Volume 11, Issue 1 (March 2024)
J. Food Qual. Hazards Control 2024, 11(1): 13-24 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Oleghe P, Akharaiyi F, Ehis-Eriakha C. Compositions Nutrient and Antinutrients of Biscuits Prepared from Fermented and Unfermented Ternary Mixture Flours. J. Food Qual. Hazards Control 2024; 11 (1) :13-24
URL: http://jfqhc.ssu.ac.ir/article-1-1119-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1119-en.html
Department of Microbiology, Edo State University Uzairue, KM 7 Auchi-Abuja Road, Iyamho Uzairue, Edo State, Nigeria , akharaiyi.fred@edouniversity.edu.ng
Full-Text [PDF 740 kb]
(502 Downloads)
| Abstract (HTML) (1062 Views)
Full-Text: (9 Views)
Compositions Nutrient and Antinutrients of Biscuits Prepared from Fermented and Unfermented Ternary Mixture Flours
P.O. Oleghe 1,2, F.C. Akharaiyi 2** , C.B. Ehis-Eriakha 2
1. Department of Biological Science Laboratory Technology, School of Applied Sciences and Technology, Auchi Polytechnic, Auchi, P.M.B. 13, Auchi, Edo State, Nigeria
2. Department of Microbiology, Edo State University Uzairue, KM 7 Auchi-Abuja Road, Iyamho Uzairue, Edo State, Nigeria
HIGHLIGHTS
Table 1: Experimental design for composite flour mixes

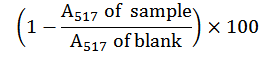
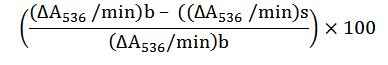
Table 2: Proximate contents in the biscuits
Data presented as mean±SD, in each column, the values having different superscript of a-c in the same column are significantly different (p<0.05).
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
Table 3: Phytochemical and antinutrient composition of biscuits
Data presented as mean±SD, in each column, the values having different superscript of a-c in the same column are significantly different (p<0.05).
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
Table 4: Minerals analysis of biscuits (ppm)
Data presented as mean±SD, in each column, the values having different superscript of a-c in the same column are significantly different (p<0.05).
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
Table 5: Antioxidants Analysis of biscuits
Data presented as mean±SD, in each column, the values having different superscript of a-c in the same column are significantly different (p<0.05).
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
OH: Hydroxyl Radical; ABTS: 2,2’–Azino-Bis-(3-Ethylbenzothiazoline-6-Sulfonic acid); DPPH: 1,1-Diphenyl-2-Picrylhydrazyl; FRAP: Ferric Reducing Antioxidant Property
Table 6: Color analysis of biscuits
*The color value was reported base on indexes of L*, a*, b*
Data presented as mean±SD, in each column, the values having different superscript of a-c in the same column are significantly different (p<0.05).
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
P.O. Oleghe 1,2, F.C. Akharaiyi 2**
1. Department of Biological Science Laboratory Technology, School of Applied Sciences and Technology, Auchi Polytechnic, Auchi, P.M.B. 13, Auchi, Edo State, Nigeria
2. Department of Microbiology, Edo State University Uzairue, KM 7 Auchi-Abuja Road, Iyamho Uzairue, Edo State, Nigeria
- Fermentation increased the contents of crude fiber, and ash but reduced the moisture and fat contents. It can have a significant impact on the nutritional composition of the different biscuits, as demonstrated by the proximate analysis results.
- The fermented and the unfermented biscuits samples had similar protein content (11.918±0.08 and 9.074±0.09%, respectively).
- Fermentation enhanced the potassium bioavailability of the products from 19.803±0.00 to 25.264±0.05 ppm).
- Flavonoid content was more in the fermented mix 5 (23.85%) than the unfermented mix 2 that has a value of (18.14%).
| Article type Original article |
ABSTRACT Background: The exorbitant cost of wheat-based foods in non-wheat growing countries has necessitated looking for more enriched and sustainable alternative flour from botanicals that can be mixed and used to produce baked products including biscuits. The study aimed to make biscuits using three different fermented and unfermented mixtures of flour (sweet potato, pigeon pea, and yellow maize). Methods: Starch-rich tubers of yellow-fleshed cultivar of sweet potato (Ipomoea batatas), yellow maize (Zea mays) grains, and pigeon peas (Cajanus cajan) seeds were purchased from food merchants in the Uchi market, located in Auchi area of Edo State, southern Nigeria in June 2022. These botanical samples were taxonomically validated. The samples were divided into two parts fermented and unfermented, prepared and produced into flour forms. The blended raw materials into flour were mixed in order of sweet potato: pigeon pea: maize (composite mix two 60.00:25.47:14.53, composite mix five 67.70:20.00:12.31 and composite mix eight 61.72:25.24:13.04) were selected to produce biscuits while 100% wheat was used as control. Biscuits were produced from the flour using a standard recipe. The obtained results were presented in mean±SD format of interpretations. Analytical significance dissimilarity between the means samples were considered based on one-way analysis of variance (ANOVA) using IBM Statistical software Results: It revealed that fermentation increased techno-functional properties containing crude fiber (3.464±0.01-3.485±0.01) and ashes (3.688±0.11-3.711±0.11), while reducing fat (17.339±0.03) and moisture contents (3.639±0.05), the control had the highest protein (12.805±0.25) and lowest carbohydrate (55.622±0.12). The fermented biscuits had more flavonoids (23.162±0.36-23.852±0.60), saponins (14.793±0.07-23.495±0.03). Additionally, fermentation enhanced the potassium bioavailability of the products (19.803±0.00-25.264±0.05). There was high free radical scavenging activity and color for all the fermented samples than unfermented and controlled biscuits. Conclusion: According to the research, ternary flour mixes from the botanicals could lead to products with improved nutritional composition, functional properties, and antioxidant attributes to further improve biscuit quality. © 2024, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
||
| Keywords Flour Fermentation Triticum Zea mays Nigeria |
|||
| Article history Received: 04 Jul 2023 Revised: 15 Oct 2023 Accepted: 25 Jan 2024 |
|||
| Acronyms and abbreviations DPPH=1,1-Diphenyl-2-Picrylhydrazyl FRAP=Ferric-Reducing Antioxidant Properties ABTS=2,2’–Azino-Bis-(3-ethylbenzothiazoline-6-Sulfonic acid) |
To cite: Oleghe P.O., Akharaiyi F.C., Ehis-Eriakha C.B. (2024). Compositions nutrient and antinutrients of biscuits prepared from fermented and unfermented ternary mixture flours. Journal of Food Quality and Hazards Control. 11: 13-25.
