Volume 12, Issue 3 (September 2025)
J. Food Qual. Hazards Control 2025, 12(3): 163-170 |
Back to browse issues page
Ethics code: Not applicable.
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Thuy C, Anh L. The Effects of Coating Made from Chitosan, Nisin, and Tannin on Some Quality Indicators of Green-skin Pomelo (Citrus grandis) During Preservation. J. Food Qual. Hazards Control 2025; 12 (3) :163-170
URL: http://jfqhc.ssu.ac.ir/article-1-1316-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1316-en.html
Ho Chi Minh City University of Industry and Trade, 140 Le Trong Tan Street, Ho Chi Minh City, 0084, Vietnam , thuycx@huit.edu.vn
Full-Text [PDF 427 kb]
(383 Downloads)
| Abstract (HTML) (500 Views)
Full-Text: (30 Views)
The Effects of Coating Made from Chitosan, Nisin, and Tannin on Some Quality Indicators of Green-skin Pomelo (Citrus grandis) During Preservation
C.X. Thuy ** , L.T.H. Anh
Ho Chi Minh City University of Industry and Trade, 140 Le Trong Tan Street, Ho Chi Minh City, 0084, Vietnam
HIGHLIGHT
Table 1: Effects of additive mixtures of chitosan, nisin, and tannin on the spoilage rate of pomelos during storage
Table 2: Effects of additive mixtures of chitosan, nisin, and tannin on the Total Soluble Solids (TSS) of pomelos during preservation
Table 3: Effects of additive mixtures of chitosan, nisin, and tannin on the Total Acid (TA) of pomelos during preservation
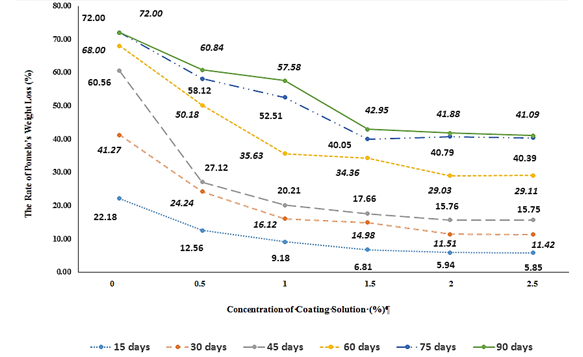
Figure 1: Effects of coating solution from chitosan, nisin, and tannin on pomelo weight loss during the preservation period
C.X. Thuy **
Ho Chi Minh City University of Industry and Trade, 140 Le Trong Tan Street, Ho Chi Minh City, 0084, Vietnam
- Coating made from chitosan, tannin, and nisin was effective in preserving pomelo.
- The most suitable concentration of the coating solution for preserving pomelo was 1.5%.
- After 90 days of preservation, pomelos experienced a 40% weight loss.
| Article type Original article |
ABSTRACT Background: Pomelos have high economic value; however if not properly preserved, their quality deteriorates rapidly. This study aimed to determine the effect of biofilms made from chitosan, nisin, and tannin at various concentrations by evaluating pomelo quality indicators during preservation. Methods: Each experimental batch was conducted on 20 ripe green-skin pomelos, collected from June to September 2024 in Mekong River Delta region, Vietnam. A mixture solution was prepared from chitosan, nisin, and tannin in a ratio of 8:1:1 at different concentrations (0.5, 1.0, 1.5, 2.0, and 2.5%) for coating. Pomelo quality indicators-including: spoilage rate, Total Soluble Solids, Total Acid (TA), and weight loss rate-were monitored at 15, 30, 45, 60, 75 and 90 days. Statistical analysis was performed using IBM SPSS Statistics 19 (IBM Corp., Armonk, NY, USA), based on the Tukey HSD. Results: After 90 days of preservation, the additive mixture at a concentration of 1.5% maintained the best quality indicators: the lowest spoilage rate of 22.20%; the highest TSS of 12.05 °Bx; TA remained stably low value at 0.68 g/100ml; and the lowest pomelo weight loss rate of 42.95%. Coating made from chitosan, tannin, and nisin (8:1:1) at a concentration of 0.15% was found to be the most suitable for maintaining pomelo quality. At this concentration, both the weight loss and spoilage rates reached the lowest value after 90 days of ambient storage; TSS increased steadily and stabilized; and TA gradually decreased during the presrevation period. Conclusion: The study findings provide valuable reference information for food manufacturers and traders in selecting appropriate storage conditions to ensure the optimal grapefruit quality. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Citrus grandis Chitosan Tannins |
||
| Article history Received: 5 Jan 2025 Revised: 30 Apr 2025 Accepted: 15 Sep 2025 |
||
| Abbreviations TA=Total Acid TSS=Total Soluble Solids |
To cite: Thuy C.X., Anh L.T.H. (2025). The effects of coating made from chitosan, nisin, and tannin on some quality indicators of green-skin pomelo (Citrus grandis) during preservation. Journal of Food Quality and Hazards Control. 12: 163-170.
Introduction
Introduction
Currently, there are various methods to preserve pomelos, including cold storage, chemical preservation, Polyvinyl Chloride (PVC) film, and biological membrane/biofilm preservation (Loi et al., 2023). Among these, biofilm preservation offers several advantages. It is simple, easy to implement, and applicable at both household and industrial scales (Kahramanoglu et al., 2020; Tietel et al., 2012). Biofilms can be made from a mixture of preservative additives such as polyethylene, carnauba wax (palm wax), chitosan, and tannin (Tuoi et al., 2021).To create an effective biofilm for pomelo preservation, it is essential to select an additive with strong film-forming ability, while other additives should have the capability to inhibit or destroy microorganisms or inhibit inside pomelo’s enzymes (Vargas et al., 2004).Chitosan, a polymer extracted from crustacean shells such as shrimp and crab, is known for its excellent film-forming properties and is almost non-toxic to humans (Saberi Riseh et al., 2024; Shiekh et al., 2013). It exhibits moisturizing, antifungal, and antibacterial effects (Loi et al., 2023). Tannin, a polyphenol found in many plants, binds firmly to proteins, thereby limiting enzyme activity (Fraga-Corral et al., 2021). In addition, tannin can form bonds with other high-molecular compounds such as amino acids and alkaloids. Tannin has astringent taste, high antibacterial ability, antifungal and antioxidant effects (Chien et al., 2007; Das et al., 2020). Nisin, a bacteriocin and hydrophobic peptide, is regarded as a potent anti-decay additive due to its ability to inhibit the growth of various spoilage-causing bacteria in fruits and vegetables (Petracek et al., 1998). While chitosan is effective at forming films, neither tannin nor nisin possesses this capability (Ali et al., 2011; Tran et al., 2020). Therefore, this study focuses on evaluating the combined effects of coatings made from chitosan and nisin at different concentrations on several quality indicators of post-harvest green-skinned pomelos during preservation.
Materials and methods
Materials and methods
Pomelo
The ripe green-skin pomelos (about 230 to 245 days from flowering) were purchased between June and September 2024 in Ben Tre province, Mekong River Delta, Vietnam. Each experimental batch was conducted on 20 ripe green-skin pomelos. The average pomelo weight was about 1.5-2.0 kg/fruit (Each experimental batch was 30-40 kg). The external skin color ranged from green to slightly yellowish green. The peel was thin, with a thickness varying from 4 to 18 mm. The pomelo segments were pink-red, and the taste was sweet or slightly sour, with Total Soluble Solids (TSS) ranging from 9.5 to 12.3 °Brix. On average, each fruit contained 35 to 40 seeds. After collection, the pomelos were stored at ambient temperatures (from 16 to 20 °C) before performing experiments.
Film-forming compounds
The biofilm was prepared from a mixture of additives including:
- Nisin: supplied by Sigma, USA. Nisin has an activity of 900 I.U/g (1 I.U is equivalent to 0.025 µg of nisin).
- Chitosan: purchased from Sigma (USA), in powder form with 99.7% purity, moisture content <10%, pH of 7.0-9.0, and molecular weight of 9-10 kDa.
- Tannin: purchased from Merck (Germany), in powder form with 98.8% purity.
