Volume 12, Issue 2 (June 2025)
J. Food Qual. Hazards Control 2025, 12(2): 139-149 |
Back to browse issues page
Ethics code: Not applicable.
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Al-Farsi M, Al-Hoqani H, Ali H, Al-Hattali D, Shah Y, Al-Abri R. Enhancing the Shelf-Life of Date Fruits by Application of Chitosan-Based Nanoemulsion Enriched with Grape Seed Oil. J. Food Qual. Hazards Control 2025; 12 (2) :139-149
URL: http://jfqhc.ssu.ac.ir/article-1-1280-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1280-en.html
Natural and Medical Sciences Research Center, University of Nizwa, Nizwa, Oman , malfarsi@unizwa.edu.om
Full-Text [PDF 619 kb]
(270 Downloads)
| Abstract (HTML) (622 Views)
Full-Text: (224 Views)
Enhancing the Shelf-Life of Date Fruits by Application of Chitosan-Based Nanoemulsion Enriched with Grape Seed Oil
M. Al-Farsi [1]** , H. Al-Hoqani 2, H.M. Ali 1, D. Al-Hattali 3, Y.A. Shah 1, R. Al-Abri 2
1. Natural and Medical Sciences Research Center, University of Nizwa, Nizwa, Oman
2. School of Pharmacy, College of Health Science, University of Nizwa, Nizwa, Oman
3. Department of Agricultural Research, Ministry of Ministry of Agriculture, Fisheries and Water Resources, Oman
HIGHLIGHTS
Recently, nanotechnology has introduced an enhanced coating method utilizing nanoemulsion, where particles with a radius of less than 100 nm are used to apply a thin layer of edible coating on the fruit's surface. This nanosystem employs a low concentration of active agents to achieve effective results without adversely affecting the fruit's sensory qualities. The edible coating must be composed of ingredients such as polysaccharides, lipids, and proteins that are classified as Generally Recognized as Safe (GRAS) by the Food and Drug Administration (FDA) (De Oliveira Filho et al., 2021; Han et al., 2018).
Nanoscale particles are utilized to encapsulate fruits, enhance their stability and bioavailability while controlling enzyme release. This method also protects fruits from degradation caused by prolonged storage, high temperatures, and light exposure. By inhibiting microbial growth and reducing the impact of free radicals that shorten shelf-life, this approach can significantly extend storage periods.
Nanoemulsion is a colloidal dispersion involving two immiscible liquids, where one liquid is dispersed within the other. It comprises an oil phase, a liquid phase, and surfactants or co-surfactants that are homogenized and mixed using external energy. The oil-in-water (O/W) nanoemulsion is the most commonly used type in industry. Two main methods for producing nanoemulsions are low-energy emulsification systems that utilize phase inversion temperature and high-energy techniques like ultra-sonication and homogenization (Aswathanarayan et al., 2019; Ramos et al., 2021).
Chitosan-based films are well-regarded for their selective permeability to O2 and CO2 gases, excellent mechanical properties, and high water vapor permeability, which helps regulate moisture transfer (Butnaru et al., 2019).
Recently, the incorporation of essential oils into nanoemulsion food coatings has gained popularity due to their benefits, nutritional value, flavour enhancement, and antimicrobial properties (Pandey et al., 2022). Grape Seed Oil (GSO) has garnered attention for its nutraceutical benefits, containing phenolic compounds and lipophilic constituents like vitamin E, Unsaturated Fatty Acids (UFAs), and phytosterols (Garavaglia et al., 2016). As a by-product of grape juice production, GSO also possesses antibacterial and antioxidant properties due to its polyphenol compounds, including catechins, epicatechin, gallic acid, monomeric flavanols, and procyanidins (Chen et al., 2020). The antimicrobial activity of GSO is attributed to its key components, which demonstrate efficacy against a broad range of microorganisms. Some studies have shown that active films made from coarse emulsions of GSO exhibit antibacterial properties against both Gram-negative and Gram-positive bacteria (Mutlu, 2023).
Dates hold a revered status in Oman's agricultural landscape, symbolizing both tradition and economic significance. According to the statistical year book (NCSI, 2024), Oman is a significant contributor to the global date market, with a production volume of 394,945 tons recorded in 2023. This robust output underscores the country's prowess in date cultivation, supported by favourable climatic conditions and dedicated agricultural practices. The preservation of fresh dates through fast nanoemulsion techniques is a pivotal process that enhances their shelf-life while retaining their natural sweetness, texture, and nutritional value.
This study aimed to assess the effects of two newly developed chitosan-based edible coatings on the shelf-life and nutritional value of date fruits. The coatings contained 0.5 and 1.0% high molecular weight chitosan in 1% acetic acid and were enriched with 3.5% GSO.
.PNG)
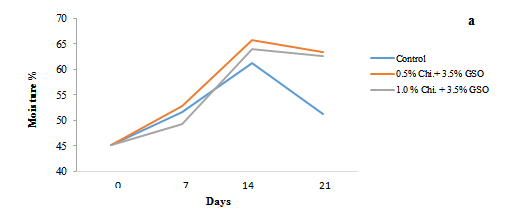
Figure 1: Changes in moisture content (a) and Total Soluble Solids (TSS) (b), measured in °Brix of date fruits over a 21day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
.PNG)
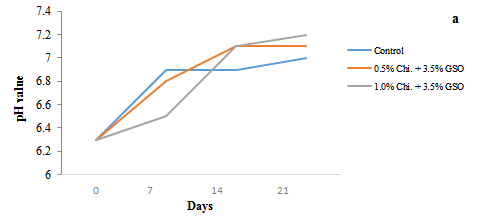
Figure 2: Changes in pH levels (a) and acidity (b) of date fruits over a 21 day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
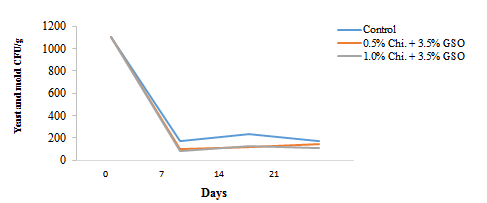
Figure 3: Changes in yeast and mold counts (Colony Forming Unit (CFU)/g) of date fruits during a 21 day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
.PNG)
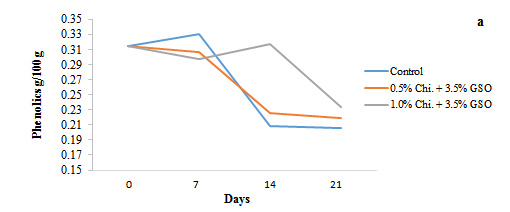
Figure 4: Changes in phenolic content (a) and antioxidant activity (b) of date fruits over a 21 day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
.PNG)
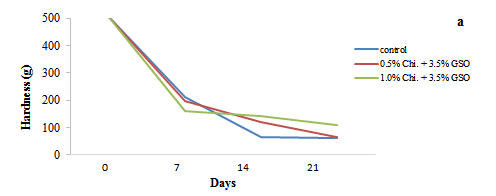
Figure 5: Changes in Hardness (a) and Elasticity (b) of date fruits over a 21 day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
The values are the mean of triplicate measurements, followed by the Standard Deviation (SD).
Chi=Chitosan; GSO=Grape Seed Oil
M. Al-Farsi [1]**
1. Natural and Medical Sciences Research Center, University of Nizwa, Nizwa, Oman
2. School of Pharmacy, College of Health Science, University of Nizwa, Nizwa, Oman
3. Department of Agricultural Research, Ministry of Ministry of Agriculture, Fisheries and Water Resources, Oman
- Chitosan nanoemulsion coatings extended date fruit shelf-life and preserved quality over 21 days.
- One percent chitosan coating maintained 63% moisture by day 21, compared to 51% in control.
- Coatings preserved antioxidant activity, retaining 65% of 2,2-Diphenyl-1-Picrylhydrazyl inhibition in coated dates versus 1.4% in control.
- Yeast and mold counts reduced to ~100 CFU/g by day seven in coated fruits.
- Coatings improved texture, color stability, and reduced quality degradation in stored date fruits.
| Article type Original article |
ABSTRACT Background: This study evaluated the effectiveness of chitosan-based nanoemulsion coatings, enriched with 3.5% Grape Seed Oil, in extending the shelf-life and preserving the quality of date fruits over a 21-day storage period. Methods: Fresh date fruits (totalling 20 kg) harvested in August 2024 were coated with 0.5 and 1.0% high molecular weight chitosan. Quality parameters including moisture content, pH, acidity, Total Soluble Solids (°Brix), phenolic content, antioxidant activity (2,2-diphenyl-1-picrylhydrazyl inhibition), texture (hardness and elasticity), color, and microbial load (yeast and mold counts) were monitored during storage. Statistical analysis was performed using Excel 2016 and two-way ANOVA, with significance at p<0.05. Results: The 1.0% chitosan coating was particularly effective; maintaining moisture levels at 63% by day 21 versus51% in the control. The pH of coated samples increased more gradually, reaching 7.2 in the 1.0% chitosan group, while the control’s increased to 7.0. Acidity decreased more slowly in coated samples, with lower levels than controls. Phenolic content and antioxidant activity were also better preserved in the coated fruits, with the 1.0% chitosan treatment retaining 65% 2,2-diphenyl-1-picrylhydrazyl inhibition by day 21, compared to 1.4% in the control. Yeast and mold counts were drastically reduced in coated fruits, dropping from 1,100 Colony Forming Unit (CFU)/g to around 100 CFU/g by day seven and remaining low throughout the storage period. In terms of texture, the coated fruits exhibited better firmness and elasticity retention, with slower degradation of hardness compared to the uncoated control. The coatings also contributed to less discoloration, with the 1.0% chitosan treatment maintaining higher lightness (L value) and color stability (a and b values) throughout the storage period. Conclusions: These newly developed chitosan-based nanoemulsion coatings demonstrated significant potential in preserving the postharvest quality of date fruits, maintaining moisture, texture, color, and antioxidant activity during storage. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Phoeniceae Chitosan Emulsions Food Preservation Oils, Volatile |
||
| Article history Received: 23 Dec 2024 Revised: 3 Mar 2025 Accepted: 5 Jun 2025 |
||
| Abbreviations CFU=Colony Forming Unit DPPH=2,2-Diphenyl-1-Picrylhydrazyl GSO=Grape Seed Oil TPC=Total Phenolic Content TSS=Total Soluble Solids |
To cite: Al-Farsi M., Al-Hoqani H., Ali H.M., Al-Hattali D., Shah Y.A., Al-Abri R. (2025). Enhancing the shelf-life of date fruits by application of chitosan-based nanoemulsion enriched with grape seed oil. Journal of Food Quality and Hazards Control. 12: 139-149.