Introduction
Introduction
Worldwide, the replacement of gluten-rich whole wheat-based flours that are appropriate and readily available as botanical flours has increased for baking food products (Hasmadi et al., 2020).
Among the notable Ready-to-Eat (RTE) snacks globally available, biscuit is one of the most popular. It is a low-cost baked snack product with numerous advantages including a wide-range consumer base, reasonably longer durability, superior handiness, nutritionally enriched bulk, and excellent mouth feel. There is a growing interest in biscuit products not only because of their nutritional enhancement status but also their potential to be used for various anti-hunger food initiatives as well as an intervention food meal, particularly in catastrophic circumstances resulting from human and natural causes (Chandra et al., 2015).
The magnitude of Nigeria’s biscuit manufacturing industry is calculated to be approximately 617 million American dollars, growing annually at 16% over the last five years. This prosperous industry is bolstered by imported Russian wheat as the country can barely produce up to 10% of the 5-6 million metric tons of wheat required annually for sustainable consumption as at year 2022 (Oleghe et al., 2023). All efforts to mitigate this, including the implementation of various baked wheat intervention programs supported by the Federal Government of Nigeria (FGN) have not been successful (Okojie, 2022); the FGN is left with little choice but to import the commodity. Records manifest that the Central Bank of Nigeria (CBN) expends over 2 billion American dollars of its insufficient foreign resources annually importing wheat (Azeez, 2021).
The exorbitant price of wheat-based food products has necessitated looking inwards for more enriched suitable and sustainable comparatively advantageous composite botanicals as alternative substitutes to wheat-based diets. Additionally, the continuous intake of wheat-based products has been connected to celiac disease, this has made the utilization of flours from mixed botanicals imperative in producing oven-baked food staples like biscuits (Kiin-Kabari and Giami, 2015). In order to replace wheat flour in the production of baked food staples, research studies in a number of developing countries are now concentrating on enhancing and analyzing the techno-functional as well as the physicochemical properties of indigenously cultivated crop flours (Kwofie et al., 2020).
Much literature has highlighted the usefulness of integrating fermentation procedures in improving the dietary and techno-functional characteristics of healthy gluten-free flour-based staple diet alternatives. The appropriateness of processing fermented and unfermented composite blends of botanical flours as another flour option, based on their techno-functional and physiochemical properties cannot be overlooked (Oleghe et al., 2023).
This study aimed to produce biscuits using three different ratios of composite-based botanical flours from sweet potato, pigeon pea, and maize; and carrying out techno-functional and physicochemical assessments on these products.
Materials and methods
Collection of botanical samples
Dry starch-rich tubers of yellow-fleshed cultivar of sweet potato (Ipomoea batatas) and yellow maize (Zea mays) grains as well as pigeon peas (Cajanus cajan) seeds, were procured locally from food merchants within the main local market in Auchi area of Edo State, southern Nigeria. These botanical samples were taxonomically validated. The botanical samples were divided into two parts. One part is for fermentation and the other is not to be fermented. The parts for fermentation were fermented for three days using the liquid-state fermentation method. After washing the samples with water, they were steeped in clean water for three days to ferment. After which, it was drained and spread out to dry. However, both the fermented and unfermented samples were dried prior to milling. Grinding machine (Thomas Wiley, model 5, United States of America) was utilized to grind the samples to obtain smooth flour.
Preparation and production of flour samples
The fermented and unfermented botanical samples were prepared and produced into their respective flour forms according to the methods of Oleghe et al. (2023). Using the Design Expert 13.0 D-optimal software for mixture in the food sector as explained by Squeo et al. (2021), the various formulations of the flour were obtained by selecting and blending the individual botanical flour portions by the developed design for the experiment. The obtained composite flours were ground with a grinding machine and then sealed properly in dry transparent containers.
Experimental design
Results from the experimental design software for the composite flour mixes obtained from sweet potato, maize, and pigeon pea yielded 11 runs (Table 1), the different combinations of these botanicals were measured and utilized in formulating the respective fermented and unfermented composite mixes. Based on the proximate and the techno-functional results, three composite mixes (2, 5, and 8) were further selected and used in producing the biscuit products.
Among the notable Ready-to-Eat (RTE) snacks globally available, biscuit is one of the most popular. It is a low-cost baked snack product with numerous advantages including a wide-range consumer base, reasonably longer durability, superior handiness, nutritionally enriched bulk, and excellent mouth feel. There is a growing interest in biscuit products not only because of their nutritional enhancement status but also their potential to be used for various anti-hunger food initiatives as well as an intervention food meal, particularly in catastrophic circumstances resulting from human and natural causes (Chandra et al., 2015).