Handling and treating the pomelos during experiments
After harvesting, green-skinned pomelos were selected and classified by size (from 1.5 to 2.0 kg/fruit). Only the intact fruits were transported by refrigerated truck (temperature <16 °C) to the laboratory. For each shipment, about 300-400 kg of green-skin pomelo (10 experimental batchs) were left to stabilize for 12 h in the laboratory. Next, the pomelos were rinsed under water tap and then sterilized by dipping them in a solution of thiabendazole and natrisunfit (200 ppm) at laboratory temperature for 3 min. Afterward, the water on the surface of the grapefruit was drained. Although, the outer surface of pomelo is relatively smooth and shiny, but it has many concave areas where dipping or spraying the additive mixture solution (comprising chitosan, tannin, and nisin), tends to either run off or accumulate unevenly. To ensure uniform distribution of the additive mixture solution on pomelo’s surface; a clean and soft cloth soaked in the solution was used to evenly rub the fruit peel 4-5 times, covering the entire outer surface. The pomelos were then naturally dried, and subsequently stored in the laboratory room (in a dry, cool, well-ventilated room). Samples were periodically taken for testing and analysis at designated intervals.
Preparation of the coating solution
The coating solution for preserving pomelos was prepared by mixing chitosan, nisin, and tannin in an 8:1:1 ratio, which has been found to yield optimum preservation effects. Solutions at varying concentrations were employed to create biofilms for the preservation of green-skinned pomelos (Loi et al., 2023).
Arranging the experiments
The preservative mixture solution was applied at different concentrations 0.5, 1.0, 1.5, 2.0, and 2.5% to form a biofilm on the outer skin of the green-skinned pomelos. Throughout the experiments, treated pomelo samples were compared with untreated controls (original pomelos not coated with the additive mixture solution). Each experimental batch consisted of 20 pomelos, and the experiment was repeated three times for replication.
After treatment with the additive mixture solution, the pomelos were stored at room temperature and monitored at intervals of 15, 30, 45, 60, 75, and 90 days. Four parameters were evaluated during the monitoring period: spoilage rate, TSS, Total Acid (TA), and weight loss rate.
Determining the spoilage rate of pomelos
Pomelos were inspected at each time point (from 15 to 90 days), and spoiled fruits were counted and removed. Rotting or spoiled pomelos were identified by the presence of rotten, soft, or blackened skin and watery, decayed segments inside. The rotting/spoilage rate (%) of green-skinned pomelos was calculated using the following formula (Tran et al., 2020):
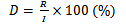
Where,
D: the spoilage rate (Visual assessment) in %
R: number of spoilage pomelos
I: total initial number of pomelos
Determining the TSS
- Principle: when light passes through a solution that contains various soluble solids, it is refracted at different angles. From this phenomina, the concentration of solids in the analyzed solution can be deduced. The measurement unit of soluble solids is “°Bx”. The TSS content is determined using an ATAGO N-1α refractometer (Japan). When measuring TSS, samples are prepared at a temperature of 20 to 22 °C (using thermostatic baths to keep the temperature of the samples stable).
- Implementation: pomelos were peeled, segmented, and seeds were removed. then the segments were pureed, and the pulp filtered to collect the juice. One to two drops of the juice were placed onto the glass surface of the ATAGO N-1α refractometer, which was then covered and read to determine the TSS value (Nguyen et al., 2014).
Determining the TA
The TA content of the green-skinned pomelos was measured according to Official Methods of Analysis - AOAC 942.15. This method was carried out on the principle of direct titration of the acids present in the samples using a sodium hydroxide solution with phenolphthalein as the indicator.
Determining pomelo weight loss rate
The rate of pomelo weight loss (%) was determined by weighing each pomelo in each experiment before and after storage period (according to timeline from 15 to 90 days). The weight loss rate was calculated using the formula below (Ahmed et al., 2018):
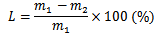
Where,
L: rate of pomelo weight loss (%)
m1: initial weight before storage (g)
m2: weight after storage (g)
- Nisin: supplied by Sigma, USA. Nisin has an activity of 900 I.U/g (1 I.U is equivalent to 0.025 µg of nisin).
- Chitosan: purchased from Sigma (USA), in powder form with 99.7% purity, moisture content <10%, pH of 7.0-9.0, and molecular weight of 9-10 kDa.
- Tannin: purchased from Merck (Germany), in powder form with 98.8% purity.
Handling and treating the pomelos during experiments
After harvesting, green-skinned pomelos were selected and classified by size (from 1.5 to 2.0 kg/fruit). Only the intact fruits were transported by refrigerated truck (temperature <16 °C) to the laboratory. For each shipment, about 300-400 kg of green-skin pomelo (10 experimental batchs) were left to stabilize for 12 h in the laboratory. Next, the pomelos were rinsed under water tap and then sterilized by dipping them in a solution of thiabendazole and natrisunfit (200 ppm) at laboratory temperature for 3 min. Afterward, the water on the surface of the grapefruit was drained. Although, the outer surface of pomelo is relatively smooth and shiny, but it has many concave areas where dipping or spraying the additive mixture solution (comprising chitosan, tannin, and nisin), tends to either run off or accumulate unevenly. To ensure uniform distribution of the additive mixture solution on pomelo’s surface; a clean and soft cloth soaked in the solution was used to evenly rub the fruit peel 4-5 times, covering the entire outer surface. The pomelos were then naturally dried, and subsequently stored in the laboratory room (in a dry, cool, well-ventilated room). Samples were periodically taken for testing and analysis at designated intervals.
Preparation of the coating solution
The coating solution for preserving pomelos was prepared by mixing chitosan, nisin, and tannin in an 8:1:1 ratio, which has been found to yield optimum preservation effects. Solutions at varying concentrations were employed to create biofilms for the preservation of green-skinned pomelos (Loi et al., 2023).
Arranging the experiments
The preservative mixture solution was applied at different concentrations 0.5, 1.0, 1.5, 2.0, and 2.5% to form a biofilm on the outer skin of the green-skinned pomelos. Throughout the experiments, treated pomelo samples were compared with untreated controls (original pomelos not coated with the additive mixture solution). Each experimental batch consisted of 20 pomelos, and the experiment was repeated three times for replication.
After treatment with the additive mixture solution, the pomelos were stored at room temperature and monitored at intervals of 15, 30, 45, 60, 75, and 90 days. Four parameters were evaluated during the monitoring period: spoilage rate, TSS, Total Acid (TA), and weight loss rate.
Determining the spoilage rate of pomelos
Pomelos were inspected at each time point (from 15 to 90 days), and spoiled fruits were counted and removed. Rotting or spoiled pomelos were identified by the presence of rotten, soft, or blackened skin and watery, decayed segments inside. The rotting/spoilage rate (%) of green-skinned pomelos was calculated using the following formula (Tran et al., 2020):
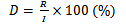
Where,
D: the spoilage rate (Visual assessment) in %
R: number of spoilage pomelos
I: total initial number of pomelos
Determining the TSS
- Principle: when light passes through a solution that contains various soluble solids, it is refracted at different angles. From this phenomina, the concentration of solids in the analyzed solution can be deduced. The measurement unit of soluble solids is “°Bx”. The TSS content is determined using an ATAGO N-1α refractometer (Japan). When measuring TSS, samples are prepared at a temperature of 20 to 22 °C (using thermostatic baths to keep the temperature of the samples stable).
- Implementation: pomelos were peeled, segmented, and seeds were removed. then the segments were pureed, and the pulp filtered to collect the juice. One to two drops of the juice were placed onto the glass surface of the ATAGO N-1α refractometer, which was then covered and read to determine the TSS value (Nguyen et al., 2014).
Determining the TA
The TA content of the green-skinned pomelos was measured according to Official Methods of Analysis - AOAC 942.15. This method was carried out on the principle of direct titration of the acids present in the samples using a sodium hydroxide solution with phenolphthalein as the indicator.
Determining pomelo weight loss rate
The rate of pomelo weight loss (%) was determined by weighing each pomelo in each experiment before and after storage period (according to timeline from 15 to 90 days). The weight loss rate was calculated using the formula below (Ahmed et al., 2018):
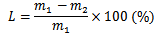
Where,
L: rate of pomelo weight loss (%)
m1: initial weight before storage (g)
m2: weight after storage (g)
Statistical analysis
In this study, the experiments were randomly arranged and repeated three times (p<0.05). Data were recorded and processed using Microsoft Excel software (Redmond, WA, USA), IBM SPSS Statistics software (19, IBM Corp., Armonk, NY, USA) was employed for statistical analysis based on the Tukey HSD.