Introduction
Today's consumers increasingly seek natural and healthy fresh foods that are nutritionally rich and have a long shelf-life, presenting a significant challenge. During processing and storage, fruit products can develop undesirable traits due to the instability and low bioavailability of their bioactive compounds. This can alter key properties like texture, flavor, and appearance, posing major technological hurdles in creating nutritional foods that meet consumer demands (Yu et al., 2018).
Historically, plastic and paper bags were used to protect and package fruits. Many food manufactures began applying waxy coatings made from paraffin, beeswax, and carnauba wax on fruits to reduce postharvest losses and extend shelf-life. While wax coatings proved effective in protecting fruits, there has consistently been a demand for more advanced, safer, and innovative coating solutions that also preserve the taste and quality of the fruit (De Oliveira Filho et al., 2021).Recently, nanotechnology has introduced an enhanced coating method utilizing nanoemulsion, where particles with a radius of less than 100 nm are used to apply a thin layer of edible coating on the fruit's surface. This nanosystem employs a low concentration of active agents to achieve effective results without adversely affecting the fruit's sensory qualities. The edible coating must be composed of ingredients such as polysaccharides, lipids, and proteins that are classified as Generally Recognized as Safe (GRAS) by the Food and Drug Administration (FDA) (De Oliveira Filho et al., 2021; Han et al., 2018).
Nanoscale particles are utilized to encapsulate fruits, enhance their stability and bioavailability while controlling enzyme release. This method also protects fruits from degradation caused by prolonged storage, high temperatures, and light exposure. By inhibiting microbial growth and reducing the impact of free radicals that shorten shelf-life, this approach can significantly extend storage periods.
Nanoemulsion is a colloidal dispersion involving two immiscible liquids, where one liquid is dispersed within the other. It comprises an oil phase, a liquid phase, and surfactants or co-surfactants that are homogenized and mixed using external energy. The oil-in-water (O/W) nanoemulsion is the most commonly used type in industry. Two main methods for producing nanoemulsions are low-energy emulsification systems that utilize phase inversion temperature and high-energy techniques like ultra-sonication and homogenization (Aswathanarayan et al., 2019; Ramos et al., 2021).
Chitosan-based films are well-regarded for their selective permeability to O2 and CO2 gases, excellent mechanical properties, and high water vapor permeability, which helps regulate moisture transfer (Butnaru et al., 2019).
Recently, the incorporation of essential oils into nanoemulsion food coatings has gained popularity due to their benefits, nutritional value, flavour enhancement, and antimicrobial properties (Pandey et al., 2022). Grape Seed Oil (GSO) has garnered attention for its nutraceutical benefits, containing phenolic compounds and lipophilic constituents like vitamin E, Unsaturated Fatty Acids (UFAs), and phytosterols (Garavaglia et al., 2016). As a by-product of grape juice production, GSO also possesses antibacterial and antioxidant properties due to its polyphenol compounds, including catechins, epicatechin, gallic acid, monomeric flavanols, and procyanidins (Chen et al., 2020). The antimicrobial activity of GSO is attributed to its key components, which demonstrate efficacy against a broad range of microorganisms. Some studies have shown that active films made from coarse emulsions of GSO exhibit antibacterial properties against both Gram-negative and Gram-positive bacteria (Mutlu, 2023).
Dates hold a revered status in Oman's agricultural landscape, symbolizing both tradition and economic significance. According to the statistical year book (NCSI, 2024), Oman is a significant contributor to the global date market, with a production volume of 394,945 tons recorded in 2023. This robust output underscores the country's prowess in date cultivation, supported by favourable climatic conditions and dedicated agricultural practices. The preservation of fresh dates through fast nanoemulsion techniques is a pivotal process that enhances their shelf-life while retaining their natural sweetness, texture, and nutritional value.
This study aimed to assess the effects of two newly developed chitosan-based edible coatings on the shelf-life and nutritional value of date fruits. The coatings contained 0.5 and 1.0% high molecular weight chitosan in 1% acetic acid and were enriched with 3.5% GSO.
Materials and methods
Dates
Fresh Nshu-Kharma dates at Rutab stage (totalling 20 kg) were obtained from local farmer in Nizwa during August 2024, and various trials were promptly conducted on date samples. Fruits were inspected to remove damaged fruits and only good quality fruits were selected for further treatment and experiment; taking into account the similarity in shape, color, and size. The fruits were stored at refrigerated temperatures (five °C) until treatments and during storage.
Materials
High molecular weight chitosan with a 90% degree of deacetylation (Product No. 96266), acetic acid (90,868), and Tween 80 (28,940) were obtained from Sisco Research Lab (Maharashtra, India) and used without further purification. Grape seed essential oil was procured from Now Solutions (IL, USA). Folin-Ciocalteu reagent (F9252), sodium bicarbonate (S6014), sodium acetate (S8750), and 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) (247,642) were supplied by Merck (Darmstadt, Germany).
Preparation of chitosan coatings
Chitosan-based edible coating solutions were prepared following the method described by Hamedi et al. (2019), with minor modifications. High molecular weight chitosan was dissolved at concentrations of 0.5 and 1% (w/v) in 1% (w/v) acetic acid under constant stirring using a magnetic stirrer (Biobase, Shandong, China) at 80 °C for approximately one h. The solutions were then allowed to cool at room temperature for one h. To facilitate the incorporation of essential oil, two ml of Tween 80 was added as an emulsifier, followed by stirring for five min. GSO (3.5%) was then added to each solution and stirred until fully emulsified. The resulting mixtures were subjected to ultrasonication at 40 kHz for 30 min using a sonicator (Labman, LMUE-6, India).
Coating application
Prior to coating, the dates were washed with water for one min and air-dried at room temperature for one h to remove surface moisture. The fruits were then immersed in the chitosan coating solutions for 20 s, followed by air drying at room temperature for another one h to eliminate excess coating. Once dried, the coated dates were packed into perforated Polyethylene Terephthalate (PET) containers, with each container holding approximately 400 g of fruit (Popescu et al., 2022), and stored at five °C. Uncoated dates served as control samples. Physicochemical and microbiological analyses were conducted weekly over a 21 day storage period.
Physicochemical characterization
The moisture content was measured using the oven drying method according to the AOAC (2023). The Total Soluble Solids (TSS) contents of the samples, either uncoated or coated (◦Brix) were determined at 20 °C using a digital refractometer (BK-RZT, Biobase, Shandong, China). The pH determination involved homogenization five g of sample with 50 ml of deionized distilled water, followed by pH measurements using a pH meter (pH-950, Biobase, Shandong, China). Acidity was measured using the titration method according to the AOAC (2023).
Color measurement
The color attributes of the date fruit surface were measured with a chroma Meter (CR-410, Konica Minolta, Japan) using the color parameters L*, a*, b* as described by Adiamo et al. (2017). The L* indicates lightness co-efficient and it ranges from 0 to 100 (black to white), a* and b* indicate color ranges from green to red and blue to yellow along the horizontal and vertical axes, respectively, with negative to positive values. All the measurements were done in triplicates.
Materials
High molecular weight chitosan with a 90% degree of deacetylation (Product No. 96266), acetic acid (90,868), and Tween 80 (28,940) were obtained from Sisco Research Lab (Maharashtra, India) and used without further purification. Grape seed essential oil was procured from Now Solutions (IL, USA). Folin-Ciocalteu reagent (F9252), sodium bicarbonate (S6014), sodium acetate (S8750), and 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) (247,642) were supplied by Merck (Darmstadt, Germany).
Preparation of chitosan coatings
Chitosan-based edible coating solutions were prepared following the method described by Hamedi et al. (2019), with minor modifications. High molecular weight chitosan was dissolved at concentrations of 0.5 and 1% (w/v) in 1% (w/v) acetic acid under constant stirring using a magnetic stirrer (Biobase, Shandong, China) at 80 °C for approximately one h. The solutions were then allowed to cool at room temperature for one h. To facilitate the incorporation of essential oil, two ml of Tween 80 was added as an emulsifier, followed by stirring for five min. GSO (3.5%) was then added to each solution and stirred until fully emulsified. The resulting mixtures were subjected to ultrasonication at 40 kHz for 30 min using a sonicator (Labman, LMUE-6, India).