The magnitude of Nigeria’s biscuit manufacturing industry is calculated to be approximately 617 million American dollars, growing annually at 16% over the last five years. This prosperous industry is bolstered by imported Russian wheat as the country can barely produce up to 10% of the 5-6 million metric tons of wheat required annually for sustainable consumption as at year 2022 (Oleghe et al., 2023). All efforts to mitigate this, including the implementation of various baked wheat intervention programs supported by the Federal Government of Nigeria (FGN) have not been successful (Okojie, 2022); the FGN is left with little choice but to import the commodity. Records manifest that the Central Bank of Nigeria (CBN) expends over 2 billion American dollars of its insufficient foreign resources annually importing wheat (Azeez, 2021).
The exorbitant price of wheat-based food products has necessitated looking inwards for more enriched suitable and sustainable comparatively advantageous composite botanicals as alternative substitutes to wheat-based diets. Additionally, the continuous intake of wheat-based products has been connected to celiac disease, this has made the utilization of flours from mixed botanicals imperative in producing oven-baked food staples like biscuits (Kiin-Kabari and Giami, 2015). In order to replace wheat flour in the production of baked food staples, research studies in a number of developing countries are now concentrating on enhancing and analyzing the techno-functional as well as the physicochemical properties of indigenously cultivated crop flours (Kwofie et al., 2020).
Much literature has highlighted the usefulness of integrating fermentation procedures in improving the dietary and techno-functional characteristics of healthy gluten-free flour-based staple diet alternatives. The appropriateness of processing fermented and unfermented composite blends of botanical flours as another flour option, based on their techno-functional and physiochemical properties cannot be overlooked (Oleghe et al., 2023).
This study aimed to produce biscuits using three different ratios of composite-based botanical flours from sweet potato, pigeon pea, and maize; and carrying out techno-functional and physicochemical assessments on these products.
Materials and methods
Collection of botanical samples
Dry starch-rich tubers of yellow-fleshed cultivar of sweet potato (Ipomoea batatas) and yellow maize (Zea mays) grains as well as pigeon peas (Cajanus cajan) seeds, were procured locally from food merchants within the main local market in Auchi area of Edo State, southern Nigeria. These botanical samples were taxonomically validated. The botanical samples were divided into two parts. One part is for fermentation and the other is not to be fermented. The parts for fermentation were fermented for three days using the liquid-state fermentation method. After washing the samples with water, they were steeped in clean water for three days to ferment. After which, it was drained and spread out to dry. However, both the fermented and unfermented samples were dried prior to milling. Grinding machine (Thomas Wiley, model 5, United States of America) was utilized to grind the samples to obtain smooth flour.
Preparation and production of flour samples
The fermented and unfermented botanical samples were prepared and produced into their respective flour forms according to the methods of Oleghe et al. (2023). Using the Design Expert 13.0 D-optimal software for mixture in the food sector as explained by Squeo et al. (2021), the various formulations of the flour were obtained by selecting and blending the individual botanical flour portions by the developed design for the experiment. The obtained composite flours were ground with a grinding machine and then sealed properly in dry transparent containers.
Experimental design
Results from the experimental design software for the composite flour mixes obtained from sweet potato, maize, and pigeon pea yielded 11 runs (Table 1), the different combinations of these botanicals were measured and utilized in formulating the respective fermented and unfermented composite mixes. Based on the proximate and the techno-functional results, three composite mixes (2, 5, and 8) were further selected and used in producing the biscuit products.
Table 1: Experimental design for composite flour mixes
| Run | Component 1 A: sweet potato g/100 g |
Component 2 B: pigeon per g/100 g |
Component 3 C: corn g/100 g |
| 1 | 60.000 | 29.998 | 10.002 |
| *2 | 60.000 | 25.470 | 14.530 |
| 3 | 64.787 | 20.000 | 15.000 |
| 4 | 64.630 | 25.370 | 10.000 |
| *5 | 67.695 | 20.000 | 12.305 |
| 6 | 63.223 | 23.493 | 13.274 |
| 7 | 61.576 | 26.929 | 11.497 |
| *8 | 61.716 | 25.241 | 13.044 |
| 9 | 67.233 | 22.767 | 10.000 |
| 10 | 69.997 | 20.003 | 10.000 |
| 11 | 65.554 | 21.958 | 12.288 |
* selected
Preparation of biscuits
The recipes stated by Onabanjo and Ighere (2014) were adjusted and adopted here. The used recipe was: flour (100 g), fat (63 g), honey (25 ml), 1 g of table salt, 20 ml of whole egg, 5 g of powdered milk, biscuits baking powder (5 g), 5 ml egg white and water (20-60 ml). The egg white was used as gluten replacement while the control was whole wheat flour (100%). The butter and honey were mixed using a hand mixer (Guangzhou Home Machinery Co., Ltd, China), thereafter, to achieve the desired texture for biscuit dough, flour, egg white, baking powder, and milk were added and properly combined. The desired thickness of the dough was attained by hand-kneaded on a rotating table. A biscuit cutter (Surya Bakery Machine, India) was used to cut the dough into sizes and arrange it into different shapes on a baking tray lined with parchment paper. It was then oven-baked with 2 Deck 4 Tray+8 Tray Proofer, China for 15 min at 160 oC, then allowed to cool down, and packed.
Determination of proximate composition
-Ash content determination
The method of AOAC (2012) was adopted. In a clean and dry crucible, 5 g of sample was weighed. The crucible with the sample was then placed on a hot plate for the removal of organic matter and thereafter, transferred to a muffle furnace (IndiaMART, India) regulated at 600 oC for 5 h. After the heating process, the sample was placed in a desiccator to cool down before taking the weight.