Results and discussion
Spoilage rate of pomelos during storage
The experimental results demonstrating the effects of additive mixtures (chitosan, nisin, and tannin) at various concentrations on the spoilage rate of pomelos during storage are presented in Table 1.
Results and discussion
Spoilage rate of pomelos during storage
The experimental results demonstrating the effects of additive mixtures (chitosan, nisin, and tannin) at various concentrations on the spoilage rate of pomelos during storage are presented in Table 1.
Table 1: Effects of additive mixtures of chitosan, nisin, and tannin on the spoilage rate of pomelos during storage
| Concentrations of additive mixtures | Pomelos spoilage rate (%) | |||||
| 15 days | 30 days | 45 days | 60 days | 75 days | 90 days | |
| 0% | 17.18±0.68 a | 40.18±0.89 a | 65.32±0.92 a | 78.90±0.91 a | 89.99±0.88 a | 100±0.00 a |
| 0.5% | 15.03±0.51 b | 25.16±0.47 b | 29.32±0.71 b | 39.90±0.52 b | 45.75±0.58 b | 68.30±0.78 b |
| 1.0% | 10.21±0.32 c | 23.27±0.41 c | 24.11±0.56 c | 31.12±0.46 c | 35.42±0.47 c | 53.29±0.67 c |
| 1.5% | 4.21±0.11 d | 10.30±0.32 d | 13.78±0.32 d | 14.68±0.38 d | 16.42±0.36 d | 22.20±0.47 d |
| 2.0% | 4.34±0.12 d | 11.67±0.34 d | 15.56±0.55 e | 19.65±0.39 e | 23.92±0.41 e | 33.64±0.51 e |
| 2.5% | 8.99±0.09e | 18.59±0.58 e | 22.01±0.64 f | 29.12±0.52 f | 33.06±0.45 f | 49.37±0.71 f |
| Data are presented as mean±Standard Deviation (SD) (n=3), within each column, values with different superscript of a-f indicate significant differences(p<0.05) | ||||||
The results presented in Table 1 show that in the untreated control samples, the spoilage rate after 15 days of storage was relatively low (approximately 17%). However, by 30 days, the spoilage rate had risen sharply to 40.18%, and continued to increase rapidly at 45, 60, and 75 days.
Biofilms formed from additive mixture concentrations of 0.5% and 1.0% significantly reduced the spoilage rates of the pomelos. After 30 days of storage, the spoilage rate decreased noticeably in these treated samples. Furthermore, at extended storage periods, the 1.0% concentration proved more effective than 0.5% in preventing spoilage, especially between 75 and 90 days.
At higher preservative mixture concentrations of 1.5 and 2.0%, the biofilms were particularly effective in suppressing pomelo spoilage. Although the spoilage rate gradually increased with longer storage times, it remained relatively low even after 90 days. In contrast, at the highest concentration tested (2.5%), the spoilage rate after 90 days was comparatively high at 49.37%.These results can be explained as follows: for the control batch (additive mixture concentration of 0%) pomelos maintained some biological activities during the first 15 days of sotrage such as metabolism, physiological, and biochemical processes. These activities lead to the pomelo’s low spoilage rate. However, as the storage time prolonged, microorganisms developed rapidly, followed by the transformation and decomposition of pomelo nutrients. This intensification of degradation processes led to increased rotting over time. Consequently, by 90 days of storage, all pomelos in the control batch were spoiled. This observation aligns with previous studies on chitosan-based preservation of pomelos, which reported that without biofilm treatment, all fruits deteriorate after 12 weeks of storage (Nguyen et al., 2014). At the additive mixture concentrations of 0.5 and 1.0%, the components in the additive mixture (tannin and nisin) inhibited the growth of microorganisms but did not achieve complete suppression. As a result, the pomelos were significantly rotten. These findings concur with published data on rotting and spoilage rates in citrus fruits such as yellow grapefruit and orange, where chitosan and tannin mixtures at 0.5–1.0% concentrations resulted in approximately 12% spoilage after 2 weeks and about 50.05% after 10 weeks of storage (Pichaiyongvongdee et al., 2014; Sawant and Panhekar, 2017). At higher concentrations of 1.5% and 2.0%, the amount of nisin and tannin immobilized within the chitosan film was sufficient to effectively kill microorganisms and inhibit both nutrient metabolism and decomposition within the pomelo. Additionally, tannin suppressed certain enzymatic activities (Vargas et al., 2004), which enhanced preservation efficiency and maintained a very low spoilage rate. However, at the highest additive mixture concentration of 2.5%, the resulting biofilm was notably thick. While the preservation effect remained strong during shorter storage periods, prolonged storage caused water evaporated from the pomelos to become trapped under the dense biofilm. This condensation on the fruit surface fostered increased and accelerated rotting and spoilage at extended storage times (up to 90 days). This phenomenon is consistent with previously reported effects of chitosan–nisin biofilms (concentrations from 1.5 to 2.5%). Accordingly, the spoilage rate of pomelos after 10 to 12 weeks of storage was about 35-40% (Loi et. al., 2023; Sawant and Panhekar et al., 2017; Sirisomboon and Theamprateep, 2012).
The pomelo TSS during preservation
Table 2 presents the effects of additive mixtures composed of chitosan, nisin, and tannin at different concentrations on the TSS of pomelos during preservation.
Biofilms formed from additive mixture concentrations of 0.5% and 1.0% significantly reduced the spoilage rates of the pomelos. After 30 days of storage, the spoilage rate decreased noticeably in these treated samples. Furthermore, at extended storage periods, the 1.0% concentration proved more effective than 0.5% in preventing spoilage, especially between 75 and 90 days.
At higher preservative mixture concentrations of 1.5 and 2.0%, the biofilms were particularly effective in suppressing pomelo spoilage. Although the spoilage rate gradually increased with longer storage times, it remained relatively low even after 90 days. In contrast, at the highest concentration tested (2.5%), the spoilage rate after 90 days was comparatively high at 49.37%.These results can be explained as follows: for the control batch (additive mixture concentration of 0%) pomelos maintained some biological activities during the first 15 days of sotrage such as metabolism, physiological, and biochemical processes. These activities lead to the pomelo’s low spoilage rate. However, as the storage time prolonged, microorganisms developed rapidly, followed by the transformation and decomposition of pomelo nutrients. This intensification of degradation processes led to increased rotting over time. Consequently, by 90 days of storage, all pomelos in the control batch were spoiled. This observation aligns with previous studies on chitosan-based preservation of pomelos, which reported that without biofilm treatment, all fruits deteriorate after 12 weeks of storage (Nguyen et al., 2014). At the additive mixture concentrations of 0.5 and 1.0%, the components in the additive mixture (tannin and nisin) inhibited the growth of microorganisms but did not achieve complete suppression. As a result, the pomelos were significantly rotten. These findings concur with published data on rotting and spoilage rates in citrus fruits such as yellow grapefruit and orange, where chitosan and tannin mixtures at 0.5–1.0% concentrations resulted in approximately 12% spoilage after 2 weeks and about 50.05% after 10 weeks of storage (Pichaiyongvongdee et al., 2014; Sawant and Panhekar, 2017). At higher concentrations of 1.5% and 2.0%, the amount of nisin and tannin immobilized within the chitosan film was sufficient to effectively kill microorganisms and inhibit both nutrient metabolism and decomposition within the pomelo. Additionally, tannin suppressed certain enzymatic activities (Vargas et al., 2004), which enhanced preservation efficiency and maintained a very low spoilage rate. However, at the highest additive mixture concentration of 2.5%, the resulting biofilm was notably thick. While the preservation effect remained strong during shorter storage periods, prolonged storage caused water evaporated from the pomelos to become trapped under the dense biofilm. This condensation on the fruit surface fostered increased and accelerated rotting and spoilage at extended storage times (up to 90 days). This phenomenon is consistent with previously reported effects of chitosan–nisin biofilms (concentrations from 1.5 to 2.5%). Accordingly, the spoilage rate of pomelos after 10 to 12 weeks of storage was about 35-40% (Loi et. al., 2023; Sawant and Panhekar et al., 2017; Sirisomboon and Theamprateep, 2012).
The pomelo TSS during preservation
Table 2 presents the effects of additive mixtures composed of chitosan, nisin, and tannin at different concentrations on the TSS of pomelos during preservation.