Coating application
Prior to coating, the dates were washed with water for one min and air-dried at room temperature for one h to remove surface moisture. The fruits were then immersed in the chitosan coating solutions for 20 s, followed by air drying at room temperature for another one h to eliminate excess coating. Once dried, the coated dates were packed into perforated Polyethylene Terephthalate (PET) containers, with each container holding approximately 400 g of fruit (Popescu et al., 2022), and stored at five °C. Uncoated dates served as control samples. Physicochemical and microbiological analyses were conducted weekly over a 21 day storage period.
Physicochemical characterization
The moisture content was measured using the oven drying method according to the AOAC (2023). The Total Soluble Solids (TSS) contents of the samples, either uncoated or coated (◦Brix) were determined at 20 °C using a digital refractometer (BK-RZT, Biobase, Shandong, China). The pH determination involved homogenization five g of sample with 50 ml of deionized distilled water, followed by pH measurements using a pH meter (pH-950, Biobase, Shandong, China). Acidity was measured using the titration method according to the AOAC (2023).
Color measurement
The color attributes of the date fruit surface were measured with a chroma Meter (CR-410, Konica Minolta, Japan) using the color parameters L*, a*, b* as described by Adiamo et al. (2017). The L* indicates lightness co-efficient and it ranges from 0 to 100 (black to white), a* and b* indicate color ranges from green to red and blue to yellow along the horizontal and vertical axes, respectively, with negative to positive values. All the measurements were done in triplicates.
Texture measurement
The samples before and during storage were subjected to texture profile analysis using a texture analyser (TA.XTplusC, Micro Systems, England). The hardness (g) and elasticity (mm) were measured in triplicate (Ghafoor et al., 2022).
Total Phenolic Content (TPC)
Polyphenols were extracted using 75% ethanol with a sample-to-solvent ratio of 1:5 (w/v) by macerating the samples in the dark at room temperature. After filtration, the resulting extracts were stored at −20 °C for further analysis. The TPC was determined following the Folin-Ciocalteu method described by Singleton and Rossi (1965). Briefly, 20 µl of the ethanolic extract was combined with 1,580 µl of distilled water and 100 µl of Folin-Ciocalteu reagent and mixed vigorously. After one min., 300 µl of 20% (w/v) aqueous sodium carbonate was added, and the mixture was stirred again. The reaction was allowed to proceed in the dark at room temperature for two h. Absorbance was measured at 765 nm using a UV/Vis spectrophotometer (Lambda-950, Thermo Helios Alpha, Waltham, MA, USA). TPC was calculated from a gallic acid calibration curve and expressed as grams of Gallic Acid Equivalents (GAE)/100 g of sample (Singleton and Rossi, 1965).
Total Phenolic Content (TPC)
Polyphenols were extracted using 75% ethanol with a sample-to-solvent ratio of 1:5 (w/v) by macerating the samples in the dark at room temperature. After filtration, the resulting extracts were stored at −20 °C for further analysis. The TPC was determined following the Folin-Ciocalteu method described by Singleton and Rossi (1965). Briefly, 20 µl of the ethanolic extract was combined with 1,580 µl of distilled water and 100 µl of Folin-Ciocalteu reagent and mixed vigorously. After one min., 300 µl of 20% (w/v) aqueous sodium carbonate was added, and the mixture was stirred again. The reaction was allowed to proceed in the dark at room temperature for two h. Absorbance was measured at 765 nm using a UV/Vis spectrophotometer (Lambda-950, Thermo Helios Alpha, Waltham, MA, USA). TPC was calculated from a gallic acid calibration curve and expressed as grams of Gallic Acid Equivalents (GAE)/100 g of sample (Singleton and Rossi, 1965).
Antioxidant activity
The antioxidant capacity of the date fruit samples was assessed using the DPPH radical scavenging method described by Lee et al. (1998). One ml of extract was diluted in methanol and mixed with two ml of DPPH solution. The mixture was incubated in the dark at room temperature for 30 min, after which absorbance was recorded at 517 nm using a UV/Vis spectrophotometer. Methanol was used as the blank. The percentage of DPPH inhibition was calculated using the following equation:
DPPH inhibition (%)=Abscontrol 517–Abssample 517/Abscontrol 517×100
where Abs is the absorbance recorded and DPPH inhibition was expressed in percentage.
Microbial assessment
-Yeast and mold counts
Microbial evaluation of yeast and mold was conducted under aseptic conditions using sterile gloves, scalpels, and a laminar flow cabinet. The method was based on protocols by Eshghi et al. (2022) and Jafari et al. (2021). In brief, 10 g of each sample was homogenized with 90 ml of sterile distilled water in a 100 ml Erlenmeyer flask. Appropriate serial dilutions were prepared, and one ml from each dilution was plated on Malt Extract Agar (MEA; Scharlau, Spain) using the standard pour plate technique. The plates were incubated at 25 °C for five days, and colony counts were expressed as Colony Forming Units (CFU)/g of sample. All microbiological assessments were performed in triplicate.
Statistical analysis
All experiments were performed in triplicates for each sample and the results were expressed as mean ± Standard Deviation (SD). Statistical analysis, including calculation of averages, SDs, and two-way ANOVA, was performed using Microsoft Excel (Version 2016, Microsoft Corp., USA). The least significant difference (LSD) at p<0.05 was used to determine statistical significance.
The antioxidant capacity of the date fruit samples was assessed using the DPPH radical scavenging method described by Lee et al. (1998). One ml of extract was diluted in methanol and mixed with two ml of DPPH solution. The mixture was incubated in the dark at room temperature for 30 min, after which absorbance was recorded at 517 nm using a UV/Vis spectrophotometer. Methanol was used as the blank. The percentage of DPPH inhibition was calculated using the following equation:
DPPH inhibition (%)=Abscontrol 517–Abssample 517/Abscontrol 517×100
where Abs is the absorbance recorded and DPPH inhibition was expressed in percentage.
Microbial assessment
-Yeast and mold counts
Microbial evaluation of yeast and mold was conducted under aseptic conditions using sterile gloves, scalpels, and a laminar flow cabinet. The method was based on protocols by Eshghi et al. (2022) and Jafari et al. (2021). In brief, 10 g of each sample was homogenized with 90 ml of sterile distilled water in a 100 ml Erlenmeyer flask. Appropriate serial dilutions were prepared, and one ml from each dilution was plated on Malt Extract Agar (MEA; Scharlau, Spain) using the standard pour plate technique. The plates were incubated at 25 °C for five days, and colony counts were expressed as Colony Forming Units (CFU)/g of sample. All microbiological assessments were performed in triplicate.
Statistical analysis
All experiments were performed in triplicates for each sample and the results were expressed as mean ± Standard Deviation (SD). Statistical analysis, including calculation of averages, SDs, and two-way ANOVA, was performed using Microsoft Excel (Version 2016, Microsoft Corp., USA). The least significant difference (LSD) at p<0.05 was used to determine statistical significance.
Results and discussion
Effect of coating on moisture and TSS
The moisture content and TSS, measured in °Brix, of the date fruits exhibited related trends over the 21 day storage period. Before coating, samples displayed around 45% moisture and approximately 55 °Brix value as shown in Figure1 (a and b). As storage progressed after coating, the control sample showed a steady increase in moisture content, peaking at 61% by day 14 before decreasing to 51% by day 21. In contrast, the dates treated with 0.5 and 1.0% chitosan exhibited better moisture retention, with both treatments showing a more consistent increase in moisture content. The 0.5% chitosan-treated sample reached 66% moisture on day 14 and maintained 64% by day 21, while the 1.0% chitosan-treated sample reached 64% and retained 63% moisture. Our moisture content results align with the findings reported by Wang and Rhim (2016), who observed that chitosan biopolymer-coated apricots showed less weight loss than the uncoated apricot samples. This improved moisture retention was reflected in the °Brix values, where both chitosan treatments demonstrated a less drastic decline in TSS compared to the control. Figure 1b shows that the 0.5% chitosan-treated sample experienced a decrease in °Brix to 41 by day 14, followed by a moderate recovery to 44 by day 21. °Brix values dropped to 44 by day 14 and recovered significantly to 52 by day 21 in a 1.0% chitosan-treated sample. The °Brix values in our study showed no increase over time; however, the control showed the highest °Brix recovery by day 21. The relationship between moisture content and TSS in fruits plays a key role in understanding their quality, ripening process, and overall composition. TSS mainly consists of sugars, acids, vitamins, and other dissolved substances, with sugars being the predominant component. Higher moisture levels typically result in lower TSS concentrations due to dilution. As fruits lose moisture through ripening, dehydration, or storage, TSS levels rise, leading to sweeter, and more concentrated flavors. This balance between moisture and TSS directly impacts a fruit's ripeness, taste, and quality. The ability of chitosan to act as a moisture barrier likely minimized dehydration and sugar concentration fluctuations, contributing to the prolonged quality of the coated fruits compared to the uncoated control (Chen et al., 2016).
