales-Figueroa G., Sánchez-Guerrero M., Castro-García M., Esparza-Romero J., López-Mata M., Quihui-Cota L. (2021).Occurrence of intestinal parasites in fruits and vegetables from markets of Northwest Mexico. Journal of Food Quality and Hazards Control. 8: 81-88.
Mufida D.C., Armiyanti Y., Putri E.R.M., Agustina D., Suswati E., Shodikin M.A., Utami W.S., Hermansyah B., Raharjo A.M. (2022). Bacterial and parasitic contamination of raw vegetable: potential risk for food-borne diseases. International Journal of Public Health. 11: 1516-1524.
Nouroozi R.V. (2015). Detection of parasitic contamination in ready- to - eat fresh packaged herbs sold in Tehran, Iran. Journal of Community Health Research. 4: 99-104.
Rodrigues A.C., Da Silva M.D.C., Pereira R.Â.S., Pinto L.C. (2020). Prevalence of contamination by intestinal parasites in vegetables (Lactuca sativa L. and Coriandrum sativum L.) sold in markets in Belém, northern Brazil. Journal of the Science of Food and Agriculture. 100: 2859-2865. [DOI: 10.1002/jsfa.10282]
Said D.E.S. (2012). Detection of parasites in commonly consumed raw vegetables. Alexandria Journal of Medicine. 48: 345-352. [DOI: 10.1016/j.ajme.2012.05.005]
Tosin O.T., Ogunniyi T., Seun O.J. (2017). Human enteric parasitic pathogens in fresh fruits and vegetables consumed in ile-ife, osun state. Food Science and Quality Management. 67: 34-41.
Yavari M.R., Mirzaei F., Shahcheraghi S.H., Bafghi A.F. (2019). Parasitic contamination on fresh raw vegetables consumed in Yazd city, Iran, In during 2017–2018. Chinese Journal of Medical Research. 2: 70-73.
According to the results, 49% of vegetable samples were contaminated, with lettuce showing the highest rate of contamination (14%) and white cabbage the lowest (5%). The pattern of parasitic contamination of samples was shown as lettuce > parsley = basil > chives = red cabbage > white cabbage. The infection rate of the present work was higher than studies in Egypt (31.7%) (Said, 2012), Gezira state in Sudan (27.7%) (Alnor and Younis, 2020), Lower Dir and Peshawar districts in Pakistan (19.7%) (Khan et al., 2021), Accra metropolis of Ghana (32%) (Amissah-Reynolds et al., 2019), Ashanti region of Ghana (22.7%) (Amoah et al., 2023), Northeast of Addis Ababa (41.7%) (Asfaw et al., 2023), UAE (15.1%) (El Bakri et al., 2020), and Tehran, Iran (8.5%) (Nouroozi, 2015). Nonetheless, the parasitic contamination rates in Caborca region of Northwest Mexico (45%) (Morales-Figueroa et al., 2021), Tabriz (76%) (Garedaghi et al., 2011), south-western Nigeria (69.7%) (Tosin et al., 2017), Khorramabad (52.8% in spring), and Asante-Mampong Municipal of Ashanti Region, Ghana (48.7%) (Amissah-Reynolds et al., 2020) were estimated higher than those in the current study. These differences in intestinal parasitic infections are related to geographical condition and environmental situations (Amoah et al., 2023; Asfaw et al., 2023). As could be seen, the lettuce showed the highest parasitic contamination rate. The higher incidence of parasites in lettuce could be related to its rough surface and open shoot architecture, which facilitates parasite attachment (Marček et al., 2018; Morales-Figueroa et al., 2021). Furthermore, the soft, tender structure of lettuce makes it more susceptible to damage during washing and enhances cyst adherence compared to cabbage. (Marček et al., 2018). The surface of leafy vegetables (lettuce and parsley) is in the direct contact of soil, increasing the odds of parasitic infection (Bahramian et al., 2021). Moreover, the higher contamination rate of lettuce could be related to higher irrigation process to keep plant fresh (Marček et al., 2018). These results are consistent with those of Morales-Figueroa et al. (2021), El Bakri et al. (2020), Mohemeed et al. (2021), Marček et al. (2018), Mufida et al. (2022), and Rodrigues et al. (2020). The lower infection rate of cabbage could be related to the closed-shoot structure of cabbage, which has limited the parasitic infections during improper transportation and handling (Marček et al., 2018). Furthermore, the smooth surface of chives makes the attachment of parasites harder and slipping easier during washing (Asfaw et al., 2023). In addition, the outer leaves of cabbage, which may be contaminated by parasites, are usually peeled off after harvest (Amissah-Reynolds et al., 2020). Hence, proper vegetable washing could reduce the risk of food-borne parasitic infections (Amoah et al., 2023). In a research by Ezatpour et al. (2013) leek (80%) followed by garden cress (54.5 %) were the highest contaminated vegetables. The highest and least contaminated samples in a study by Said (2012) were rocket or arugula (46.7%) and green onion (13.3 %), respectively.