Table 2: Effects of additive mixtures of chitosan, nisin, and tannin on the Total Soluble Solids (TSS) of pomelos during preservation
| Concentrations of additive mixtures (%) |
Total Soluble Solids (0Bx) | ||||||
| first day | 15 days | 30 days | 45 days | 60 days | 75 days | 90 days | |
| 0% | 10.08±0.22 a | 11.02±0.36 a | 11.18±0.37 a | 10.10±0.26 a | 9.35±0.21 a | 9.10±0.20 | - |
| 0.5% | 10.08±0.24 a | 10.93±0.34 b | 11.23±0.25 b | 11.20±0.28 b | 11.10±0.27 b | 11.05±0.29 b | 11.00±0.36 b |
| 1.0% | 10.08±0.23 a | 10.90±0.33 b | 11.33±0.26 c | 11.30±0.29 c | 10.28±0.23 c | 10.25±0.33 c | 11.23±0.38 c |
| 1.5% | 10.08±0.22 a | 10.99±0.35 c | 11.45±0.41 d | 11.59±0.31 d | 12.03±0.32 d | 12.05±0.31 d | 12.05±0.40 d |
| 2.0% | 10.08±0.24 a | 10.99±0.35 c | 11.44±0.41 d | 11.37±0.30 e | 11.32±0.29 e | 11.29±0.33 c | 11.25±0.38 c |
| 2,50% | 10.08±0.22 a | 10.98±0.35 c | 11.44±0.41 d | 11.30±0.29 c | 11.27±0.29 e | 11.22±0.32 c | 11.18±0.37 c |
| Data are presented as mean±Standard Deviation (SD) (n=3), within each column, values with different superscript of a-d indicate significant differences (p<0.05) | |||||||
At the additive mixture concentration of 0% (in the original samples or control batch), as the storage time was extended, TSS values decreased sharply. For all additive mixture concentrations, TSS initially increased during the first 30 days of preservation. However, with further extension of the storage period, samples treated with low additive concentrations of 0.5% and 1.0% exhibited a slight decline in TSS. Notably, at the 1.5% additive concentration, TSS demonstrated the greatest advantage throughout storage, reaching a peak value and stabilizing around 12.05 °Bx.
These results can be explained as follows: During the pomelo storage, TSS can increase or decrease depending on storage conditions (Kahramanoglu et al., 2020). In all experiments, at the initial preservative period (up to 30 days), TSS increased due to the hydrolysis of various components in the pomelo’s cell walls by various internal enzymes such as pectinase, cellulase, hemicellulose, and pectinesterase. These enzymes convert insoluble substances into soluble ones. This subject is consistent with the previous research findings (Cisneros-Zevallos and Krochta, 2003). When the preservative time was further extended (more than 30 days), TSS tended to decrease in the control batch and batches with low additive mixture concentrations (0.5 and 1.0%). Although tannin and nisin have anti-bacterial properties, their concentration is not high enough to completely inhibit microorganisms over long storage durations. Microorganisms fermented soluble sugars, leading to a significant reduction in TSS. This is consistent with some previous research results (Loi et al., 2023; Nguyen et al., 2022). These authors concluded that pomelos coated with biofilms made from chitosan and acetic acid at 0.5% to 1.0% concentrations exhibited TSS levels of approximately 10.04 °Bx after 90 days of storage. At a 1.5% additive mixture concentration, the content of tannin and nisin was sufficient to effectively suppress fermentative bacteria. Thus, the fermentation process was almost delayed, and TSS remained stable throughout storage. When the additive mixture concentrations were increased to 2.0 and 2.5%, although the microorganisms were completely inhibited, the thick biofilm itself prevented the evaporation of water from the pomelo, causing a slight decrease in TSS. This result is consistent with previous research evaluating the effects of chitosan film on TSS as well as antioxidant capacity of some bioactive substances in pomelos (Barrion et al., 2014).
Overall, an additive mixture concentration of 1.5% appears to be optimal for maintaining TSS during preservation as it allows a slight initial increase followed by stable TSS levels throughout storage.
TA of pomelos during preservation
TA content is one of the important quality criteria of pomelo during storage. TA largely determines the flavor and other sensory evaluation values of pomelo (Susanto et al., 2018; Tuoi et al., 2021). The changes in pomelo TA over the preservation period are presented in Table 3.
These results can be explained as follows: During the pomelo storage, TSS can increase or decrease depending on storage conditions (Kahramanoglu et al., 2020). In all experiments, at the initial preservative period (up to 30 days), TSS increased due to the hydrolysis of various components in the pomelo’s cell walls by various internal enzymes such as pectinase, cellulase, hemicellulose, and pectinesterase. These enzymes convert insoluble substances into soluble ones. This subject is consistent with the previous research findings (Cisneros-Zevallos and Krochta, 2003). When the preservative time was further extended (more than 30 days), TSS tended to decrease in the control batch and batches with low additive mixture concentrations (0.5 and 1.0%). Although tannin and nisin have anti-bacterial properties, their concentration is not high enough to completely inhibit microorganisms over long storage durations. Microorganisms fermented soluble sugars, leading to a significant reduction in TSS. This is consistent with some previous research results (Loi et al., 2023; Nguyen et al., 2022). These authors concluded that pomelos coated with biofilms made from chitosan and acetic acid at 0.5% to 1.0% concentrations exhibited TSS levels of approximately 10.04 °Bx after 90 days of storage. At a 1.5% additive mixture concentration, the content of tannin and nisin was sufficient to effectively suppress fermentative bacteria. Thus, the fermentation process was almost delayed, and TSS remained stable throughout storage. When the additive mixture concentrations were increased to 2.0 and 2.5%, although the microorganisms were completely inhibited, the thick biofilm itself prevented the evaporation of water from the pomelo, causing a slight decrease in TSS. This result is consistent with previous research evaluating the effects of chitosan film on TSS as well as antioxidant capacity of some bioactive substances in pomelos (Barrion et al., 2014).
Overall, an additive mixture concentration of 1.5% appears to be optimal for maintaining TSS during preservation as it allows a slight initial increase followed by stable TSS levels throughout storage.
TA of pomelos during preservation
TA content is one of the important quality criteria of pomelo during storage. TA largely determines the flavor and other sensory evaluation values of pomelo (Susanto et al., 2018; Tuoi et al., 2021). The changes in pomelo TA over the preservation period are presented in Table 3.
Table 3: Effects of additive mixtures of chitosan, nisin, and tannin on the Total Acid (TA) of pomelos during preservation
| Concentrations of additive mixtures (%) | Total Organic Acids (g/100 ml) | ||||||
| first day | 15 days | 30 days | 45 days | 60 days | 75 days | 90 days | |
| 0% | 0.82±0.03 a | 0.68±0.02 a | 0.67±0.02 a | 0.70±0.02 a | 0.84±0.04 a | 0.97±0.04 a | - |
| 0.5% | 0.82±0.03 a | 0.68±0.02 a | 0.72±0.03 b | 0.72±0.03 b | 0.74±0.03 b | 0.74±0.03 b | 0.78±0.04 a |
| 1.0% | 0.82±0.03 a | 0.67±0.01 a | 0.68±0.02 a | 0.70±0.02 a | 0.71±0.03 c | 0.73±0.03 b | 0.75±0.03 b |
| 1.5% | 0.82±0.03 a | 0.68±0.02 a | 0.67±0.02 a | 0.67±0.01 c | 0.68±0.02 d | 0.68±0.02 c | 0.68±0.02 c |
| 2.0% | 0.82±0.03 a | 0.69±0.02 a | 0.70±0.03 c | 0.71±0.03 d | 0.72±0.03 c | 0.72±0.03 d | 0.73±0.03 d |
| 2.5% | 0.82±0.03 a | 0.68±0.02 a | 0.70±0.03 c | 0.70±0.02 a | 0.72±0.03 c | 0.72±0.03 d | 0.73±0.03 d |
| Data are presented as mean±Standard Deviation (SD) (n=3), within each column, values with different superscript of a-d indicating significant differences (p<0.05) | |||||||
Table 3 shows that TA gradually decreased and then remained relatively stable or increased slightly during preservation (with the exception of the control batch). Specifically, in the first 45 days of preservation, TA tended to decrease in all experiments (at all concentration levels). This trend can be explained as follows: In the early stages of storage, pomelos begin to ripen, during which a certain amount of starch in pomelo is converted into sugars, making the fruit sweeter. This raises the pH in pomelo. As the pH increases, the bond between tannin and some alkaloids in pomelo weakens, leading to the release of some alkaloids into the surrounding environment. The released alkaloids react with organic acids, which reduces TA in pomelo. These indings are consistent with previous studies (Arnon et al., 2014; Xing et al., 2011), which assessed the impacts of other additives such as chitosan, polyethylene, acetic acid, and tannin on TA in various citrus fruits such as oranges, tangerines, and yellow pomelo.