The recipes stated by Onabanjo and Ighere (2014) were adjusted and adopted here. The used recipe was: flour (100 g), fat (63 g), honey (25 ml), 1 g of table salt, 20 ml of whole egg, 5 g of powdered milk, biscuits baking powder (5 g), 5 ml egg white and water (20-60 ml). The egg white was used as gluten replacement while the control was whole wheat flour (100%). The butter and honey were mixed using a hand mixer (Guangzhou Home Machinery Co., Ltd, China), thereafter, to achieve the desired texture for biscuit dough, flour, egg white, baking powder, and milk were added and properly combined. The desired thickness of the dough was attained by hand-kneaded on a rotating table. A biscuit cutter (Surya Bakery Machine, India) was used to cut the dough into sizes and arrange it into different shapes on a baking tray lined with parchment paper. It was then oven-baked with 2 Deck 4 Tray+8 Tray Proofer, China for 15 min at 160 oC, then allowed to cool down, and packed.
Determination of proximate composition
-Ash content determination
The method of AOAC (2012) was adopted. In a clean and dry crucible, 5 g of sample was weighed. The crucible with the sample was then placed on a hot plate for the removal of organic matter and thereafter, transferred to a muffle furnace (IndiaMART, India) regulated at 600 oC for 5 h. After the heating process, the sample was placed in a desiccator to cool down before taking the weight.
Ash percentage=(crucible’s weight+ash)-(empty crucible’s weight)×100/Sample’s weight
-Moisture content estimation
The criteria described by AOAC (2012) were used. Five g sample of the flour was obtained and placed in a crucible with a specified load and thereafter positioned in an oven regulated at 650 oC and left for 1 h. After the heating period, it was removed from the oven and weighed. This process was repeated for a consistent weight to be achieved.
Moisture content=(W2-W1)-(W3-W1)×100/W2-W1
Where, W1 is the empty weight of crucible; W2 is the total weight of sample and crucible; W3 is the mass of crucible with dry sample.
-Crude fat determination
The described criterion by AOAC (2012) was adopted. Five g of sample was weighed into a fat-free extraction thimble and sealed tightly. The thimble containing the sample was then positioned in an extractor that was attached to a reflux condenser (1,508 condenser reflux, India). A 250 ml soxhlet flask (Pirex, England) was filled with petroleum ether to the third quarter (¾) of the flask. The set-up was placed in a muffle furnace for 6 h for extraction under a flow of tap water to aid the condensation of the petroleum ether. After this, the flask containing the sample was removed and transferred to an oven regulated at 65 oC for 4 h. Thereafter, the flask was removed from the oven to cool down before weighing.
-Crude fiber determination
-Moisture content estimation
The criteria described by AOAC (2012) were used. Five g sample of the flour was obtained and placed in a crucible with a specified load and thereafter positioned in an oven regulated at 650 oC and left for 1 h. After the heating period, it was removed from the oven and weighed. This process was repeated for a consistent weight to be achieved.
Moisture content=(W2-W1)-(W3-W1)×100/W2-W1
Where, W1 is the empty weight of crucible; W2 is the total weight of sample and crucible; W3 is the mass of crucible with dry sample.
-Crude fat determination
The described criterion by AOAC (2012) was adopted. Five g of sample was weighed into a fat-free extraction thimble and sealed tightly. The thimble containing the sample was then positioned in an extractor that was attached to a reflux condenser (1,508 condenser reflux, India). A 250 ml soxhlet flask (Pirex, England) was filled with petroleum ether to the third quarter (¾) of the flask. The set-up was placed in a muffle furnace for 6 h for extraction under a flow of tap water to aid the condensation of the petroleum ether. After this, the flask containing the sample was removed and transferred to an oven regulated at 65 oC for 4 h. Thereafter, the flask was removed from the oven to cool down before weighing.
-Crude fiber determination
The AOAC (2012) method for the determination of crude fiber was adopted. Five g of the flour sample was measured into a 500 ml beaker. Two hundred ml of preheated 1.25% sulfuric acid (H2SO4; Merk, Darmstadt, Germany) was mixed with the sample and then placed in a regulated digestion apparatus. It was then refluxed for 30 m and filtered with No 1 Whatman filter paper. The obtained residue was boiled in hot distilled water until a neutral filtrate was acquired. The residue was then conveyed into a clean crucible and dried for 24 h at 60 oC. The combined weight of the sample which was labelled as “A” was recorded. Thereafter, the crucible with the sample was placed in a furnace regulated at 600 oC. Afterwards, it was re-weighed and labelled as “B”
% Crude fiber=(DWR-WR)×100/SW
WR: weight of the residue; DWR: denotes WR weight of the residue after ashing; and SW represents the sample’s weight.
-Protein content determination
The Kjeldahl nitrogen criterion as described by AOAC (2012) was used. Ten ml of concentrated H2SO4 and one kjeldahl catalyst tablet were mixed with the sample. The mixture was then digested for 4 h. Thereafter, the sample was cooled and transferred into a conical flask and combined with 5 ml of 40% sodium hydroxide (NaOH; Merk, Darmstadt, Germany) and 10 ml of boric acid was then add up with indicator solution and placed at the receiving top of the condenser. The sample was later titrated against a 0.01 N concentration of hydrochloric acid (HCl; Merk, Darmstadt, Germany).
% Nitrogen=14×VA×0.1×W×100/100
VA=volume of used acid, while
W=sample’s weight
The percentage crude protein is calculated as Nitrogen×6.25.
WR: weight of the residue; DWR: denotes WR weight of the residue after ashing; and SW represents the sample’s weight.
-Protein content determination
The Kjeldahl nitrogen criterion as described by AOAC (2012) was used. Ten ml of concentrated H2SO4 and one kjeldahl catalyst tablet were mixed with the sample. The mixture was then digested for 4 h. Thereafter, the sample was cooled and transferred into a conical flask and combined with 5 ml of 40% sodium hydroxide (NaOH; Merk, Darmstadt, Germany) and 10 ml of boric acid was then add up with indicator solution and placed at the receiving top of the condenser. The sample was later titrated against a 0.01 N concentration of hydrochloric acid (HCl; Merk, Darmstadt, Germany).