In the current study, the parasites observed in raw legumes were E. coli (12%), B. hominis (9%), G. lamblia (8%), H. nana (5%), and E. nana (3%). In the study by El Bakri et al. (2020), the most prevalent parasites were reported as Entamoeba complex (30.3%), Strongyloides (12.1%), Trichuris trichiura (12.1%), Ascaris lumbricoides eggs (9.1%), Enterobius vermicularis egg (6.1%), E. nana cyst (6.1%), H. nana (3%), and G. lamblia (3%). According to the results, the most frequent parasitic contamination in all samples except for chives and white cabbage belonged to E. coli. The presence of E. coli in vegetables is due to human fecal contamination of waste waters or organic fertilizers used for vegetable irrigation (Gharavi et al., 2002). Moreover, the ability to survive in cold or humid storage condition would increase the incidence rate (Bahramian et al., 2021). The existence of pseudopods for motility as well as Trophozoite and cyst stages in life cycle resulted in its higher prevalence (Agbalaka et al., 2018). B. hominis, a fecal parasite with good resistance to environmental condition and disinfectants, was the second most frequent parasites in the current study, which is consistent with the results of Barua et al. (2023). In the current research, G. lamblia incidence ranged from 0 to 18.2% in white cabbage and chives, respectively. The global prevalence of 5 to 20% were observed for G. lamblia (Bahramian et al., 2021). The most prevalent parasitic pathogens in vegetables of Iraq was G. lamblia (21.9%) followed by E. histolytica (21.4%) (Mohemeed et al., 2021). G. lamblia cyst was the most prevalent parasites in studies by Alnor and Younis (2020) (48% of total parasites), Amissah-Reynolds et al. (2019), and Gharavi et al. (2002) (18.6%), which was attributed to the poor sanitary condition and probable surface or water contamination by fecal sources (Amissah-Reynolds et al., 2019; Bahramian et al., 2021) as well as higher sensivity of iodine wet mount method to detect them (Asfaw et al., 2023). Furthermore, G. cysts are resistant to hard environmental situations including acidic condition of stomach, chlorinated or UV treated water, and cold storage conditions (Agbalaka et al., 2018). The A. lumbricoides eggs (12.3%) in a study by Khan et al. (2021), E. histolytica (26.9%) as reported by Al-Sanabani et al. (2016), and Cystoisospora belli (38%) by Barua et al. (2023), were the most frequent parasites. According to a study by Morales-Figueroa (2021), the most frequent parasites were Cryptosporidium spp. (11.7%), Cyclospora spp., (11.0%), and B. hominis (9.2%). Hymenolepis is a common cestodes infection that belongs to Helminthes spp., generally found in tropical or subtropical countries (Morales-Figueroa et al., 2021); therefore, compatible with the climate of Yazd, Iran. The results of the current study, reported 5% prevalence of H. nana in vegetables, which was lower than that in Barua et al. (2023) (6%), and Bekele and Shumbej (2019) (11.9%) and higher than that in the study by Lawal et al. (2015) (3.05 %). The simultaneous presence of E. nana and E. coli confirms the vegetables contamination with human waste (El Bakri et al., 2020).