When the storage period was prolonged to 60 and 75 days, TA (at mixture concentration of 0%) tended to increase significantly. Whereas at other concentrations, TA was significantly decreased before stabilizing. This pattern parallels results reported by Mayuoni et al. (2011) and Nie et al. (2020) who investigated the impact of polyethylene-supplemented biofilms on TA of some citrus fruits. This phenomenon can be explained as follows: after 15 days of preservation, in batches with low additive mixture concentrations (0.5 and 1.0%), the additives inhibited but did not completely eradicate microorganisms. The activity of anaerobic microorganisms facilitated the conversion of some sugars into organic acids, causing an increase in TA. Conversely, at higher concentrations (1.5%, 2.0%, and 2.5%), TA decreased during the initial 15 days of storage. Over extended preservation periods, microbial populations were fully suppressed, preventing fermentation and sugar-to-acid conversion. This resulted in the stabilization of TA levels. These findings align with previous research (Saberi Riseh et al., 2024; Tran et al., 2020).
pomelo weight loss rate during preservation
Weight loss is an inevitable problem during the preservation of pomelo, primarily caused by water evaporation, respiration, and physiological and biochemical changes in pomelos (Hossain et al., 2018). To maintain the stable quality of pomelos, it is necessary to find the best preservation methods that minimize weight loss. The effects of biofilms made from chitosan, nisin, and tannin on pomelo weight loss are presented in Figure 1.
The results presented in Figure 1 show that the rate of weight loss gradually decreases over the preservation period. Among all treatments, the control batch (0% coating solution) exhibited the highest weight loss at every storage interval. This can be explained by the high water content in pomelos: approximately 74.7% in the peel and up to 87.2% in the segments (Tuoi et al., 2021).
While the pomelo is still attached to the tree, the amount of water evaporated is continuously replenished by the water absorption from the roots. In the control batch, the weight loss reaches the highest value because the evaporation occurs most intensely.
When the storage period was prolonged to 60 and 75 days, TA (at mixture concentration of 0%) tended to increase significantly. Whereas at other concentrations, TA was significantly decreased before stabilizing. This pattern parallels results reported by Mayuoni et al. (2011) and Nie et al. (2020) who investigated the impact of polyethylene-supplemented biofilms on TA of some citrus fruits. This phenomenon can be explained as follows: after 15 days of preservation, in batches with low additive mixture concentrations (0.5 and 1.0%), the additives inhibited but did not completely eradicate microorganisms. The activity of anaerobic microorganisms facilitated the conversion of some sugars into organic acids, causing an increase in TA. Conversely, at higher concentrations (1.5%, 2.0%, and 2.5%), TA decreased during the initial 15 days of storage. Over extended preservation periods, microbial populations were fully suppressed, preventing fermentation and sugar-to-acid conversion. This resulted in the stabilization of TA levels. These findings align with previous research (Saberi Riseh et al., 2024; Tran et al., 2020).
pomelo weight loss rate during preservation
Weight loss is an inevitable problem during the preservation of pomelo, primarily caused by water evaporation, respiration, and physiological and biochemical changes in pomelos (Hossain et al., 2018). To maintain the stable quality of pomelos, it is necessary to find the best preservation methods that minimize weight loss. The effects of biofilms made from chitosan, nisin, and tannin on pomelo weight loss are presented in Figure 1.
The results presented in Figure 1 show that the rate of weight loss gradually decreases over the preservation period. Among all treatments, the control batch (0% coating solution) exhibited the highest weight loss at every storage interval. This can be explained by the high water content in pomelos: approximately 74.7% in the peel and up to 87.2% in the segments (Tuoi et al., 2021).
While the pomelo is still attached to the tree, the amount of water evaporated is continuously replenished by the water absorption from the roots. In the control batch, the weight loss reaches the highest value because the evaporation occurs most intensely.

Figure 1: Effects of coating solution from chitosan, nisin, and tannin on pomelo weight loss during the preservation period
As storage time increases, pomelos experience weight loss due to water evaporation and activities of microorganisms, leading to fruit wilting. In addition, the internal enzymes modify the structure of the pomelo segments (for example, pomelo become more porous, dry, fibrous, and etc.). According to some previous reports (Ali et al., 2011; Shiekh et al., 2013), Previous studies have reported that without chemical preservation methods (such as lime water treatment) or Polyvinyl Chloride (PVC) films, pomelo weight loss can range from 25.36% to 26.13% after just 15 days of storage At coating solution concentrations of 0.5 and 1.0%, the rate of pomelo’s weight loss was relatively high: after 90 days of storage, the loss rate exceeded 50%. This is because the film-forming ability of chitosan exerted its anti-evaporation effect. However, at this concentration, the amount of chitosan was low, so water loss was still relatively high. As a result, the pomelo continued to lose weight, but to a lesser extent than in the control batch. These findings are consistent with previously published results studying pomelo weight loss using biofilms made from chitosan and acetic acid (Chien et al., 2007; Cisneros-Zevallos and Krochta., 2003; Nguyen et al., 2014; Nie et al., 2020).
At the additive mixture concentrations from 1.5 to 2.5%, the weight loss rate remained stable even when the storage period was extended from 2 to 3 months. The reasons for this change are as follws: at these concentrations, the coating had a thickness enough to effectively prevent water evaporation. Moreover, the amount of tannin and nisin is large enough to inhibit or destroy a large number of microorganisms, leading to significantly reducing heat generation within pomelo. In this circumstance, the preservation bio-film not only prevented water loss, but also effectively destroyed microorganisms. Thus, the pomelo weight loss was significantly improved. However, at additive concentrations of 2.0 and 2.5%, the pomelo– spoilage rate was relatively high. Based on the above experimental results, an additive mixture concentration of 1.5% is the most suitable for minimizing pomelo weight loss. These findings are consistent with previously published studies on the weight loss of some citrus fruits during storage (Sawant and Panhekar et al., 2017; Petracek et al., 1998; Vargas et al., 2004).
Conclusions
The coating composed of chitosan, nisin, and tannin proved to be a highly effective method for pomelo preservation. The most suitable coating solution concentration was 1.5%. At this concentration, after 90 days of storage, the pomelos exhibited the lowest spoilage rate of 22.20%. TSS increased steadily before stabilizing at approximately 12.03 °Bx. TA reached a minimum value of 0.68 g/100 ml, and the weight loss rate was about 40% compared to the initial weight. Therefore, when preserving pomelos using a coating made from chitosan, tannin, and nisin, a 1.5% concentration is optimal for maintaining the best quality indicators.
Author contributions
C.X.T. contributed in designing the study, experimental work, data analysis, and writing of the manuscript; L.T.H.A. designed the study and experimental work. Both authors read and approved the final manuscript.
Acknowledgements
The authors would like to express sincere thanks to leaders of the Experimental Center, Ho Chi Minh City University of Industry and Trade; Mekong Delta Fruit Research Institute, Vietnam for supporting and allowing us to conduct the relevant experiments.
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Ethical consideration
Not applicable.
References
Ahmed W., Azmat R., Qayyum A., Mehmood A., Khan S.M., Liaquat M., Ahmed S., Moin S. (2018). The role of chitosan to prolonge the fresh fruit quality during storage of grapefruit cv. ray ruby. Pakistan Journal of Botany. 50: 151-159.
Ali A., Muhammad M.T.M., Sijam K., Siddiqui Y. (2011). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chemistry. 124: 620-626. [DOI: 10.1016/j.foodchem. 2010.06.085]
AOAC 942.15 Acidity Titratable of Fruit Products (1980). Official methods of analysis, online edition. Association of official analytical chemists, Washington, DC, USA.