% Nitrogen=14×VA×0.1×W×100/100
VA=volume of used acid, while
W=sample’s weight
The percentage crude protein is calculated as Nitrogen×6.25.
-Carbohydrate determination
Difference was used to determine the carbohydrate content of the flour samples. This was performed by calculating the difference as stated in the method described by AOAC (2016). The content of the carbohydrate was estimated by subtraction of the total percentage of other components which include ash, moisture, fat, and protein from 100 employing the calculation stated below.
-Determination of tannin
The criteria of Govindappa et al. (2011) were adopted where 2 g each of flour sample was weighed and placed into a beaker of 250 ml capacity. Subsequently, 200 ml of 0.004 M K3Fe (CN)6 (Merk, Darmstadt, Germany) and 10 ml of 0.008 M ferric chloride (FeCl3; Merk, Darmstadt, Germany) in 1.00 M HCl were add up to the flask containing the sample. The flask with the sample was stood for 20 min, with occasional stirring at intervals of 10 min. Subsequently, 1 ml aliquots were withdrawn from the mixture, and a cocktail containing 2 ml of 0.008 M FeCl3 in 0.008 M HCl and 10 ml of 0.0015 M K3Fe (CN)6 was added. After 30 s, the absorbance was measured at 720 nm, using a blank for comparison:
Difference was used to determine the carbohydrate content of the flour samples. This was performed by calculating the difference as stated in the method described by AOAC (2016). The content of the carbohydrate was estimated by subtraction of the total percentage of other components which include ash, moisture, fat, and protein from 100 employing the calculation stated below.
-Determination of tannin
The criteria of Govindappa et al. (2011) were adopted where 2 g each of flour sample was weighed and placed into a beaker of 250 ml capacity. Subsequently, 200 ml of 0.004 M K3Fe (CN)6 (Merk, Darmstadt, Germany) and 10 ml of 0.008 M ferric chloride (FeCl3; Merk, Darmstadt, Germany) in 1.00 M HCl were add up to the flask containing the sample. The flask with the sample was stood for 20 min, with occasional stirring at intervals of 10 min. Subsequently, 1 ml aliquots were withdrawn from the mixture, and a cocktail containing 2 ml of 0.008 M FeCl3 in 0.008 M HCl and 10 ml of 0.0015 M K3Fe (CN)6 was added. After 30 s, the absorbance was measured at 720 nm, using a blank for comparison:
Tannin (mg/g)=Sample’s absorbance×concentration of the standard×dilution factor/The standard absorbance×size of sample
-Determination of flavonoid
The aluminium chloride (AlCl3) (ProChem, Inc, USA) colorimetric assay as stated by Singh et al. (2012) was adopted for the estimation of total flavonoid in the biscuit samples. In a 10 ml conical flask, 5 g of biscuit sample was mixed with 20 ml of methanol. Five ml of 10% AlCl3 and 5 ml of 1 M of potassium acetate (CH3COOK) (Vizag chemical, India) were add up to the mixture. Distilled water was added up to the mixture to make up a volume of 2,500 ml. Thereafter, it was incubated for 30 min at a room temperature of 27±2 oC. The sample’s absorbance was read at 415 nm against a blank with a spectrophotometer (SP-UV 5,100, Model: SP-UV 5,100, China). Using quercetin as the standard, the flavonoid in the sample was calculated with the below equation:
Flavonoids content (mg Quercetin Equivalent (QE)/g)=Abssample×conc. of the standard (mg/ml)/Absstandard×con. of the sample (mg/g)
The standard absorbance is the absorbance of a containing 500 µl quercetin in 50 µl of 100% AlCl3 and 1 M of ethanoic acid (CH2COOK). The blank represents the mixtures of 500 µl of distilled water, 50 µl of distilled water, 500 µl of methanol, and 1 M CH3COOK.
-Determination of flavonoid
The aluminium chloride (AlCl3) (ProChem, Inc, USA) colorimetric assay as stated by Singh et al. (2012) was adopted for the estimation of total flavonoid in the biscuit samples. In a 10 ml conical flask, 5 g of biscuit sample was mixed with 20 ml of methanol. Five ml of 10% AlCl3 and 5 ml of 1 M of potassium acetate (CH3COOK) (Vizag chemical, India) were add up to the mixture. Distilled water was added up to the mixture to make up a volume of 2,500 ml. Thereafter, it was incubated for 30 min at a room temperature of 27±2 oC. The sample’s absorbance was read at 415 nm against a blank with a spectrophotometer (SP-UV 5,100, Model: SP-UV 5,100, China). Using quercetin as the standard, the flavonoid in the sample was calculated with the below equation:
Flavonoids content (mg Quercetin Equivalent (QE)/g)=Abssample×conc. of the standard (mg/ml)/Absstandard×con. of the sample (mg/g)
The standard absorbance is the absorbance of a containing 500 µl quercetin in 50 µl of 100% AlCl3 and 1 M of ethanoic acid (CH2COOK). The blank represents the mixtures of 500 µl of distilled water, 50 µl of distilled water, 500 µl of methanol, and 1 M CH3COOK.
-Determination of saponin
The criterion described by Mhada et al. (2020) was adopted for saponin content determination in the biscuits. Twenty g of each biscuit sample was dissolved in 100 ml of 20% ethanol and heated in a water bath regulated at a temperature of 55 oC for 4 h and with a continuous stirring. The mixture was filtered to obtain residue. The residue was then re-extracted by the addition of 200 ml of 20% ethanol. The filtrates were put together and concentrated to 40 ml in a water bath regulated at a temperature of 90 oC. Thereafter, the concentrated residue was removed and placed in a 250 ml separating funnel where it was mixed with 20 ml of diethyl ether by vigorously shaking. By this, we recovered the aqueous layer and discarded the ether layer. The process was repeated and 60 ml of n-butanol was then added. The aqueous layer mixed with butanol was then washed two times by adding 10 ml of 5% aqueous solution of sodium chloride (NaCl). The left solution was evaporated by heating water. After this, the samples were oven-dried to obtain a constant weight. The content of the saponin was then estimated in mg/g.