The isolation of multiple parasite species from vegetable samples suggests a high likelihood of fecal contamination (Tosin et al., 2017). Based on our findings, the incidence of multiple parasitic contaminations of E. coli- B. hominis and B. hominis- G. lamblia were higher than other parasites. The multiple parasitic contamination of E. coli- B. hominis was highest in chives (9.6%), followed by lettuce (9.1%), and basil (3.1%). The multiple parasitic contaminations of E. coli- G. lamblia and E. coli- H. nana were only reported in lettuce (3.1%) and parsley (6.2%) samples, respectively. Furthermore, the highest incidence of multiple parasitic contaminations of B. hominis -G. lamblia and B. hominis- H. nana were shown in white cabbage. According to a study by El Bakri (2020), the multiple parasitic contamination were reported in all of vegetable samples except for chard and tomato.
In the present study, the samples gathered from North of Yazd had lower contamination rate of E. coli, B. hominis, and E. nana. The vegetable samples gathered from East of Yazd showed higher contamination rate with B. hominis, G. lamblia, and H. nana. E. nana was only detected in samples gathered from the South of Yazd following by East samples. The prevalence of multiple parasitic contaminations of E. coli- B. hominis, B. hominis, G. lamblia, B. hominis, and H. nana was only detected in samples gathered from Central part of Yazd. E. coli- H. nana was found in samples gathered from North and West of Yazd. Such contaminations could be attributed to improper storage procedures, inadequate packaging, as well as the poor hygienic conditions of agricultural farms where the samples were prepared (Rodrigues et al., 2020). Furthermore, the agricultural practices of different locations and the environmental conditions could affect the type of parasitic contamination (Amoah et al., 2023).
Regarding sandwich bars, the highest prevalence was reported by E. coli and G. lamblia (12.9%) followed by multiple parasitic contaminations of B. hominis, and G. lamblia. The differences in the type of parasitic prevalence in sandwich bars are related to type of used vegetables, improper handling, presence of dust, low quality of raw materials, cross-contamination, and storage conditions (Morales-Figueroa et al., 2021).
Generally, the difference in parasitic contamination could be attributed to poor hygienic procedure, improper handling and transportation, geographical situation, intestinal parasites detection methods, sampling size, sampling season, the type of water used in irrigation, type of vegetables, and contamination of soil with human or animal feces (Al-Sanabani et al., 2016; Bahramian et al., 2021; Ezatpour et al., 2013; Morales-Figueroa et al., 2021).
Although common vegetable washing methods—using pure water, salt water, or vinegar water—are widely practiced, none are sufficient to ensure the safety and absence of contaminants.
Conclusion
The results of this study highlight the necessity of proper preparation, processing, and handling of vegetables in delicatessens to ensure their safety and reduce infections from both pathogenic and non-pathogenic enteric parasites. Given the high year-round consumption of vegetables in the community, it is essential to implement strict hygienic controls, alongside public education on safe consumption, cultivation, and harvesting practices.
Author contributions
A.F.B. and M.R.Y. collaborated on the study design, literature review, and data collection; A.F.B. and E.K.S. contributed to writing the manuscript; M.V. supervised the data analysis; K.B. prepared the English version of the manuscript. All authors read and approved the final version.
Acknowledgments
We appreciate Mrs. Modarres Sanavi M. and Mrs. Gholami. M., technicians of the Department of Parasitology and Mycology, Shahid Sadoughi Medical School, Yazd for their technical during this research.
Conflicts of interest
The authors declared that there is no conflict of interest.
Funding
This study project was bestowed a specific grant based on a thesis submitted (No. 1202) in the Infectious Diseases Research Center, School of Medicine, Yazd Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Ethical considerations
The experiments were confirmed by the Ethics Committee at Vice-Chancellor of Research, with ethics code: No: IR.SSU.REC.1402.333.
References
Abougrain A.K., Nahaisi M.H., Madi N.S., Saied M.M., Ghenghesh K.S. (2010). Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control. 21: 760-762. [DOI: 10.1016/j.foodcont.2009.11.005]
Agbalaka P., Obeta M., Daniel K. (2018). Food-Safety regarding intestinal parasites on edible fruits and vegetables. The Diagnostics. 1: 13-24.
Alnor A., Younis M.S. (2020). Prevalence of pathogenic intestinal parasites in common raw edible vegetables in gezira state, Sudan: A cross-sectional study. Prevalence. 4: 1-8.
Al-Sanabani A.-W., AbdAlgalil F.M., Radman B.A., Al-Manusori R.T. (2016). Prevalence of intestinal parasites in fresh leafy vegetables in some farms at Dhamar city, Yemen. Prevalence. 1.