Arnon H., Zaitsev Y., Porat R., Poverenov E. (2014). Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biology and Technology. 87: 21-26. [DOI: 10.1016/j.postharvbio. 2013.08.007]
Barrion A.S.A., Hurtada W.A., Papa I.A., Zulayvar T.O., Yee M.G. (2014). Phytochemical composition, antioxidant and antibacterial properties of pummelo (Citrus maxima (Burm.) Merr. Against Escherichia coli and Salmonella typhimurium. Food and Nutrition Sciences. 5: 749-758. [DOI: 10.4236/fns.2014.59085]
Chien P.-J., Sheu F., Lin H.-R. (2007). Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chemistry. 100: 1160-1164. [DOI: 10.1016/j.foodchem.2005.10.068]
Cisneros-Zevallos L., Krochta J.M. (2003). Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. Journal of Food Science. 68: 503-510. [DOI: 10.1111/j.1365-2621.2003.tb05702.x]
Das A.K., Islam M.N., Faruk M.O., Ashaduzzaman M., Dungani R. (2020). Review on tannins: extraction processes, applications and possibilities. South African Journal of Botany. 135: 58-70. [DOI: 10.1016/j.sajb.2020.08.008]
Fraga-Corral M., Otero P., Cassani L., Echave J., Garcia-Oliveira P., Carpena M., Chamorro F., Lourenço-Lopes C., Prieto M.A., Simal-Gandara J. (2021). Traditional applications of tannin rich extracts supported by scientific data: chemical composition, bioavailability and bioaccessibility. Foods. 10: 251-260. [DOI: 10.3390/foods10020251]
Hossain M.M., Disha R.F., Rahim M.A. (2018). Physio-morphological variations of pummelo genotype (Citrus grandis L. Osbeck). Advances in Horticultural Science. 32: 93-103. [DOI: 10.13128/ahs-21874].
Kahramanoglu I., Chen C., Gan Z., Chen J., Wan C. (2020). The effects of edible coatings on the postharvest quality of Citrus fruits as affected by granulation. Journal of Food Quality. 88:1-8. [DOI: 10.1155/2020/8819233]
Loi N.V., Binh P.T., Sam V.K. (2023). Change of chemical and biochemical indicators of Phuc Trach pomelo in the preservation process with chitosan-based coating with tannin and vinegar. Food Research. 7: 170-177. [DOI: 10.26656/fr.2017.7(2).167]
Mayuoni L., Tietel Z., Patil B.S., Porat R. (2011). Does ethylene degreening affect internal quality of citrus fruit? Postharvest Biology and Technology. 62: 50-58. [DOI: 10.1016/j. postharvbio.2011.04.005]
Nguyen D.T., Ha Q.V., Ta T.M. (2014). Research on the effect of concentration of chitosan on quality and preservation time of Doan Hung grapefruit (Citrus grandis Osbeck). Journal of Agriculture and Rural Development. 11: 80-83.
Nguyen N.H.K., Tran M.T., Le T.D., Nguyen M.V., Tran T.T. (2022). Chemical properties and biological properties of four varieties of pomelo (Citrus grandis (L) Osbeck) in the Mekong Delta of Vietnam. Food Research. 6: 267-272. [DOI: 10.26656/ fr.2017.6(4).479]
Nie Z., Huang Q., Chen C., Wan C., Chen J. (2020). Chitosan coating alleviates postharvest juice sac granulation by mitigating ROS accumulation in harvested pomelo (Citrus grandis L. Osbeck) during room temperature storage. Postharvest Biology and Technology. 169: 111309. [DOI: 10.1016/j.postharvbio. 2020.111309]
Petracek P.D., Dou H., Pao S. (1998). The influence of applied waxes on postharvest physiological behavior and pitting of grapefruit. Postharvest Biology and Technology. 14: 99-106. [DOI: 10.1016/S0925-5214(98)00018-0]
Pichaiyongvongdee S., Rattanapun B., Haruenkit R. (2014). Total polyphenol content and antioxidant properties in different tissues of seven pomelo (Citrus grandis (L.) osbeck) cultivars. Kasetsart Journal - Natural Science. 48: 989-996.
Saberi Riseh R., Vatankhah M., Hassanisaadi M., Shafiei-Hematabad Z., Kennedy J.F. (2024). Advancements in coating technologies: unveiling the potential of chitosan for the preservation of fruits and vegetables. International Journal of Biological Macromolecules. 254: 127677. [DOI: 10.1016/j.ijbiomac. 2023.127677]
Sawant T.P., Panhekar D. (2017). A brief reveiw on recent advances of Citrus maxima (Chakota). International Journal of Recent Scientific Research. 8: 19400-19416.
Shiekh R.A., Malik M.A., Al-Thabaiti S.A., Shiekh M.A. (2013). Chitosan as a novel edible coating for fresh fruits. Food Science Technology Research. 19: 139-155. [DOI: 10.3136/fstr.19.139]
Sirisomboon P., Theamprateep C. (2012). Physicochemical and textural properties of pomelo (Citrus maxima Merr. cv. Kao Nam Pueng) fruit at preharvest, postharvest and during the commercial harvest period. Philippine Agricultural Scientist. 95: 43-52.
Susanto S., Hermansah D., Amanda F. (2018). The growth and quality of fruit of three pummelo (Citrus maxima (Burn.) Merr.) accessions. IOP Conference Series: Earth and Environmental Science. 196: 012014. [DOI: 10.1088/1755-1315/196/1/012014]
Tietel Z., Lewinsohn E., Fallik E., Porat R. (2012). Importance of storage temperatures in maintaining flavor and quality of mandarins. Postharvest Biology and Technology. 64: 175-182. [DOI: 10.1016/j.postharvbio.2011.07.009]
Tran C.V., Mai T.P.T., Luong T.T., Son H.L. (2020). Study on the appropriate mixing ratio between chitosan and nisin to preserve post-harvest oranges at room temperate. Scientific Journal of Tan Trao University. 6: 47-53. [DOI: 10.51453/2354-1431/2020/387]
Tuoi N.T.K., Nguyen N.H.K., Truc T.T., Toan H.T. (2021). Physicochemical properties of green-skinned grapefruit and Nam Roi grapefruit grown in the Mekong Delta. Can Tho University Science Journal. 57: 118-126. [DOI: 10.22144/ctu.jsi.2021.013]
Vargas M., Gillabert M., Albors C., Chiralt A. (2004). Effect of film coating on fruit quality and changes. Journal of Food Science. 23: 746-753. [DOI: 10.1016/j.fochx.2024.101169]
Xing Y., Xu Q., Che Z., Li X., Li W. (2011). Effects of chitosan-oil coating on blue mold disease and quality attributes of jujube fruits. Food and Function. 2: 466-474. [DOI: 10.1039/ c1fo10073d]
At the additive mixture concentrations from 1.5 to 2.5%, the weight loss rate remained stable even when the storage period was extended from 2 to 3 months. The reasons for this change are as follws: at these concentrations, the coating had a thickness enough to effectively prevent water evaporation. Moreover, the amount of tannin and nisin is large enough to inhibit or destroy a large number of microorganisms, leading to significantly reducing heat generation within pomelo. In this circumstance, the preservation bio-film not only prevented water loss, but also effectively destroyed microorganisms. Thus, the pomelo weight loss was significantly improved. However, at additive concentrations of 2.0 and 2.5%, the pomelo– spoilage rate was relatively high. Based on the above experimental results, an additive mixture concentration of 1.5% is the most suitable for minimizing pomelo weight loss. These findings are consistent with previously published studies on the weight loss of some citrus fruits during storage (Sawant and Panhekar et al., 2017; Petracek et al., 1998; Vargas et al., 2004).
Conclusions
The coating composed of chitosan, nisin, and tannin proved to be a highly effective method for pomelo preservation. The most suitable coating solution concentration was 1.5%. At this concentration, after 90 days of storage, the pomelos exhibited the lowest spoilage rate of 22.20%. TSS increased steadily before stabilizing at approximately 12.03 °Bx. TA reached a minimum value of 0.68 g/100 ml, and the weight loss rate was about 40% compared to the initial weight. Therefore, when preserving pomelos using a coating made from chitosan, tannin, and nisin, a 1.5% concentration is optimal for maintaining the best quality indicators.
Author contributions
C.X.T. contributed in designing the study, experimental work, data analysis, and writing of the manuscript; L.T.H.A. designed the study and experimental work. Both authors read and approved the final manuscript.
Acknowledgements
The authors would like to express sincere thanks to leaders of the Experimental Center, Ho Chi Minh City University of Industry and Trade; Mekong Delta Fruit Research Institute, Vietnam for supporting and allowing us to conduct the relevant experiments.