-Determination of oxalate
The criteria analyzed by Karamad et al. (2019) were employed. A 2 g portion of the biscuit samples was subjected to digestion using 10 ml of 6M HCl for duration of 1 h. The resulting mixture was then brought to make a volume of 250 ml in a volumetric flask. A concentrated solution of NH4OH was utilized to adjust the pH of the filtrate until the color changed from pink to pale yellow. Following this, a 5% CaCl2 aqueous solution was added for the precipitation of insoluble oxalate that might be present in the mixture. The obtained suspension was thereafter centrifuged at a speed of 2,500 rpm for 10 min the supernatant was carefully emptied into a container. The obtained precipitate from the centrifugation step was mixed with 10 ml of 20% (v/v) H2SO4 and the resulting solution obtained was subsequently brought to a final volume of 300 ml. A measure of 125 ml solution was subjected to heat up to nearly its point of boiling and thereafter was titrated against a standardized solution of 0.05 M of potassium permanganate (KMnO4) for 30 s to reach a faintly pink color. The observation on the burette at this point was recorded and used to estimate the content of oxalate in the biscuit sample.
Oxalate (mg/g)=titre value×volume of KMnO4×dilution factor/Sample size
The criterion described by Mhada et al. (2020) was adopted for saponin content determination in the biscuits. Twenty g of each biscuit sample was dissolved in 100 ml of 20% ethanol and heated in a water bath regulated at a temperature of 55 oC for 4 h and with a continuous stirring. The mixture was filtered to obtain residue. The residue was then re-extracted by the addition of 200 ml of 20% ethanol. The filtrates were put together and concentrated to 40 ml in a water bath regulated at a temperature of 90 oC. Thereafter, the concentrated residue was removed and placed in a 250 ml separating funnel where it was mixed with 20 ml of diethyl ether by vigorously shaking. By this, we recovered the aqueous layer and discarded the ether layer. The process was repeated and 60 ml of n-butanol was then added. The aqueous layer mixed with butanol was then washed two times by adding 10 ml of 5% aqueous solution of sodium chloride (NaCl). The left solution was evaporated by heating water. After this, the samples were oven-dried to obtain a constant weight. The content of the saponin was then estimated in mg/g.
-Determination of oxalate
The criteria analyzed by Karamad et al. (2019) were employed. A 2 g portion of the biscuit samples was subjected to digestion using 10 ml of 6M HCl for duration of 1 h. The resulting mixture was then brought to make a volume of 250 ml in a volumetric flask. A concentrated solution of NH4OH was utilized to adjust the pH of the filtrate until the color changed from pink to pale yellow. Following this, a 5% CaCl2 aqueous solution was added for the precipitation of insoluble oxalate that might be present in the mixture. The obtained suspension was thereafter centrifuged at a speed of 2,500 rpm for 10 min the supernatant was carefully emptied into a container. The obtained precipitate from the centrifugation step was mixed with 10 ml of 20% (v/v) H2SO4 and the resulting solution obtained was subsequently brought to a final volume of 300 ml. A measure of 125 ml solution was subjected to heat up to nearly its point of boiling and thereafter was titrated against a standardized solution of 0.05 M of potassium permanganate (KMnO4) for 30 s to reach a faintly pink color. The observation on the burette at this point was recorded and used to estimate the content of oxalate in the biscuit sample.
Oxalate (mg/g)=titre value×volume of KMnO4×dilution factor/Sample size
Determination of trypsin inhibitor
The trypsin inhibitor in the biscuits was evaluated with the procedure established by Szmigielski et al. 2010. Fifty ml of 0.01 N NaOH was applied to mix 1 g of the biscuit sample. This was performed at a pH that ranged from 8.4 to 10.00. This was turned continuously for 3 h. Two ml of the solution was pipetted into a test tube. Subsequently, 2 ml of cold trypsin solution (4 mg in 200 ml of 0.001 M HCl) were added to clean test tubes. The test tubes with the biscuit samples were placed in an incubator at a temperature of 37 oC. A substrate solution, Benzoyl-DL-arginine-P-nitroanilide hydrochloride (BAPNA; Sigma-Aldrich, USA) was prepared by dissolving 40 mg in 1 ml of dimethyl sulfoxide and adding more of 0.05 M tris buffer at pH of 8.2 to make up to 100 ml. Five ml of the BAPNA substrate solution was added up to the test tubes for 10 m and hindered the reaction by adding a volume of 30% acetic acid, and the contents of each test tube were stirred to mix very well. The mixtures contained in the tubes were subjected to centrifugation at 3,000 rpm for 10 min, and the supernatant so obtained was read in a spectrophotometer at a wavelength of 410 nm against a blank of reagent. A reference solution was prepared in the same manner as the sample, only that 2 ml of distilled water was add up instead of the biscuit samples.
Mineral analysis
Using the dry ash criteria, 1 g of each of the biscuit samples was weighed precisely into various crucibles and then transferred to a muffle kiln for ashing at a temperature of 550 oC until all carbon substances were completely burnt. The crucible (containing the ash) was taken off and transferred into separate desiccators for cooling. Thereafter, 10 ml of 0.1 M HCl solution was pipetted into each crucible containing the biscuit sample for the dissociation of ash and percolation of the mineral elements. The crucibles were washed three times with 0.1 M HCl solution, the volume was subsequently adjusted to 100 ml using deionized water.