Amissah-Reynolds P.K., Yar D.D., Aboagye V., Monney I., Nuamah F., Ndego E.A. (2019). Parasitic contamination in ready-to-eat salads in the Accra metropolis, Ghana. South Asian Journal of Parasitology. 3: 1-11. [DOI: 10.9734/ejnfs/2020/v12i930308]
Amissah-Reynolds P.K., Yar D.D., Gyamerah I., Apenteng O.Y., Sakyi S. (2020). Fresh vegetables and ready-to-eat salads: sources of parasitic zoonoses in mampong-Ashanti, Ghana. European Journal of Nutrition and Food Safety. 12: 47-55.
Amoah B.D., Effah-Yeboah E., Owusu-Asenso C.M., Aduhene E., Mensah A., Dzotefe G.B., Obeng B.C., Dumev C.Y., Arhin G.D., Asante C. (2023). Gastrointestinal parasite contamination of ready-to-eat vegetables sold in selected markets in Ashanti region, Ghana. South Asian Journal of Parasitology. 6: 161-171.
Asfaw T., Genetu D., Shenkute D., Shenkutie T.T., Amare Y.E., Yitayew B. (2023). Parasitic contamination and microbiological quality of commonly consumed fresh vegetables marketed in debreberhan town, Ethiopia. Environmental Health Insights. 17: 11786302231154755. [DOI: 10.1177/11786302231154755]
Bafghi A.F., Mirzaei F., Yavari M.R., Siyadatpanah A., Mitsuwan W., Nissapatorn V., De Lourdes Pereira M., Norouzi R., Hosseini S.A. (2020). Prevalence and risk factors associated with cryptosporidium infection in raw vegetables in Yazd District, Iran. World's Veterinary Journal. 10: 260-266. [DOI: 10.54203/scil.2020.wvj33]
Bahramian B., Afshari A., Kiani B., Sani M.A., Hashemi M. (2021). The prevalence of foodborne parasites in raw vegetables in Iran: a comprehensive systematic review and meta-analysis. Journal of Environmental Health Science and Engineering. 19: 2027-2045. [DOI: 10.1007/s40201-021-00748-0]
Barua P., Banik K.S., Saha S., Musa S. (2023). Parasitic contamination of street food samples from school-based food vendors of Dhaka city, Bangladesh. Bangladesh Journal of Zoology. 51: 217-229.
Bekele F., Shumbej T. (2019). Fruit and vegetable contamination with medically important helminths and protozoans in Tarcha town, Dawuro zone, South West Ethiopia. Research and Reports in Tropical Medicine. 10: 19-23. [DOI: 10.2147/RRTM.S205350]
Bier J.W. (1991).Isolation of parasites on fruits and vegetables. The Southeast Asian Journal of Tropical Medicine and Public Health. 22: 144-145.
El Bakri A., Hussein N.M., Ibrahim Z.A., Hasan H., AbuOdeh R. (2020). Intestinal parasite detection in assorted vegetables in the United Arab Emirates. Oman Medical Journal. 35: e128.
Ezatpour B., Chegeni A.S., Abdollahpour F., Aazami M., Alirezaei M. (2013). Prevalence of parasitic contamination of raw vegetables in Khorramabad, Iran. Food Control. 34: 92-95. [DOI: 10.1016/j.foodcont.2013.03.026]
Faria C.P., Pereira A., Almeida D., Pinto M., Lourenço Á., Do Céu Sousa M. (2023). Molecular investigation of ready-to-eat salads for Giardia duodenalis and Cryptosporidium spp. in Portugal. Food and Waterborne Parasitology. 30: e00190. [DOI: 10.1016/j.fawpar.2023.e00190]
Garedaghi Y., Farhang H.H., Pooryagoobi S. (2011). Parasitic contamination of fresh vegetables consumed in Tabriz, Iran. Researcher. 3: 76-79.
Gharavi M., Jahani M., Rokni M. (2002). Parasitic contamination of vegetables from farms and markets in Tehran. Iranian Journal of Public Health. 31: 83-86.