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Ethical consideration
Not applicable.
References
Ahmed W., Azmat R., Qayyum A., Mehmood A., Khan S.M., Liaquat M., Ahmed S., Moin S. (2018). The role of chitosan to prolonge the fresh fruit quality during storage of grapefruit cv. ray ruby. Pakistan Journal of Botany. 50: 151-159.
Ali A., Muhammad M.T.M., Sijam K., Siddiqui Y. (2011). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chemistry. 124: 620-626. [DOI: 10.1016/j.foodchem. 2010.06.085]
AOAC 942.15 Acidity Titratable of Fruit Products (1980). Official methods of analysis, online edition. Association of official analytical chemists, Washington, DC, USA.
Arnon H., Zaitsev Y., Porat R., Poverenov E. (2014). Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biology and Technology. 87: 21-26. [DOI: 10.1016/j.postharvbio. 2013.08.007]
Barrion A.S.A., Hurtada W.A., Papa I.A., Zulayvar T.O., Yee M.G. (2014). Phytochemical composition, antioxidant and antibacterial properties of pummelo (Citrus maxima (Burm.) Merr. Against Escherichia coli and Salmonella typhimurium. Food and Nutrition Sciences. 5: 749-758. [DOI: 10.4236/fns.2014.59085]
Chien P.-J., Sheu F., Lin H.-R. (2007). Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chemistry. 100: 1160-1164. [DOI: 10.1016/j.foodchem.2005.10.068]
Cisneros-Zevallos L., Krochta J.M. (2003). Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. Journal of Food Science. 68: 503-510. [DOI: 10.1111/j.1365-2621.2003.tb05702.x]
Das A.K., Islam M.N., Faruk M.O., Ashaduzzaman M., Dungani R. (2020). Review on tannins: extraction processes, applications and possibilities. South African Journal of Botany. 135: 58-70. [DOI: 10.1016/j.sajb.2020.08.008]
Fraga-Corral M., Otero P., Cassani L., Echave J., Garcia-Oliveira P., Carpena M., Chamorro F., Lourenço-Lopes C., Prieto M.A., Simal-Gandara J. (2021). Traditional applications of tannin rich extracts supported by scientific data: chemical composition, bioavailability and bioaccessibility. Foods. 10: 251-260. [DOI: 10.3390/foods10020251]
Hossain M.M., Disha R.F., Rahim M.A. (2018). Physio-morphological variations of pummelo genotype (Citrus grandis L. Osbeck). Advances in Horticultural Science. 32: 93-103. [DOI: 10.13128/ahs-21874].
Kahramanoglu I., Chen C., Gan Z., Chen J., Wan C. (2020). The effects of edible coatings on the postharvest quality of Citrus fruits as affected by granulation. Journal of Food Quality. 88:1-8. [DOI: 10.1155/2020/8819233]
Loi N.V., Binh P.T., Sam V.K. (2023). Change of chemical and biochemical indicators of Phuc Trach pomelo in the preservation process with chitosan-based coating with tannin and vinegar. Food Research. 7: 170-177. [DOI: 10.26656/fr.2017.7(2).167]
Mayuoni L., Tietel Z., Patil B.S., Porat R. (2011). Does ethylene degreening affect internal quality of citrus fruit? Postharvest Biology and Technology. 62: 50-58. [DOI: 10.1016/j. postharvbio.2011.04.005]
Nguyen D.T., Ha Q.V., Ta T.M. (2014). Research on the effect of concentration of chitosan on quality and preservation time of Doan Hung grapefruit (Citrus grandis Osbeck). Journal of Agriculture and Rural Development. 11: 80-83.
Nguyen N.H.K., Tran M.T., Le T.D., Nguyen M.V., Tran T.T. (2022). Chemical properties and biological properties of four varieties of pomelo (Citrus grandis (L) Osbeck) in the Mekong Delta of Vietnam. Food Research. 6: 267-272. [DOI: 10.26656/ fr.2017.6(4).479]
Nie Z., Huang Q., Chen C., Wan C., Chen J. (2020). Chitosan coating alleviates postharvest juice sac granulation by mitigating ROS accumulation in harvested pomelo (Citrus grandis L. Osbeck) during room temperature storage. Postharvest Biology and Technology. 169: 111309. [DOI: 10.1016/j.postharvbio. 2020.111309]
Petracek P.D., Dou H., Pao S. (1998). The influence of applied waxes on postharvest physiological behavior and pitting of grapefruit. Postharvest Biology and Technology. 14: 99-106. [DOI: 10.1016/S0925-5214(98)00018-0]
Pichaiyongvongdee S., Rattanapun B., Haruenkit R. (2014). Total polyphenol content and antioxidant properties in different tissues of seven pomelo (Citrus grandis (L.) osbeck) cultivars. Kasetsart Journal - Natural Science. 48: 989-996.
Saberi Riseh R., Vatankhah M., Hassanisaadi M., Shafiei-Hematabad Z., Kennedy J.F. (2024). Advancements in coating technologies: unveiling the potential of chitosan for the preservation of fruits and vegetables. International Journal of Biological Macromolecules. 254: 127677. [DOI: 10.1016/j.ijbiomac. 2023.127677]
Sawant T.P., Panhekar D. (2017). A brief reveiw on recent advances of Citrus maxima (Chakota). International Journal of Recent Scientific Research. 8: 19400-19416.
Shiekh R.A., Malik M.A., Al-Thabaiti S.A., Shiekh M.A. (2013). Chitosan as a novel edible coating for fresh fruits. Food Science Technology Research. 19: 139-155. [DOI: 10.3136/fstr.19.139]
Sirisomboon P., Theamprateep C. (2012). Physicochemical and textural properties of pomelo (Citrus maxima Merr. cv. Kao Nam Pueng) fruit at preharvest, postharvest and during the commercial harvest period. Philippine Agricultural Scientist. 95: 43-52.
Susanto S., Hermansah D., Amanda F. (2018). The growth and quality of fruit of three pummelo (Citrus maxima (Burn.) Merr.) accessions. IOP Conference Series: Earth and Environmental Science. 196: 012014. [DOI: 10.1088/1755-1315/196/1/012014]
Tietel Z., Lewinsohn E., Fallik E., Porat R. (2012). Importance of storage temperatures in maintaining flavor and quality of mandarins. Postharvest Biology and Technology. 64: 175-182. [DOI: 10.1016/j.postharvbio.2011.07.009]
Tran C.V., Mai T.P.T., Luong T.T., Son H.L. (2020). Study on the appropriate mixing ratio between chitosan and nisin to preserve post-harvest oranges at room temperate. Scientific Journal of Tan Trao University. 6: 47-53. [DOI: 10.51453/2354-1431/2020/387]
Tuoi N.T.K., Nguyen N.H.K., Truc T.T., Toan H.T. (2021). Physicochemical properties of green-skinned grapefruit and Nam Roi grapefruit grown in the Mekong Delta. Can Tho University Science Journal. 57: 118-126. [DOI: 10.22144/ctu.jsi.2021.013]
Vargas M., Gillabert M., Albors C., Chiralt A. (2004). Effect of film coating on fruit quality and changes. Journal of Food Science. 23: 746-753. [DOI: 10.1016/j.fochx.2024.101169]
Xing Y., Xu Q., Che Z., Li X., Li W. (2011). Effects of chitosan-oil coating on blue mold disease and quality attributes of jujube fruits. Food and Function. 2: 466-474. [DOI: 10.1039/ c1fo10073d]
[*]Corresponding author (C.X. Thuy)
* E-mail: thuycx@huit.edu.vn
ORCID ID: https://orcid.org/0009-0005-6219-5418
* E-mail: thuycx@huit.edu.vn
ORCID ID: https://orcid.org/0009-0005-6219-5418
Type of Study: Original article |
Subject:
Special
Received: 25/01/05 | Accepted: 25/09/15 | Published: 25/09/30
Received: 25/01/05 | Accepted: 25/09/15 | Published: 25/09/30
References
1. Ahmed W., Azmat R., Qayyum A., Mehmood A., Khan S.M., Liaquat M., Ahmed S., Moin S. (2018). The role of chitosan to prolonge the fresh fruit quality during storage of grapefruit cv. ray ruby. Pakistan Journal of Botany. 50: 151-159.