Atomic Absorption Spectroscopy (AAS) (Infitek, China) was used to analyze iron (Fe), magnesium (Mg), zinc (Zn), and calcium (Ca), while the flame photometer (Dongguan Xin Bao Instrument Co. Ltd, China) was employed to analyze potassium (K) and sodium (Na) and the Vanodo-molybdate method was utilized to analyze phosphorus (P). A stock solution was made ready for each metal utilizing their relevant metal salts to set up their standard curve (AOAC, 2012).
Determination of antioxidant activities
-Free radical scavenging activity
The authority of Matamane et al. (2020) was followed. Ten g of the biscuit sample was dissolved in 1 ml of buffer containing 0.1 M sodium phosphate and 1% (w/v) Triton X-100 at a pH of 7.0. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH; Wuhan Golden Wing Idustry & Trade Co. Ltd, China) was mixed up in a methanol solution to make up a final concentration of 100 μM. Then, 100 μl of the protein fractions were dissolved in 100 μl of the DPPH suspension in a 96-well plate to reach a final assay concentration of 1 mg/ml, and the mixture was left for 30 min in the dark at a room temperature of 27±2 oC. The value of the absorbance of the blank, the control Glutathione (GSH), and samples were read at 517 nm. The negative control consisted of sodium phosphate buffer in place of the protein fractions, while GSH served as the positive control. The percent DPPH radical scavenging activity of the samples was calculated with the below formula:
The trypsin inhibitor in the biscuits was evaluated with the procedure established by Szmigielski et al. 2010. Fifty ml of 0.01 N NaOH was applied to mix 1 g of the biscuit sample. This was performed at a pH that ranged from 8.4 to 10.00. This was turned continuously for 3 h. Two ml of the solution was pipetted into a test tube. Subsequently, 2 ml of cold trypsin solution (4 mg in 200 ml of 0.001 M HCl) were added to clean test tubes. The test tubes with the biscuit samples were placed in an incubator at a temperature of 37 oC. A substrate solution, Benzoyl-DL-arginine-P-nitroanilide hydrochloride (BAPNA; Sigma-Aldrich, USA) was prepared by dissolving 40 mg in 1 ml of dimethyl sulfoxide and adding more of 0.05 M tris buffer at pH of 8.2 to make up to 100 ml. Five ml of the BAPNA substrate solution was added up to the test tubes for 10 m and hindered the reaction by adding a volume of 30% acetic acid, and the contents of each test tube were stirred to mix very well. The mixtures contained in the tubes were subjected to centrifugation at 3,000 rpm for 10 min, and the supernatant so obtained was read in a spectrophotometer at a wavelength of 410 nm against a blank of reagent. A reference solution was prepared in the same manner as the sample, only that 2 ml of distilled water was add up instead of the biscuit samples.
Mineral analysis
Using the dry ash criteria, 1 g of each of the biscuit samples was weighed precisely into various crucibles and then transferred to a muffle kiln for ashing at a temperature of 550 oC until all carbon substances were completely burnt. The crucible (containing the ash) was taken off and transferred into separate desiccators for cooling. Thereafter, 10 ml of 0.1 M HCl solution was pipetted into each crucible containing the biscuit sample for the dissociation of ash and percolation of the mineral elements. The crucibles were washed three times with 0.1 M HCl solution, the volume was subsequently adjusted to 100 ml using deionized water.
Atomic Absorption Spectroscopy (AAS) (Infitek, China) was used to analyze iron (Fe), magnesium (Mg), zinc (Zn), and calcium (Ca), while the flame photometer (Dongguan Xin Bao Instrument Co. Ltd, China) was employed to analyze potassium (K) and sodium (Na) and the Vanodo-molybdate method was utilized to analyze phosphorus (P). A stock solution was made ready for each metal utilizing their relevant metal salts to set up their standard curve (AOAC, 2012).
Determination of antioxidant activities
-Free radical scavenging activity
The authority of Matamane et al. (2020) was followed. Ten g of the biscuit sample was dissolved in 1 ml of buffer containing 0.1 M sodium phosphate and 1% (w/v) Triton X-100 at a pH of 7.0. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH; Wuhan Golden Wing Idustry & Trade Co. Ltd, China) was mixed up in a methanol solution to make up a final concentration of 100 μM. Then, 100 μl of the protein fractions were dissolved in 100 μl of the DPPH suspension in a 96-well plate to reach a final assay concentration of 1 mg/ml, and the mixture was left for 30 min in the dark at a room temperature of 27±2 oC. The value of the absorbance of the blank, the control Glutathione (GSH), and samples were read at 517 nm. The negative control consisted of sodium phosphate buffer in place of the protein fractions, while GSH served as the positive control. The percent DPPH radical scavenging activity of the samples was calculated with the below formula:

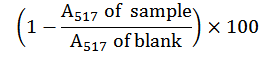
-Determination of metal chelating activity
The criterion of Wong et al. (2014) was employed for the determination of the metal chelating potential of the biscuits samples. It was measured in the samples by adding 0.2 ml of 0.1 mM FeSO4 (Merk, Darmstadt, Germany) and 0.4 ml of 0.25 ml of mM ferrozine subsequently into 0.2 ml of biscuit solution. The mixture was placed in an incubator at a temperature of 27±2 oC for 10 m. Thereafter, the absorbance of the mixture was measured at 562 nm and recorded. The chelating potential of the biscuit samples was evaluated with the formula below:

Where, Acontrol is the control reaction absorbance (without the biscuit sample); and Asample is the absorbance in the presence of the biscuit sample.