Khan W., Rafiq N., Nawaz M., Kabir M., Farooqi Z.U.R., Romman M., Parvez R., Alfarraj S., Noor A., Ujjan A. (2021). Parasitic contamination of fresh vegetables sold in open markets: a public health threat. Brazilian Journal of Biology. 82: e242614. [DOI: 10.1590/1519-6984.242614]
Lawal S., Wada Y., Ifraimu D. (2015). Parasitic contamination of commonly consumed fresh fruits and vegetables sold in open-air markets in Zaria Metropolis, Nigeria. Journal of Tropical Biosciences. 10: 68-75.
Marček T., Čorluka S., Gložinić M., Jažić E., Radman P., Sučić M., Ižaković M., Banjari I. (2018). A comparative survey on the prevalence of parasite elements in fresh vegetables and ready-to-eat salads. Hrana u Zdravlju i Bolesti: Znanstveno-StručniČasopiszaNutricionizam i Dijetetiku. 7: 26-30.
Mohemeed A.A., Alrawi Z.A.A., Mahdi N.K., Salih T.A. (2021). Immunological and molecular investigation of the level of parasite contamination of some vegetables sold in the local markets of Ramadi City–Iraq. Annals of the Romanian Society for Cell Biology. 25: 5964-5973.
Morales-Figueroa G., Sánchez-Guerrero M., Castro-García M., Esparza-Romero J., López-Mata M., Quihui-Cota L. (2021).Occurrence of intestinal parasites in fruits and vegetables from markets of Northwest Mexico. Journal of Food Quality and Hazards Control. 8: 81-88.
Mufida D.C., Armiyanti Y., Putri E.R.M., Agustina D., Suswati E., Shodikin M.A., Utami W.S., Hermansyah B., Raharjo A.M. (2022). Bacterial and parasitic contamination of raw vegetable: potential risk for food-borne diseases. International Journal of Public Health. 11: 1516-1524.
Nouroozi R.V. (2015). Detection of parasitic contamination in ready- to - eat fresh packaged herbs sold in Tehran, Iran. Journal of Community Health Research. 4: 99-104.
Rodrigues A.C., Da Silva M.D.C., Pereira R.Â.S., Pinto L.C. (2020). Prevalence of contamination by intestinal parasites in vegetables (Lactuca sativa L. and Coriandrum sativum L.) sold in markets in Belém, northern Brazil. Journal of the Science of Food and Agriculture. 100: 2859-2865. [DOI: 10.1002/jsfa.10282]
Said D.E.S. (2012). Detection of parasites in commonly consumed raw vegetables. Alexandria Journal of Medicine. 48: 345-352. [DOI: 10.1016/j.ajme.2012.05.005]
Tosin O.T., Ogunniyi T., Seun O.J. (2017). Human enteric parasitic pathogens in fresh fruits and vegetables consumed in ile-ife, osun state. Food Science and Quality Management. 67: 34-41.
Yavari M.R., Mirzaei F., Shahcheraghi S.H., Bafghi A.F. (2019). Parasitic contamination on fresh raw vegetables consumed in Yazd city, Iran, In during 2017–2018. Chinese Journal of Medical Research. 2: 70-73.
[*] Corresponding author (A. Fattahi Bafghi)
Email: a.fattahi@ssu.ac.ir
Orchid ID: https://orcid.org/0000-0001-8469-2187
Email: a.fattahi@ssu.ac.ir
Orchid ID: https://orcid.org/0000-0001-8469-2187
Type of Study: Original article |
Subject:
Special
Received: 24/06/19 | Accepted: 25/12/30 | Published: 25/12/30
Received: 24/06/19 | Accepted: 25/12/30 | Published: 25/12/30
References
1. Abougrain A.K., Nahaisi M.H., Madi N.S., Saied M.M., Ghenghesh K.S. (2010). Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control. 21: 760-762. [DOI: 10.1016/j.foodcont.2009.11.005] [DOI:10.1016/j.foodcont.2009.11.005]
2. Agbalaka P., Obeta M., Daniel K. (2018). Food-Safety regarding intestinal parasites on edible fruits and vegetables. The Diagnostics. 1: 13-24.
3. Alnor A., Younis M.S. (2020). Prevalence of pathogenic intestinal parasites in common raw edible vegetables in gezira state, Sudan: A cross-sectional study. Prevalence. 4: 1-8.