2. Ali A., Muhammad M.T.M., Sijam K., Siddiqui Y. (2011). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chemistry. 124: 620-626. [DOI: 10.1016/j.foodchem. 2010.06.085] [DOI:10.1016/j.foodchem.2010.06.085]
3. AOAC 942.15 Acidity Titratable of Fruit Products (1980). Official methods of analysis, online edition. Association of official analytical chemists, Washington, DC, USA.
4. Arnon H., Zaitsev Y., Porat R., Poverenov E. (2014). Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biology and Technology. 87: 21-26. [DOI: 10.1016/j.postharvbio. 2013.08.007] [DOI:10.1016/j.postharvbio.2013.08.007]
5. Barrion A.S.A., Hurtada W.A., Papa I.A., Zulayvar T.O., Yee M.G. (2014). Phytochemical composition, antioxidant and antibacterial properties of pummelo (Citrus maxima (Burm.) Merr. Against Escherichia coli and Salmonella typhimurium. Food and Nutrition Sciences. 5: 749-758. [DOI: 10.4236/fns.2014.59085] [DOI:10.4236/fns.2014.59085]
6. Chien P.-J., Sheu F., Lin H.-R. (2007). Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chemistry. 100: 1160-1164. [DOI: 10.1016/j.foodchem.2005.10.068] [DOI:10.1016/j.foodchem.2005.10.068]
7. Cisneros-Zevallos L., Krochta J.M. (2003). Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. Journal of Food Science. 68: 503-510. [DOI: 10.1111/j.1365-2621.2003.tb05702.x] [DOI:10.1111/j.1365-2621.2003.tb05702.x]
8. Das A.K., Islam M.N., Faruk M.O., Ashaduzzaman M., Dungani R. (2020). Review on tannins: extraction processes, applications and possibilities. South African Journal of Botany. 135: 58-70. [DOI: 10.1016/j.sajb.2020.08.008] [DOI:10.1016/j.sajb.2020.08.008]
9. Fraga-Corral M., Otero P., Cassani L., Echave J., Garcia-Oliveira P., Carpena M., Chamorro F., Lourenço-Lopes C., Prieto M.A., Simal-Gandara J. (2021). Traditional applications of tannin rich extracts supported by scientific data: chemical composition, bioavailability and bioaccessibility. Foods. 10: 251-260. [DOI: 10.3390/foods10020251] [DOI:10.3390/foods10020251] [PMID] [PMCID]
10. Hossain M.M., Disha R.F., Rahim M.A. (2018). Physio-morphological variations of pummelo genotype (Citrus grandis L. Osbeck). Advances in Horticultural Science. 32: 93-103. [DOI: 10.13128/ahs-21874].
11. Kahramanoglu I., Chen C., Gan Z., Chen J., Wan C. (2020). The effects of edible coatings on the postharvest quality of Citrus fruits as affected by granulation. Journal of Food Quality. 88:1-8. [DOI: 10.1155/2020/8819233] [DOI:10.1155/2020/8819233]
12. Loi N.V., Binh P.T., Sam V.K. (2023). Change of chemical and biochemical indicators of Phuc Trach pomelo in the preservation process with chitosan-based coating with tannin and vinegar. Food Research. 7: 170-177. [DOI: 10.26656/fr.2017.7(2).167] [DOI:10.26656/fr.2017.7(2).167]
13. Mayuoni L., Tietel Z., Patil B.S., Porat R. (2011). Does ethylene degreening affect internal quality of citrus fruit? Postharvest Biology and Technology. 62: 50-58. [DOI: 10.1016/j. postharvbio.2011.04.005] [DOI:10.1016/j.postharvbio.2011.04.005]
14. Nguyen D.T., Ha Q.V., Ta T.M. (2014). Research on the effect of concentration of chitosan on quality and preservation time of Doan Hung grapefruit (Citrus grandis Osbeck). Journal of Agriculture and Rural Development. 11: 80-83.
15. Nguyen N.H.K., Tran M.T., Le T.D., Nguyen M.V., Tran T.T. (2022). Chemical properties and biological properties of four varieties of pomelo (Citrus grandis (L) Osbeck) in the Mekong Delta of Vietnam. Food Research. 6: 267-272. [DOI: 10.26656/ fr.2017.6(4).479] [DOI:10.26656/fr.2017.6(4).479]
16. Nie Z., Huang Q., Chen C., Wan C., Chen J. (2020). Chitosan coating alleviates postharvest juice sac granulation by mitigating ROS accumulation in harvested pomelo (Citrus grandis L. Osbeck) during room temperature storage. Postharvest Biology and Technology. 169: 111309. [DOI: 10.1016/j.postharvbio. 2020.111309] [DOI:10.1016/j.postharvbio.2020.111309]
17. Petracek P.D., Dou H., Pao S. (1998). The influence of applied waxes on postharvest physiological behavior and pitting of grapefruit. Postharvest Biology and Technology. 14: 99-106. [DOI: 10.1016/S0925-5214(98)00018-0] [DOI:10.1016/S0925-5214(98)00018-0]
18. Pichaiyongvongdee S., Rattanapun B., Haruenkit R. (2014). Total polyphenol content and antioxidant properties in different tissues of seven pomelo (Citrus grandis (L.) osbeck) cultivars. Kasetsart Journal - Natural Science. 48: 989-996.
19. Saberi Riseh R., Vatankhah M., Hassanisaadi M., Shafiei-Hematabad Z., Kennedy J.F. (2024). Advancements in coating technologies: unveiling the potential of chitosan for the preservation of fruits and vegetables. International Journal of Biological Macromolecules. 254: 127677. [DOI: 10.1016/j.ijbiomac. 2023.127677] [DOI:10.1016/j.ijbiomac.2023.127677] [PMID]
20. Sawant T.P., Panhekar D. (2017). A brief reveiw on recent advances of Citrus maxima (Chakota). International Journal of Recent Scientific Research. 8: 19400-19416.
21. Shiekh R.A., Malik M.A., Al-Thabaiti S.A., Shiekh M.A. (2013). Chitosan as a novel edible coating for fresh fruits. Food Science Technology Research. 19: 139-155. [DOI: 10.3136/fstr.19.139] [DOI:10.3136/fstr.19.139]
22. Sirisomboon P., Theamprateep C. (2012). Physicochemical and textural properties of pomelo (Citrus maxima Merr. cv. Kao Nam Pueng) fruit at preharvest, postharvest and during the commercial harvest period. Philippine Agricultural Scientist. 95: 43-52.
23. Susanto S., Hermansah D., Amanda F. (2018). The growth and quality of fruit of three pummelo (Citrus maxima (Burn.) Merr.) accessions. IOP Conference Series: Earth and Environmental Science. 196: 012014. [DOI: 10.1088/1755-1315/196/1/012014] [DOI:10.1088/1755-1315/196/1/012014]
24. Tietel Z., Lewinsohn E., Fallik E., Porat R. (2012). Importance of storage temperatures in maintaining flavor and quality of mandarins. Postharvest Biology and Technology. 64: 175-182. [DOI: 10.1016/j.postharvbio.2011.07.009] [DOI:10.1016/j.postharvbio.2011.07.009]
25. Tran C.V., Mai T.P.T., Luong T.T., Son H.L. (2020). Study on the appropriate mixing ratio between chitosan and nisin to preserve post-harvest oranges at room temperate. Scientific Journal of Tan Trao University. 6: 47-53. [DOI: 10.51453/2354-1431/2020/387] [DOI:10.51453/2354-1431/2020/387]
26. Tuoi N.T.K., Nguyen N.H.K., Truc T.T., Toan H.T. (2021). Physicochemical properties of green-skinned grapefruit and Nam Roi grapefruit grown in the Mekong Delta. Can Tho University Science Journal. 57: 118-126. [DOI: 10.22144/ctu.jsi.2021.013] [DOI:10.22144/ctu.jsi.2021.013]
27. Vargas M., Gillabert M., Albors C., Chiralt A. (2004). Effect of film coating on fruit quality and changes. Journal of Food Science. 23: 746-753. [DOI: 10.1016/j.fochx.2024.101169] [DOI:10.1016/j.fochx.2024.101169] [PMID] [PMCID]
28. Xing Y., Xu Q., Che Z., Li X., Li W. (2011). Effects of chitosan-oil coating on blue mold disease and quality attributes of jujube fruits. Food and Function. 2: 466-474. [DOI: 10.1039/ c1fo10073d] [DOI:10.1039/c1fo10073d] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