The moisture content and TSS, measured in °Brix, of the date fruits exhibited related trends over the 21 day storage period. Before coating, samples displayed around 45% moisture and approximately 55 °Brix value as shown in Figure1 (a and b). As storage progressed after coating, the control sample showed a steady increase in moisture content, peaking at 61% by day 14 before decreasing to 51% by day 21. In contrast, the dates treated with 0.5 and 1.0% chitosan exhibited better moisture retention, with both treatments showing a more consistent increase in moisture content. The 0.5% chitosan-treated sample reached 66% moisture on day 14 and maintained 64% by day 21, while the 1.0% chitosan-treated sample reached 64% and retained 63% moisture. Our moisture content results align with the findings reported by Wang and Rhim (2016), who observed that chitosan biopolymer-coated apricots showed less weight loss than the uncoated apricot samples. This improved moisture retention was reflected in the °Brix values, where both chitosan treatments demonstrated a less drastic decline in TSS compared to the control. Figure 1b shows that the 0.5% chitosan-treated sample experienced a decrease in °Brix to 41 by day 14, followed by a moderate recovery to 44 by day 21. °Brix values dropped to 44 by day 14 and recovered significantly to 52 by day 21 in a 1.0% chitosan-treated sample. The °Brix values in our study showed no increase over time; however, the control showed the highest °Brix recovery by day 21. The relationship between moisture content and TSS in fruits plays a key role in understanding their quality, ripening process, and overall composition. TSS mainly consists of sugars, acids, vitamins, and other dissolved substances, with sugars being the predominant component. Higher moisture levels typically result in lower TSS concentrations due to dilution. As fruits lose moisture through ripening, dehydration, or storage, TSS levels rise, leading to sweeter, and more concentrated flavors. This balance between moisture and TSS directly impacts a fruit's ripeness, taste, and quality. The ability of chitosan to act as a moisture barrier likely minimized dehydration and sugar concentration fluctuations, contributing to the prolonged quality of the coated fruits compared to the uncoated control (Chen et al., 2016).

.PNG)
Figure 1: Changes in moisture content (a) and Total Soluble Solids (TSS) (b), measured in °Brix of date fruits over a 21day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
Effect of coating on pH and acidity
Figure 2 (a and b) demonstrate that the pH and acidity levels of the date fruits during the 21 day storage period exhibited an inverse relationship, with the pH increasing as the acidity decreased in all samples. Samples exhibited pH values around 6.3 and acidity levels of 0.04% before coating on day one. After coating, a steady increase in pH was observed, particularly in the chitosan-treated samples. By day 14, the control sample's pH raised to 6.9 and further increased to 7.0 by day 21, accompanied by a corresponding decrease in acidity to 0.025%. The 0.5% chitosan-treated samples followed a similar pattern, with the pH reaching 7.1 by day 21 and acidity decreasing to 0.021%. However, the 1.0% chitosan-treated samples exhibited 7.2 pH by day 21, while acidity dropped to 0.019% by day 14 and remained relatively stable until day 21. These results agree with the previous study of Ghafoor et al. (2022), who reported relatively lower acidity in the chitosan-coated Barhi fruits compared to the uncoated fruits. Similarly, El-Moneim et al. (2015) reported similar acidity patterns in coated-Zaghloul date fruits. These changes reflect the natural metabolic processes during storage, where organic acids are consumed or degraded, resulting in increased pH and reduced acidity (Maftoonazad et al., 2008). The slower rate of acidity reduction and the more stable pH levels observed in the chitosan-treated samples, particularly in the 1.0% concentration, suggest that the coatings effectively slowed the degradation of organic acids, thereby preserving the internal conditions of the fruits. This stabilization likely contributed to maintaining the overall quality of the dates and extending their shelf-life, as the slower increase in pH and more gradual reduction in acidity in the coated fruits indicate improved control over-ripening and metabolic activity compared to the uncoated control (Maftoonazad and Ramaswamy, 2005).
Figure 2 (a and b) demonstrate that the pH and acidity levels of the date fruits during the 21 day storage period exhibited an inverse relationship, with the pH increasing as the acidity decreased in all samples. Samples exhibited pH values around 6.3 and acidity levels of 0.04% before coating on day one. After coating, a steady increase in pH was observed, particularly in the chitosan-treated samples. By day 14, the control sample's pH raised to 6.9 and further increased to 7.0 by day 21, accompanied by a corresponding decrease in acidity to 0.025%. The 0.5% chitosan-treated samples followed a similar pattern, with the pH reaching 7.1 by day 21 and acidity decreasing to 0.021%. However, the 1.0% chitosan-treated samples exhibited 7.2 pH by day 21, while acidity dropped to 0.019% by day 14 and remained relatively stable until day 21. These results agree with the previous study of Ghafoor et al. (2022), who reported relatively lower acidity in the chitosan-coated Barhi fruits compared to the uncoated fruits. Similarly, El-Moneim et al. (2015) reported similar acidity patterns in coated-Zaghloul date fruits. These changes reflect the natural metabolic processes during storage, where organic acids are consumed or degraded, resulting in increased pH and reduced acidity (Maftoonazad et al., 2008). The slower rate of acidity reduction and the more stable pH levels observed in the chitosan-treated samples, particularly in the 1.0% concentration, suggest that the coatings effectively slowed the degradation of organic acids, thereby preserving the internal conditions of the fruits. This stabilization likely contributed to maintaining the overall quality of the dates and extending their shelf-life, as the slower increase in pH and more gradual reduction in acidity in the coated fruits indicate improved control over-ripening and metabolic activity compared to the uncoated control (Maftoonazad and Ramaswamy, 2005).

.PNG)
Figure 2: Changes in pH levels (a) and acidity (b) of date fruits over a 21 day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
The antimicrobial effect of coating
The yeast and mold counts (CFU/g) in the date fruits during the 21 day storage period are shown in Figure 3. Initially date samples exhibited a high microbial load, with counts of 1,000-1,100 CFU/g before coating treatment at day one. After coating, by day seven, a substantial reduction in microbial counts was observed across all treatments. The control sample showed a decrease to 173 CFU/g, while the 0.5 and 1.0% chitosan-treated samples dropped to 100 and 80 CFU/g, respectively. After this sharp decline, the microbial load remained relatively stable throughout the remaining storage period, with only minimal increases observed by day 21. The control sample showed a slight rise, while the chitosan-treated samples demonstrated more stability, with 1.0% treatments maintaining the lowest yeast and mold count followed by 0.5% treatment by day 21. Our findings are consistent with those of Popescu et al. (2022), who reported a more significant decrease in yeast and mold count in the chitosan-based edible-coated organic strawberries than in the uncoated samples. This sharp reduction in the fungal count could also be due to the presence of GSO in the coatings. This can be justified by the previously reported antifungal activity of essential oils by Tian et al. (2022). These results suggest that the application of chitosan coatings effectively suppressed yeast and mold growth on the date fruits during storage, with both 0.5 and 1.0% concentrations showing antimicrobial efficacy. The lower microbial counts in the chitosan-treated samples, particularly in the 1.0% treatment, indicate that chitosan may serve as an effective antimicrobial barrier, enhancing the shelf-life of the fruit by reducing the risk of spoilage caused by fungal contamination (Velickova et al., 2013).
The yeast and mold counts (CFU/g) in the date fruits during the 21 day storage period are shown in Figure 3. Initially date samples exhibited a high microbial load, with counts of 1,000-1,100 CFU/g before coating treatment at day one. After coating, by day seven, a substantial reduction in microbial counts was observed across all treatments. The control sample showed a decrease to 173 CFU/g, while the 0.5 and 1.0% chitosan-treated samples dropped to 100 and 80 CFU/g, respectively. After this sharp decline, the microbial load remained relatively stable throughout the remaining storage period, with only minimal increases observed by day 21. The control sample showed a slight rise, while the chitosan-treated samples demonstrated more stability, with 1.0% treatments maintaining the lowest yeast and mold count followed by 0.5% treatment by day 21. Our findings are consistent with those of Popescu et al. (2022), who reported a more significant decrease in yeast and mold count in the chitosan-based edible-coated organic strawberries than in the uncoated samples. This sharp reduction in the fungal count could also be due to the presence of GSO in the coatings. This can be justified by the previously reported antifungal activity of essential oils by Tian et al. (2022). These results suggest that the application of chitosan coatings effectively suppressed yeast and mold growth on the date fruits during storage, with both 0.5 and 1.0% concentrations showing antimicrobial efficacy. The lower microbial counts in the chitosan-treated samples, particularly in the 1.0% treatment, indicate that chitosan may serve as an effective antimicrobial barrier, enhancing the shelf-life of the fruit by reducing the risk of spoilage caused by fungal contamination (Velickova et al., 2013).