4. Al-Sanabani A.-W., AbdAlgalil F.M., Radman B.A., Al-Manusori R.T. (2016). Prevalence of intestinal parasites in fresh leafy vegetables in some farms at Dhamar city, Yemen. Prevalence. 1.
5. Amissah-Reynolds P.K., Yar D.D., Aboagye V., Monney I., Nuamah F., Ndego E.A. (2019). Parasitic contamination in ready-to-eat salads in the Accra metropolis, Ghana. South Asian Journal of Parasitology. 3: 1-11. [DOI: 10.9734/ejnfs/2020/v12i930308]
6. Amissah-Reynolds P.K., Yar D.D., Gyamerah I., Apenteng O.Y., Sakyi S. (2020). Fresh vegetables and ready-to-eat salads: sources of parasitic zoonoses in mampong-Ashanti, Ghana. European Journal of Nutrition and Food Safety. 12: 47-55. [DOI:10.9734/ejnfs/2020/v12i230192]
7. Amoah B.D., Effah-Yeboah E., Owusu-Asenso C.M., Aduhene E., Mensah A., Dzotefe G.B., Obeng B.C., Dumev C.Y., Arhin G.D., Asante C. (2023). Gastrointestinal parasite contamination of ready-to-eat vegetables sold in selected markets in Ashanti region, Ghana. South Asian Journal of Parasitology. 6: 161-171.
8. Asfaw T., Genetu D., Shenkute D., Shenkutie T.T., Amare Y.E., Yitayew B. (2023). Parasitic contamination and microbiological quality of commonly consumed fresh vegetables marketed in debreberhan town, Ethiopia. Environmental Health Insights. 17: 11786302231154755. [DOI: 10.1177/11786302231154755] [DOI:10.1177/11786302231154755] [PMID] [PMCID]
9. Bafghi A.F., Mirzaei F., Yavari M.R., Siyadatpanah A., Mitsuwan W., Nissapatorn V., De Lourdes Pereira M., Norouzi R., Hosseini S.A. (2020). Prevalence and risk factors associated with cryptosporidium infection in raw vegetables in Yazd District, Iran. World's Veterinary Journal. 10: 260-266. [DOI: 10.54203/scil.2020.wvj33] [DOI:10.36380/scil.2020.wvj34]
10. Bahramian B., Afshari A., Kiani B., Sani M.A., Hashemi M. (2021). The prevalence of foodborne parasites in raw vegetables in Iran: a comprehensive systematic review and meta-analysis. Journal of Environmental Health Science and Engineering. 19: 2027-2045. [DOI: 10.1007/s40201-021-00748-0] [DOI:10.1007/s40201-021-00748-0] [PMID] [PMCID]
11. Barua P., Banik K.S., Saha S., Musa S. (2023). Parasitic contamination of street food samples from school-based food vendors of Dhaka city, Bangladesh. Bangladesh Journal of Zoology. 51: 217-229. [DOI:10.3329/bjz.v51i2.70781]
12. Bekele F., Shumbej T. (2019). Fruit and vegetable contamination with medically important helminths and protozoans in Tarcha town, Dawuro zone, South West Ethiopia. Research and Reports in Tropical Medicine. 10: 19-23. [DOI: 10.2147/RRTM.S205350] [DOI:10.2147/RRTM.S205250] [PMID] [PMCID]
13. Bier J.W. (1991).Isolation of parasites on fruits and vegetables. The Southeast Asian Journal of Tropical Medicine and Public Health. 22: 144-145.
14. El Bakri A., Hussein N.M., Ibrahim Z.A., Hasan H., AbuOdeh R. (2020). Intestinal parasite detection in assorted vegetables in the United Arab Emirates. Oman Medical Journal. 35: e128. [DOI:10.5001/omj.2020.46] [PMID] [PMCID]
15. Ezatpour B., Chegeni A.S., Abdollahpour F., Aazami M., Alirezaei M. (2013). Prevalence of parasitic contamination of raw vegetables in Khorramabad, Iran. Food Control. 34: 92-95. [DOI: 10.1016/j.foodcont.2013.03.026] [DOI:10.1016/j.foodcont.2013.03.026]
16. Faria C.P., Pereira A., Almeida D., Pinto M., Lourenço Á., Do Céu Sousa M. (2023). Molecular investigation of ready-to-eat salads for Giardia duodenalis and Cryptosporidium spp. in Portugal. Food and Waterborne Parasitology. 30: e00190. [DOI: 10.1016/j.fawpar.2023.e00190] [DOI:10.1016/j.fawpar.2023.e00190] [PMID] [PMCID]
17. Garedaghi Y., Farhang H.H., Pooryagoobi S. (2011). Parasitic contamination of fresh vegetables consumed in Tabriz, Iran. Researcher. 3: 76-79.