Figure 3: Changes in yeast and mold counts (Colony Forming Unit (CFU)/g) of date fruits during a 21 day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
Effect of coating on phenolics and antioxidants
The phenolic content and antioxidant activity of the date fruits demonstrated a closely related pattern during the 21 day storage period (Figures 4a and 4b), with the decline in phenolic compounds being directly linked to the reduction in antioxidant activity. As the storage progressed, both parameters showed a noticeable decline, particularly in the uncoated control samples. By day 14, the phenolic content of the control dropped from around 0.31 to 0.21 g/100 g as shown in Figure 4a, while its antioxidant activity also decreased sharply from 54 to 4%. In contrast, the 0.5 and 1.0% chitosan-treated samples exhibited a slower reduction in both phenolic content and antioxidant capacity, with the 1.0% chitosan treatment showing the highest retention. The 1.0% chitosan-treated fruits retained higher levels of both phenolic content (0.32 g/100 g) and antioxidant activity (62%) on day 14. This dramatic increase in the antioxidant activity of coated samples can be associated with the combined oxidation inhibition potential of edible chitosan and GSOs. For instance, Harbeoui et al. (2018) reported that GSO possesses tremendous antioxidant properties. Similarly, Adiletta et al. (2019) also reported enhanced antioxidant activity in Ficus carica. L coated with chitosan and essential oils. By the end of the storage period on day 21, the control sample exhibited the lowest values for both parameters, with phenolic content reduced to 0.21 g/100 g and antioxidant activity to 1.4%. In contrast, the 1.0% chitosan-treated fruits maintained a higher phenolic content of 0.24 g/100 g and a strong antioxidant capacity of 65% followed by 0.5% treatment with phenolic content of 0.22 g/100 g and antioxidant activity of 41%. Our results are in agreement with Ghafoor et al. (2022), who also observed over 60% DPPH inhibition at day 21 in Barhi dates, coated with orange peel extract and chitosan. These results indicate that chitosan coatings, particularly at the 1.0% concentration, effectively preserved the phenolic compounds and, consequently, the antioxidant properties of the date fruits during storage. Chitosan’s ability to act as a protective barrier likely slowed down the oxidation and degradation of bioactive compounds, thereby extending the overall quality and antioxidant potential of the coated fruits.
The phenolic content and antioxidant activity of the date fruits demonstrated a closely related pattern during the 21 day storage period (Figures 4a and 4b), with the decline in phenolic compounds being directly linked to the reduction in antioxidant activity. As the storage progressed, both parameters showed a noticeable decline, particularly in the uncoated control samples. By day 14, the phenolic content of the control dropped from around 0.31 to 0.21 g/100 g as shown in Figure 4a, while its antioxidant activity also decreased sharply from 54 to 4%. In contrast, the 0.5 and 1.0% chitosan-treated samples exhibited a slower reduction in both phenolic content and antioxidant capacity, with the 1.0% chitosan treatment showing the highest retention. The 1.0% chitosan-treated fruits retained higher levels of both phenolic content (0.32 g/100 g) and antioxidant activity (62%) on day 14. This dramatic increase in the antioxidant activity of coated samples can be associated with the combined oxidation inhibition potential of edible chitosan and GSOs. For instance, Harbeoui et al. (2018) reported that GSO possesses tremendous antioxidant properties. Similarly, Adiletta et al. (2019) also reported enhanced antioxidant activity in Ficus carica. L coated with chitosan and essential oils. By the end of the storage period on day 21, the control sample exhibited the lowest values for both parameters, with phenolic content reduced to 0.21 g/100 g and antioxidant activity to 1.4%. In contrast, the 1.0% chitosan-treated fruits maintained a higher phenolic content of 0.24 g/100 g and a strong antioxidant capacity of 65% followed by 0.5% treatment with phenolic content of 0.22 g/100 g and antioxidant activity of 41%. Our results are in agreement with Ghafoor et al. (2022), who also observed over 60% DPPH inhibition at day 21 in Barhi dates, coated with orange peel extract and chitosan. These results indicate that chitosan coatings, particularly at the 1.0% concentration, effectively preserved the phenolic compounds and, consequently, the antioxidant properties of the date fruits during storage. Chitosan’s ability to act as a protective barrier likely slowed down the oxidation and degradation of bioactive compounds, thereby extending the overall quality and antioxidant potential of the coated fruits.

.PNG)
Figure 4: Changes in phenolic content (a) and antioxidant activity (b) of date fruits over a 21 day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
Effect of coating on texture
The texture analysis of the date fruits, represented by hardness and elasticity (Figures 5a and 5b), showed significant changes during the 21 day storage period. Firmness is an essential criterion that affects the overall texture quality and integrity of date fruits. Figure 5b shows that hardness consistently decreased across all samples; however, the coated fruits (treated with 0.5 and 1.0% chitosan) experienced a slower decline compared to the uncoated control. Fruits exhibited high hardness and elasticity values at day zero, indicating firm and flexible texture. However, by day seven, the uncoated control fruits showed a rapid reduction in hardness, while the coated fruits retained more firmness, particularly those treated with 1.0% chitosan, which maintained a higher level of hardness throughout the storage period. This trend continued through day 14 and 21, where the uncoated fruits had softened considerably (62 g), whereas the chitosan-coated samples remained comparatively firmer (110 g). In contrast, the uncoated fruits, which lost hardness more quickly, exhibited a higher elasticity by day 21 (6.0 mm) compared to the coated samples (5.8 mm). Our textural analysis results are consistent with those of Mannozzi et al. (2017), who reported that the coating agent significantly retained the firmness of the blueberries compared to the uncoated samples. This inverse relationship between hardness and elasticity became more evident as the storage period progressed; as the control fruits became softer, they also became more flexible. The chitosan-coated fruits, especially those treated with 1.0% chitosan, demonstrated better texture retention overall, with a more balanced preservation of both firmness and elasticity. These findings suggest that while the uncoated fruits became softer and more elastic, the chitosan coatings helped to slow the degradation of texture, maintaining both the structural integrity and flexibility of the fruits and thus prolonging their shelf-life.

.PNG)
Figure 5: Changes in Hardness (a) and Elasticity (b) of date fruits over a 21 day storage period (5 ºC). Chi=Chitosan; GSO=Grape Seed Oil
Effect of coating on color
Table 1 represents the color parameters (L*, a*, b* values) of the date fruits showing noticeable changes during the 21 day storage period. The lightness (L*) values decreased across all samples, with the control dropping significantly from its initial value of 77.1 to 64.9, while the 0.5 and 1.0% chitosan-treated samples maintained slightly better lightness with 66.8 and 66.1 L* values, respectively, by day 21, as shown in Figure 6. In terms of redness (a* value), the control initially increased from 8.8 to 11.2 by day seven but later decreased to 10.9 by day 14 and then to 8.1 by day 21. The initial increase in the redness might be due to the fast ripening of the control fruits compared to gradually ripening coated fruits, while the 0.5 and 1.0% chitosan-treated sample consistently showed an increase in the redness throughout the storage period ranging from 8.8 to 11.2, indicating a pronounced red color, while the 0.1% coated samples interestingly showed a significant decrease in the redness over the storage time. The yellowness (b* value) declined significantly for all samples from 31.1 b* value to 20.6 in control and 23.4 in 0.5% coated fruit, with the 1.0% chitosan-treated dates exhibiting the most substantial reduction in yellowness dropping to 15.2. These results suggest that the chitosan coatings, particularly at the higher concentration, were effective in slowing the degradation of lightness and influencing color retention in terms of redness and yellowness. Our results agree with those previously reported by Ghafoor et al. (2022), who reported that coated samples lost the lightness more gradually than the uncoated fruits. This decrease in discoloration in the coated samples might be due to the reduced oxidation by chitosan coating. Eissa (2007) suggests that chitosan coating can reduce the oxidative enzyme activity, which is associated with discoloration.
Table 1: Changes in lightness (L*), redness (a*), and yellowness (b*) values of date fruits during the 21 day storage period (5ºC)
Table 1: Changes in lightness (L*), redness (a*), and yellowness (b*) values of date fruits during the 21 day storage period (5ºC)
| Days | 0 | 7 | 14 | 21 |
| Control | ||||
| L* | 77.1±0.1 | 71.0±1.7 | 65.9±2.5 | 64.9±1.7 |
| a* | 8.8±1.0 | 11.2±0.4 | 10.9±1.2 | 8.1±0.8 |
| b* | 31.1±2.1 | 22.9±3.6 | 26.4±2.5 | 20.6±1.7 |
| 0.5% Chi+3.5% GSO | ||||
| L* | 77.1±0.1 | 71.3±1.0 | 67.9±2.3 | 66.8±1.3 |
| a* | 8.8±1.0 | 10.7±0.8 | 9.7±1.2 | 11.2±1.2 |
| b* | 31.1±2.1 | 27.2±1.6 | 22.4±1.9 | 23.4±1.9 |
| 1.0% Chi+3.5% GSO | ||||
| L | 77.1±0.1 | 73.6±4.7 | 67.6±1.4 | 66.1±1.4 |
| a* | 8.8±1.0 | 7.4±0.7 | 9.3±0.7 | 6.2±0.7 |
| b* | 31.1±2.1 | 26.4±1.7 | 17.8±1.0 | 15.2±1.2 |
Chi=Chitosan; GSO=Grape Seed Oil

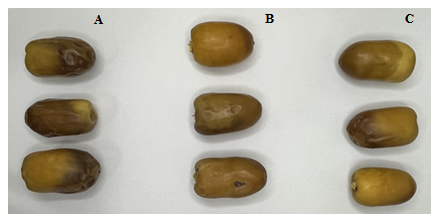
Figure 6: The Photograph of, A: control; B: 0.5% chitosan+3.5% Grape Seed Oil (GSO); and C: 1.0% chitosan+3.5% GSO after 21 day storage period (5 ºC)
Conclusion
The chitosan-based nanoemulsion coatings, enriched with GSO, effectively extended the shelf-life and preserved the quality of date fruits during the 21 day storage period. The coatings improved moisture retention and slowed down the rate of acidity reduction while maintaining more stable pH levels. Additionally, the phenolic content and antioxidant activity were better preserved, and microbial growth was significantly reduced in the coated samples compared to the control, demonstrating the ability of chitosan to protect bioactive compounds. The coatings also contributed to retaining the texture and better color preservation. These findings suggest that chitosan-based nanoemulsion coatings are a promising method for extending the shelf-life and maintaining the postharvest quality of dates. Future research could investigate the use of different essential oils, variations in chitosan concentrations, and the effects of various storage conditions, including room temperature, to optimize the coating formulations for broader applications across different date varieties and storage environments.
Author contributions
M.A.-F. designed, supervised, and reviewed the data and the manuscript; H.A.-H. and H.M.A. designed, performed the analytical work, and drafted the manuscript; D.A.-H., Y.A.S., and R.A.-A. contributed to the chemical, microbial, and physical analyses, and analysed the data. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to the Ministry of Higher Education for funding this project.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Funding
The present study was funded by the Ministry of
Higher Education GRG-TRC project, Proposal ID: BFP/GRG/EI/22/129.
Ethical consideration
Not applicable.
Author contributions
M.A.-F. designed, supervised, and reviewed the data and the manuscript; H.A.-H. and H.M.A. designed, performed the analytical work, and drafted the manuscript; D.A.-H., Y.A.S., and R.A.-A. contributed to the chemical, microbial, and physical analyses, and analysed the data. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to the Ministry of Higher Education for funding this project.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Funding
The present study was funded by the Ministry of
Higher Education GRG-TRC project, Proposal ID: BFP/GRG/EI/22/129.
Ethical consideration
Not applicable.
References
Adiamo O.Q., Ghafoor K., Al-Juhaimi F., Mohamed Ahmed I.A., Babiker E.E. (2017). Effects of thermosonication and orange by-products extracts on quality attributes of carrot (Daucus carota) juice during storage. International Journal of Food Science and Technology. 52: 2115-2125. [DOI: 10.1111/ijfs.13490]
Adiletta G., Zampella L., Coletta C., Petriccione M. (2019). Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture. 9: 84. [DOI: 10.3390/agriculture9040084]
Association of Official Analytical Chemists (AOAC). (2023). Official Methods of Analysis. 18th edition. AOAC International. Gaithersburg, USA. URL: https://www.aoac.org/official-methods-of-analysis/. Accecced 15 May 2025.
Aswathanarayan J.B., Anandan R., Mathew S., Ganesan B. (2019). Nanoemulsions: preparation techniques and applications in food systems. Current Nutrition and Food Science. 15: 104-115. [DOI: 10.2174/1573401314666181113162155]
Butnaru E., Stoleru E., Brebu M.A., Darie-Nita R.N., Bargan A., Vasile C. (2019). Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials. 12: 373. [DOI: 10.3390/ma12030373]
Chen C., Peng X., Zeng R., Chen M., Wan C., Chen J. (2016). Ficus hirta fruits extract incorporated into an alginate-based edible coating for nanfeng mandarin preservation. Scientia Horticulturae. 202: 41-48. [DOI: 10.1016/j.scienta.2015.12.046]
Chen J., Thilakarathna W.P.D.W., Astatkie T., Rupasinghe H.P.V. (2020). Optimization of catechin and proanthocyanidin recovery from grape seeds using microwave-assisted extraction. Biomolecules. 10: 243. [DOI: 10.3390/biom10020243]
De Oliveira Filho J.G., Miranda M., Ferreira M.D., Plotto A. (2021). Nanoemulsions as edible coatings: a potential strategy for fresh fruits and vegetables preservation. Foods. 10: 2438. [DOI: 10.3390/foods10102438]
Eissa H.A.A. (2007). Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. Journal of Food Quality. 30: 623-645. [DOI: 10.1111/j.1745-4557.2007.00147.x]
El-Moneim A., Eman A.A., El-Gioushy S.F., Baiea M.H.M. (2015). Effect of some natural coating materials on storability and fruit quality of Zaghloul date palm cv. under cold storage. Middle East Journal of Agriculture Research. 4: 602-612.
Eshghi S., Karimi R., Shiri A., Karami M., Moradi M. (2022). Effects of polysaccharide-based coatings on postharvest storage life of grape: measuring the changes in nutritional, antioxidant and phenolic compounds. Journal of Food Measurement and Characterization. 16: 1159-1170. [DOI: 10.1007/s11694-021-01275-0]
Garavaglia J., Markoski M.M., Oliveira A., Marcadenti A. (2016). Grape seed oil compounds: biological and chemical actions for health. Nutrition and Metabolic Insights. 9: 59-64. [DOI: 10.4137/NMI.S32910]
Ghafoor K., Al-Juhaimi F.Y., Babiker E.E., Mohamed Ahmed I.A., Shahzad S.A., Alsawmahi O.N. (2022). Quality attributes of refrigerated Barhi dates coated with edible chitosan containing natural functional ingredients. Foods. 11: 1584. [DOI: 10.3390/foods11111584]
Hamedi H., Moradi S., Tonelli A.E., Hudson S.M. (2019). Preparation and characterization of chitosan–alginate polyelectrolyte complexes loaded with antibacterial thyme oil nanoemulsions. Applied Sciences. 9: 3933. [DOI: 10.3390/app9183933]
Han J.-W., Ruiz-Garcia L., Qian J.-P., Yang X.-T. (2018). Food packaging: a comprehensive review and future trends. Comprehensive Reviews in Food Science and Food Safety 17: 860-877. [DOI: 10.1111/1541-4337.12343]
Harbeoui H., Bettaieb Rebey I., Ouerghemmi I., Aidi Wannes W., Zemni H., Zoghlami N., Khan N.A., Ksouri R., Tounsi M.S. (2018). Biochemical characterization and antioxidant activity of grape (Vitis vinifera L.) seed oils from nine Tunisian varieties. Journal of Food Biochemistry. 42: e12595. [DOI: 10.1111/jfbc.12595]
Jafari S., Jouki M., Soltani M. (2021). Modification of physicochemical, structural, rheological, and organoleptic properties of sweetened condensed milk by maltodextrin, fructose, and lactose. Journal of Food Measurement and Characterization. 15: 3800-3810. [DOI: 10.1007/s11694-021-00976-w]
Lee S.K., Mbwambo Z.H., Chung H., Luyengi L., Gamez E.J., Mehta R.G., Kinghorn A.D., Pezzuto J.M. (1998). Evaluation of the antioxidant potential of natural products. Combinatorial Chemistry and High Throughput Screening. 1: 35-46.
Maftoonazad N., Ramaswamy H.S. (2005). Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT - Food Science and Technology. 38: 617-624. [DOI: 10.1016/j.lwt.2004.08.007]
Maftoonazad N., Ramaswamy H.S., Marcotte M. (2008). Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. International Journal of Food Science and Technology. 43: 951-957. [DOI: 10.1111/j.1365-2621.2006.01444.x]
Mannozzi C., Cecchini J.P., Tylewicz U., Siroli L., Patrignani F., Lanciotti R., Rocculi P., Dalla Rosa M., Romani S. (2017). Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT - Food Science and Technology. 85: 440-444. [DOI: 10.1016/j.lwt.2016.12.056]
Mutlu N. (2023). Effects of grape seed oil nanoemulsion on physicochemical and antibacterial properties of gelatin-sodium alginate film blends. International Journal of Biological Macromolecules. 237: 124207. [DOI: 10.1016/j.ijbiomac. 2023.124207]
National Centre for Statistics and Information (NCSI). (2024). Statistical year book. 52. Sultanate of Oman. URL: https://www.ncsi.gov.om/Elibrary/Pages/PublicationDetails.aspx?ItemID=Rvd6e2TXWs61qzB1LQKG6A%3D%3D. Accecced 15 May 2025.
Pandey V.K., Islam R.U., Shams R., Dar A.H. (2022). A comprehensive review on the application of essential oils as bioactive compounds in nano-emulsion based edible coatings of fruits and vegetables. Applied Food Research. 2: 100042. [DOI: 10.1016/j.afres.2022.100042]
Popescu P.-A., Palade L.M., Nicolae I.-C., Popa E.E., Miteluț A.C., Drăghici M.C., Matei F., Popa M.E. (2022). Chitosan-based edible coatings containing essential oils to preserve the shelf life and postharvest quality parameters of organic strawberries and apples during cold storage. Foods. 11: 3317. [DOI: 10.3390/foods11213317]
Ramos M., Mellinas C., Solaberrieta I., Garrigós M.C., Jiménez A. (2021). Emulsions incorporated in polysaccharide-based active coatings for fresh and minimally processed vegetables. Foods. 10: 665. [DOI: 10.3390/foods10030665]
Singleton V.L., Rossi J.A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16: 144-158. [DOI: 10.5344/ajev.1965.16.3.144]
Tian F., Woo S.Y., Lee S.Y., Park S.B., Zheng Y., Chun H.S. (2022). Antifungal activity of essential oil and plant-derived natural compounds against Aspergillus flavus. Antibiotics. 11: 1727. [DOI: 10.3390/antibiotics11121727]
Velickova E., Winkelhausen E., Kuzmanova S., Alves V.D., Moldão-Martins M. (2013). Impact of chitosan–beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv. Camarosa) under commercial storage conditions. LWT - Food Science and Technology. 52: 80-92. [DOI: 10.1016/j.lwt. 2013.02.004]
Wang L.-F., Rhim J.-W. (2016). Grapefruit seed extract incorporated antimicrobial LDPE and PLA films: effect of type of polymer matrix. LWT - Food Science and Technology. 74: 338-345. [DOI: 10.1016/j.lwt.2016.07.066]
Yu H., Park J.-Y., Kwon C.W., Hong S.-C., Park K.-M., Chang P.-S. (2018). An overview of nanotechnology in food science: preparative methods, practical applications, and safety. Journal of Chemistry. 2018. [DOI: 10.1155/2018/5427978]
Adiletta G., Zampella L., Coletta C., Petriccione M. (2019). Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture. 9: 84. [DOI: 10.3390/agriculture9040084]
Association of Official Analytical Chemists (AOAC). (2023). Official Methods of Analysis. 18th edition. AOAC International. Gaithersburg, USA. URL: https://www.aoac.org/official-methods-of-analysis/. Accecced 15 May 2025.