18. Gharavi M., Jahani M., Rokni M. (2002). Parasitic contamination of vegetables from farms and markets in Tehran. Iranian Journal of Public Health. 31: 83-86.
19. Khan W., Rafiq N., Nawaz M., Kabir M., Farooqi Z.U.R., Romman M., Parvez R., Alfarraj S., Noor A., Ujjan A. (2021). Parasitic contamination of fresh vegetables sold in open markets: a public health threat. Brazilian Journal of Biology. 82: e242614. [DOI: 10.1590/1519-6984.242614] [DOI:10.1590/1519-6984.242614] [PMID]
20. Lawal S., Wada Y., Ifraimu D. (2015). Parasitic contamination of commonly consumed fresh fruits and vegetables sold in open-air markets in Zaria Metropolis, Nigeria. Journal of Tropical Biosciences. 10: 68-75.
21. Marček T., Čorluka S., Gložinić M., Jažić E., Radman P., Sučić M., Ižaković M., Banjari I. (2018). A comparative survey on the prevalence of parasite elements in fresh vegetables and ready-to-eat salads. Hrana u Zdravlju i Bolesti: Znanstveno-StručniČasopiszaNutricionizam i Dijetetiku. 7: 26-30.
22. Mohemeed A.A., Alrawi Z.A.A., Mahdi N.K., Salih T.A. (2021). Immunological and molecular investigation of the level of parasite contamination of some vegetables sold in the local markets of Ramadi City-Iraq. Annals of the Romanian Society for Cell Biology. 25: 5964-5973.
23. Morales-Figueroa G., Sánchez-Guerrero M., Castro-García M., Esparza-Romero J., López-Mata M., Quihui-Cota L. (2021).Occurrence of intestinal parasites in fruits and vegetables from markets of Northwest Mexico. Journal of Food Quality and Hazards Control. 8: 81-88. [DOI:10.18502/jfqhc.8.2.6469]
24. Mufida D.C., Armiyanti Y., Putri E.R.M., Agustina D., Suswati E., Shodikin M.A., Utami W.S., Hermansyah B., Raharjo A.M. (2022). Bacterial and parasitic contamination of raw vegetable: potential risk for food-borne diseases. International Journal of Public Health. 11: 1516-1524. [DOI:10.11591/ijphs.v11i4.21875]
25. Nouroozi R.V. (2015). Detection of parasitic contamination in ready- to - eat fresh packaged herbs sold in Tehran, Iran. Journal of Community Health Research. 4: 99-104.
26. Rodrigues A.C., Da Silva M.D.C., Pereira R.Â.S., Pinto L.C. (2020). Prevalence of contamination by intestinal parasites in vegetables (Lactuca sativa L. and Coriandrum sativum L.) sold in markets in Belém, northern Brazil. Journal of the Science of Food and Agriculture. 100: 2859-2865. [DOI: 10.1002/jsfa.10282] [DOI:10.1002/jsfa.10282] [PMID]
27. Said D.E.S. (2012). Detection of parasites in commonly consumed raw vegetables. Alexandria Journal of Medicine. 48: 345-352. [DOI: 10.1016/j.ajme.2012.05.005] [DOI:10.1016/j.ajme.2012.05.005]
28. Tosin O.T., Ogunniyi T., Seun O.J. (2017). Human enteric parasitic pathogens in fresh fruits and vegetables consumed in ile-ife, osun state. Food Science and Quality Management. 67: 34-41.
29. Yavari M.R., Mirzaei F., Shahcheraghi S.H., Bafghi A.F. (2019). Parasitic contamination on fresh raw vegetables consumed in Yazd city, Iran, In during 2017-2018. Chinese Journal of Medical Research. 2: 70-73. [DOI:10.37515/cjmr.091X.2404]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