Aswathanarayan J.B., Anandan R., Mathew S., Ganesan B. (2019). Nanoemulsions: preparation techniques and applications in food systems. Current Nutrition and Food Science. 15: 104-115. [DOI: 10.2174/1573401314666181113162155]
Butnaru E., Stoleru E., Brebu M.A., Darie-Nita R.N., Bargan A., Vasile C. (2019). Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials. 12: 373. [DOI: 10.3390/ma12030373]
Chen C., Peng X., Zeng R., Chen M., Wan C., Chen J. (2016). Ficus hirta fruits extract incorporated into an alginate-based edible coating for nanfeng mandarin preservation. Scientia Horticulturae. 202: 41-48. [DOI: 10.1016/j.scienta.2015.12.046]
Chen J., Thilakarathna W.P.D.W., Astatkie T., Rupasinghe H.P.V. (2020). Optimization of catechin and proanthocyanidin recovery from grape seeds using microwave-assisted extraction. Biomolecules. 10: 243. [DOI: 10.3390/biom10020243]
De Oliveira Filho J.G., Miranda M., Ferreira M.D., Plotto A. (2021). Nanoemulsions as edible coatings: a potential strategy for fresh fruits and vegetables preservation. Foods. 10: 2438. [DOI: 10.3390/foods10102438]
Eissa H.A.A. (2007). Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. Journal of Food Quality. 30: 623-645. [DOI: 10.1111/j.1745-4557.2007.00147.x]
El-Moneim A., Eman A.A., El-Gioushy S.F., Baiea M.H.M. (2015). Effect of some natural coating materials on storability and fruit quality of Zaghloul date palm cv. under cold storage. Middle East Journal of Agriculture Research. 4: 602-612.
Eshghi S., Karimi R., Shiri A., Karami M., Moradi M. (2022). Effects of polysaccharide-based coatings on postharvest storage life of grape: measuring the changes in nutritional, antioxidant and phenolic compounds. Journal of Food Measurement and Characterization. 16: 1159-1170. [DOI: 10.1007/s11694-021-01275-0]
Garavaglia J., Markoski M.M., Oliveira A., Marcadenti A. (2016). Grape seed oil compounds: biological and chemical actions for health. Nutrition and Metabolic Insights. 9: 59-64. [DOI: 10.4137/NMI.S32910]
Ghafoor K., Al-Juhaimi F.Y., Babiker E.E., Mohamed Ahmed I.A., Shahzad S.A., Alsawmahi O.N. (2022). Quality attributes of refrigerated Barhi dates coated with edible chitosan containing natural functional ingredients. Foods. 11: 1584. [DOI: 10.3390/foods11111584]
Hamedi H., Moradi S., Tonelli A.E., Hudson S.M. (2019). Preparation and characterization of chitosan–alginate polyelectrolyte complexes loaded with antibacterial thyme oil nanoemulsions. Applied Sciences. 9: 3933. [DOI: 10.3390/app9183933]
Han J.-W., Ruiz-Garcia L., Qian J.-P., Yang X.-T. (2018). Food packaging: a comprehensive review and future trends. Comprehensive Reviews in Food Science and Food Safety 17: 860-877. [DOI: 10.1111/1541-4337.12343]
Harbeoui H., Bettaieb Rebey I., Ouerghemmi I., Aidi Wannes W., Zemni H., Zoghlami N., Khan N.A., Ksouri R., Tounsi M.S. (2018). Biochemical characterization and antioxidant activity of grape (Vitis vinifera L.) seed oils from nine Tunisian varieties. Journal of Food Biochemistry. 42: e12595. [DOI: 10.1111/jfbc.12595]
Jafari S., Jouki M., Soltani M. (2021). Modification of physicochemical, structural, rheological, and organoleptic properties of sweetened condensed milk by maltodextrin, fructose, and lactose. Journal of Food Measurement and Characterization. 15: 3800-3810. [DOI: 10.1007/s11694-021-00976-w]
Lee S.K., Mbwambo Z.H., Chung H., Luyengi L., Gamez E.J., Mehta R.G., Kinghorn A.D., Pezzuto J.M. (1998). Evaluation of the antioxidant potential of natural products. Combinatorial Chemistry and High Throughput Screening. 1: 35-46.
Maftoonazad N., Ramaswamy H.S. (2005). Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT - Food Science and Technology. 38: 617-624. [DOI: 10.1016/j.lwt.2004.08.007]
Maftoonazad N., Ramaswamy H.S., Marcotte M. (2008). Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. International Journal of Food Science and Technology. 43: 951-957. [DOI: 10.1111/j.1365-2621.2006.01444.x]
Mannozzi C., Cecchini J.P., Tylewicz U., Siroli L., Patrignani F., Lanciotti R., Rocculi P., Dalla Rosa M., Romani S. (2017). Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT - Food Science and Technology. 85: 440-444. [DOI: 10.1016/j.lwt.2016.12.056]
Mutlu N. (2023). Effects of grape seed oil nanoemulsion on physicochemical and antibacterial properties of gelatin-sodium alginate film blends. International Journal of Biological Macromolecules. 237: 124207. [DOI: 10.1016/j.ijbiomac. 2023.124207]
National Centre for Statistics and Information (NCSI). (2024). Statistical year book. 52. Sultanate of Oman. URL: https://www.ncsi.gov.om/Elibrary/Pages/PublicationDetails.aspx?ItemID=Rvd6e2TXWs61qzB1LQKG6A%3D%3D. Accecced 15 May 2025.
Pandey V.K., Islam R.U., Shams R., Dar A.H. (2022). A comprehensive review on the application of essential oils as bioactive compounds in nano-emulsion based edible coatings of fruits and vegetables. Applied Food Research. 2: 100042. [DOI: 10.1016/j.afres.2022.100042]
Popescu P.-A., Palade L.M., Nicolae I.-C., Popa E.E., Miteluț A.C., Drăghici M.C., Matei F., Popa M.E. (2022). Chitosan-based edible coatings containing essential oils to preserve the shelf life and postharvest quality parameters of organic strawberries and apples during cold storage. Foods. 11: 3317. [DOI: 10.3390/foods11213317]
Ramos M., Mellinas C., Solaberrieta I., Garrigós M.C., Jiménez A. (2021). Emulsions incorporated in polysaccharide-based active coatings for fresh and minimally processed vegetables. Foods. 10: 665. [DOI: 10.3390/foods10030665]
Singleton V.L., Rossi J.A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16: 144-158. [DOI: 10.5344/ajev.1965.16.3.144]
Tian F., Woo S.Y., Lee S.Y., Park S.B., Zheng Y., Chun H.S. (2022). Antifungal activity of essential oil and plant-derived natural compounds against Aspergillus flavus. Antibiotics. 11: 1727. [DOI: 10.3390/antibiotics11121727]
Velickova E., Winkelhausen E., Kuzmanova S., Alves V.D., Moldão-Martins M. (2013). Impact of chitosan–beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv. Camarosa) under commercial storage conditions. LWT - Food Science and Technology. 52: 80-92. [DOI: 10.1016/j.lwt. 2013.02.004]
Wang L.-F., Rhim J.-W. (2016). Grapefruit seed extract incorporated antimicrobial LDPE and PLA films: effect of type of polymer matrix. LWT - Food Science and Technology. 74: 338-345. [DOI: 10.1016/j.lwt.2016.07.066]
Yu H., Park J.-Y., Kwon C.W., Hong S.-C., Park K.-M., Chang P.-S. (2018). An overview of nanotechnology in food science: preparative methods, practical applications, and safety. Journal of Chemistry. 2018. [DOI: 10.1155/2018/5427978]
* Corresponding author (M.A. Al-Farsi)
* E-mail: malfarsi@unizwa.edu.om
ORCID ID: https://orcid.org/0000-0002-5694-9691
* E-mail: malfarsi@unizwa.edu.om
ORCID ID: https://orcid.org/0000-0002-5694-9691
Type of Study: Original article |
Subject:
Special
Received: 24/12/23 | Accepted: 25/06/05 | Published: 25/06/22
Received: 24/12/23 | Accepted: 25/06/05 | Published: 25/06/22
References
1. Adiamo O.Q., Ghafoor K., Al-Juhaimi F., Mohamed Ahmed I.A., Babiker E.E. (2017). Effects of thermosonication and orange by-products extracts on quality attributes of carrot (Daucus carota) juice during storage. International Journal of Food Science and Technology. 52: 2115-2125. [DOI: 10.1111/ijfs.13490] [DOI:10.1111/ijfs.13490]
2. Adiletta G., Zampella L., Coletta C., Petriccione M. (2019). Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture. 9: 84. [DOI: 10.3390/agriculture9040084] [DOI:10.3390/agriculture9040084]
3. Association of Official Analytical Chemists (AOAC). (2023). Official Methods of Analysis. 18th edition. AOAC International. Gaithersburg, USA. URL: https://www.aoac.org/official-methods-of-analysis/. Accecced 15 May 2025.
4. Aswathanarayan J.B., Anandan R., Mathew S., Ganesan B. (2019). Nanoemulsions: preparation techniques and applications in food systems. Current Nutrition and Food Science. 15: 104-115. [DOI: 10.2174/1573401314666181113162155] [DOI:10.3389/fsufs.2019.00095]
5. Butnaru E., Stoleru E., Brebu M.A., Darie-Nita R.N., Bargan A., Vasile C. (2019). Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials. 12: 373. [DOI: 10.3390/ma12030373] [DOI:10.3390/ma12030373] [PMID] [PMCID]
6. Chen C., Peng X., Zeng R., Chen M., Wan C., Chen J. (2016). Ficus hirta fruits extract incorporated into an alginate-based edible coating for nanfeng mandarin preservation. Scientia Horticulturae. 202: 41-48. [DOI: 10.1016/j.scienta.2015.12.046] [DOI:10.1016/j.scienta.2015.12.046]
7. Chen J., Thilakarathna W.P.D.W., Astatkie T., Rupasinghe H.P.V. (2020). Optimization of catechin and proanthocyanidin recovery from grape seeds using microwave-assisted extraction. Biomolecules. 10: 243. [DOI: 10.3390/biom10020243] [DOI:10.3390/biom10020243] [PMID] [PMCID]
8. De Oliveira Filho J.G., Miranda M., Ferreira M.D., Plotto A. (2021). Nanoemulsions as edible coatings: a potential strategy for fresh fruits and vegetables preservation. Foods. 10: 2438. [DOI: 10.3390/foods10102438] [DOI:10.3390/foods10102438] [PMID] [PMCID]
9. Eissa H.A.A. (2007). Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. Journal of Food Quality. 30: 623-645. [DOI: 10.1111/j.1745-4557.2007.00147.x] [DOI:10.1111/j.1745-4557.2007.00147.x]
10. El-Moneim A., Eman A.A., El-Gioushy S.F., Baiea M.H.M. (2015). Effect of some natural coating materials on storability and fruit quality of Zaghloul date palm cv. under cold storage. Middle East Journal of Agriculture Research. 4: 602-612.
11. Eshghi S., Karimi R., Shiri A., Karami M., Moradi M. (2022). Effects of polysaccharide-based coatings on postharvest storage life of grape: measuring the changes in nutritional, antioxidant and phenolic compounds. Journal of Food Measurement and Characterization. 16: 1159-1170. [DOI: 10.1007/s11694-021-01275-0] [DOI:10.1007/s11694-021-01275-0] [PMCID]
12. Garavaglia J., Markoski M.M., Oliveira A., Marcadenti A. (2016). Grape seed oil compounds: biological and chemical actions for health. Nutrition and Metabolic Insights. 9: 59-64. [DOI: 10.4137/NMI.S32910] [DOI:10.4137/NMI.S32910] [PMID] [PMCID]
13. Ghafoor K., Al-Juhaimi F.Y., Babiker E.E., Mohamed Ahmed I.A., Shahzad S.A., Alsawmahi O.N. (2022). Quality attributes of refrigerated Barhi dates coated with edible chitosan containing natural functional ingredients. Foods. 11: 1584. [DOI: 10.3390/foods11111584] [DOI:10.3390/foods11111584] [PMID] [PMCID]
14. Hamedi H., Moradi S., Tonelli A.E., Hudson S.M. (2019). Preparation and characterization of chitosan-alginate polyelectrolyte complexes loaded with antibacterial thyme oil nanoemulsions. Applied Sciences. 9: 3933. [DOI: 10.3390/app9183933] [DOI:10.3390/app9183933]
15. Han J.-W., Ruiz-Garcia L., Qian J.-P., Yang X.-T. (2018). Food packaging: a comprehensive review and future trends. Comprehensive Reviews in Food Science and Food Safety 17: 860-877. [DOI: 10.1111/1541-4337.12343] [DOI:10.1111/1541-4337.12343] [PMID]
16. Harbeoui H., Bettaieb Rebey I., Ouerghemmi I., Aidi Wannes W., Zemni H., Zoghlami N., Khan N.A., Ksouri R., Tounsi M.S. (2018). Biochemical characterization and antioxidant activity of grape (Vitis vinifera L.) seed oils from nine Tunisian varieties. Journal of Food Biochemistry. 42: e12595. [DOI: 10.1111/jfbc.12595] [DOI:10.1111/jfbc.12595]
17. Jafari S., Jouki M., Soltani M. (2021). Modification of physicochemical, structural, rheological, and organoleptic properties of sweetened condensed milk by maltodextrin, fructose, and lactose. Journal of Food Measurement and Characterization. 15: 3800-3810. [DOI: 10.1007/s11694-021-00976-w] [DOI:10.1007/s11694-021-00976-w]
18. Lee S.K., Mbwambo Z.H., Chung H., Luyengi L., Gamez E.J., Mehta R.G., Kinghorn A.D., Pezzuto J.M. (1998). Evaluation of the antioxidant potential of natural products. Combinatorial Chemistry and High Throughput Screening. 1: 35-46. [PMID]
19. Maftoonazad N., Ramaswamy H.S. (2005). Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT - Food Science and Technology. 38: 617-624. [DOI: 10.1016/j.lwt.2004.08.007] [DOI:10.1016/j.lwt.2004.08.007]
20. Maftoonazad N., Ramaswamy H.S., Marcotte M. (2008). Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. International Journal of Food Science and Technology. 43: 951-957. [DOI: 10.1111/j.1365-2621.2006.01444.x] [DOI:10.1111/j.1365-2621.2006.01444.x]
21. Mannozzi C., Cecchini J.P., Tylewicz U., Siroli L., Patrignani F., Lanciotti R., Rocculi P., Dalla Rosa M., Romani S. (2017). Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT - Food Science and Technology. 85: 440-444. [DOI: 10.1016/j.lwt.2016.12.056] [DOI:10.1016/j.lwt.2016.12.056]
22. Mutlu N. (2023). Effects of grape seed oil nanoemulsion on physicochemical and antibacterial properties of gelatin-sodium alginate film blends. International Journal of Biological Macromolecules. 237: 124207. [DOI: 10.1016/j.ijbiomac. 2023.124207] [DOI:10.1016/j.ijbiomac.2023.124207] [PMID]
23. National Centre for Statistics and Information (NCSI). (2024). Statistical year book. 52. Sultanate of Oman. URL: https://www.ncsi.gov.om/Elibrary/Pages/PublicationDetails.aspx?ItemID=Rvd6e2TXWs61qzB1LQKG6A%3D%3D. Accecced 15 May 2025.
24. Pandey V.K., Islam R.U., Shams R., Dar A.H. (2022). A comprehensive review on the application of essential oils as bioactive compounds in nano-emulsion based edible coatings of fruits and vegetables. Applied Food Research. 2: 100042. [DOI: 10.1016/j.afres.2022.100042] [DOI:10.1016/j.afres.2022.100042]
25. Popescu P.-A., Palade L.M., Nicolae I.-C., Popa E.E., Miteluț A.C., Drăghici M.C., Matei F., Popa M.E. (2022). Chitosan-based edible coatings containing essential oils to preserve the shelf life and postharvest quality parameters of organic strawberries and apples during cold storage. Foods. 11: 3317. [DOI: 10.3390/foods11213317] [DOI:10.3390/foods11213317] [PMID] [PMCID]
26. Ramos M., Mellinas C., Solaberrieta I., Garrigós M.C., Jiménez A. (2021). Emulsions incorporated in polysaccharide-based active coatings for fresh and minimally processed vegetables. Foods. 10: 665. [DOI: 10.3390/foods10030665] [DOI:10.3390/foods10030665] [PMID] [PMCID]
27. Singleton V.L., Rossi J.A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16: 144-158. [DOI: 10.5344/ajev.1965.16.3.144] [DOI:10.5344/ajev.1965.16.3.144]
28. Tian F., Woo S.Y., Lee S.Y., Park S.B., Zheng Y., Chun H.S. (2022). Antifungal activity of essential oil and plant-derived natural compounds against Aspergillus flavus. Antibiotics. 11: 1727. [DOI: 10.3390/antibiotics11121727] [DOI:10.3390/antibiotics11121727] [PMID] [PMCID]
29. Velickova E., Winkelhausen E., Kuzmanova S., Alves V.D., Moldão-Martins M. (2013). Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv. Camarosa) under commercial storage conditions. LWT - Food Science and Technology. 52: 80-92. [DOI: 10.1016/j.lwt. 2013.02.004] [DOI:10.1016/j.lwt.2013.02.004]
30. Wang L.-F., Rhim J.-W. (2016). Grapefruit seed extract incorporated antimicrobial LDPE and PLA films: effect of type of polymer matrix. LWT - Food Science and Technology. 74: 338-345. [DOI: 10.1016/j.lwt.2016.07.066] [DOI:10.1016/j.lwt.2016.07.066]
31. Yu H., Park J.-Y., Kwon C.W., Hong S.-C., Park K.-M., Chang P.-S. (2018). An overview of nanotechnology in food science: preparative methods, practical applications, and safety. Journal of Chemistry. 2018. [DOI: 10.1155/2018/5427978] [DOI:10.1155/2018/5427978]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