-Hydroxyl radical scavenging
This was performed by the criteria of Girgih et al. (2011). The biscuit samples, glutathione and 1, 10-phenanthroline (3 mM) were mixed up in 0.1 M phosphate buffer (pH 7.4) separately while FeSO4 (3.0 mM) and 0.01% hydrogen peroxide were mixed up in distilled water separately. A sample solution of 50 μl was added first to a clean-bottomed 96-well plate, following the addition of 50 μl of 1, 10-phenanthroline and subsequently, the addition of 50 μl of FeSO4. To commence the Fenton response in the wells, the addition of 50 μl of hydrogen peroxide was made to the mixture which was covered and placed in an incubator that was regulated at 37 oC for 1 h that involved agitation. The absorbance was read with a spectrophotometer at 536 nm for 1 h at 10 min intervals. The potential of the hydroxyl radical scavenging of the biscuit sample was evaluated with the reaction rate (ΔA/min) of the below equation:

The criterion of Wong et al. (2014) was employed for the determination of the metal chelating potential of the biscuits samples. It was measured in the samples by adding 0.2 ml of 0.1 mM FeSO4 (Merk, Darmstadt, Germany) and 0.4 ml of 0.25 ml of mM ferrozine subsequently into 0.2 ml of biscuit solution. The mixture was placed in an incubator at a temperature of 27±2 oC for 10 m. Thereafter, the absorbance of the mixture was measured at 562 nm and recorded. The chelating potential of the biscuit samples was evaluated with the formula below:

Where, Acontrol is the control reaction absorbance (without the biscuit sample); and Asample is the absorbance in the presence of the biscuit sample.
-Hydroxyl radical scavenging
This was performed by the criteria of Girgih et al. (2011). The biscuit samples, glutathione and 1, 10-phenanthroline (3 mM) were mixed up in 0.1 M phosphate buffer (pH 7.4) separately while FeSO4 (3.0 mM) and 0.01% hydrogen peroxide were mixed up in distilled water separately. A sample solution of 50 μl was added first to a clean-bottomed 96-well plate, following the addition of 50 μl of 1, 10-phenanthroline and subsequently, the addition of 50 μl of FeSO4. To commence the Fenton response in the wells, the addition of 50 μl of hydrogen peroxide was made to the mixture which was covered and placed in an incubator that was regulated at 37 oC for 1 h that involved agitation. The absorbance was read with a spectrophotometer at 536 nm for 1 h at 10 min intervals. The potential of the hydroxyl radical scavenging of the biscuit sample was evaluated with the reaction rate (ΔA/min) of the below equation:

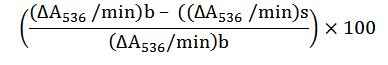
-Ferric Reducing Antioxidant Properties (FRAP)
The modified criteria described by Zhang et al. (2013) were used. The experimental biscuit sample was dissociated in 0.2 M phosphate buffer with a pH of 6.6. An aliquot of 250 μl from this solution was mingled with 250 μl of the buffer and 250 μl of a 1% potassium ferricyanide mixture. The mixture was vortexed with a machine (LAB M, England) and then incubated for 20 m at 50 °C. At the end of incubation, 250 μl of 10% Trichloroacetic Acid (TCA) was added up. This was followed by the addition of 50 μl of a 0.1% FeCl3 solution dissociated in double distilled water, and ultimately, 200 μl of double distilled water was add up. The resulting mixture was stood for 10 m at 27 oC and thereafter, centrifuged at 1,000 rpm for 10 min. An aliquot of 200 μl from the supernatant was transferred to a clean-bottomed 96-well plate, and the absorbance was read at 700 nm.
-2,2’–Azino-Bis-(3-ethylbenzothiazoline-6-Sulfonic acid) (ABTS) radical scavenging assay
The ABTS activity was estimated following a modification of the criteria described by Sochor et al. (2013). The initial step involved preparing a stock solution consisting of 7 mM ABTS and 2.45 mM potassium persulfate. This stock solution was then mixed in the same proportions and left to react for 14 h at 27 oC in the absence of light. To create a working solution, the resulting mixture was diluted by combining 1 ml of the ABTS solution with 60 ml of methanol until the absorbance reached a specific value of 0.706±0.01 units at 734 nm with a spectrophotometer. A fresh ABTS solution was made ready for each test. In the assay itself, 1 ml of the biscuit sample was mixed with 1 ml of the ABTS solution, and the absorbance was measured at 734 nm after a 7 min incubation period with a spectrophotometer. The ABTS scavenging activity of the samples was then compared to that of Butylated Hydroxytoluene (BHT)/Trolox and ascorbic acid, and the % of inhibition was measured as the ABTS radical scavenging capacity.
ABTS radical scavenging effect (100%)=[(Acontrol-Asample/Acontrol)]×100
Where, Acontrol is the absorbance of ABTS radical in methanol; Asample is the absorbance of ABTS radical solution mixed with biscuit sample/standard.
Determination of color
The modified procedures outlined by Ntrallou et al. (2020) were used to determine the color of the composite biscuit and control product. The spectrophotometer (model PU8,740, Mettler Toledo) was employed which has glass cells of 2 mm wavelength to confine the spectra of absorption. Their L*, a*, b* values were measured with illuminant D65 and a 100 observer. The L* value represents the lightness, ranging from a complete opaque (0) to a complete transparent (100). The a* value indicates the level of preparedness, while the b* value represents the level of yellowness. The hue angle (H) was estimated with the formula: H=tan-1(b*/a*), and the chroma (C) was measured as C=[(a*)2+(b*)2]0.5.
Statistical analysis
Data collected from all samples were obtained in triplicates. The obtained results were presented in mean±SD format of interpretations. Analytical significance dissimilarity between the means samples were determined based on one-way analysis of variance (ANOVA) using IBM Statistical software known as Statistical Package for Social Science (SPSS) version 23.0 for Windows and Microsoft Office Excel 2010. The Least Significance Difference (LSD) test of means was a significant difference if p<0.05 at a 5% level of importance at a 95% confidence level.
Results
Experimental design
The different combinations of the botanicals were measured and used in formulating the respective fermented and unfermented composite mixes. Based on the proximate and the techno-functional results three composite mixes (2, 5, and 8) were further selected and utilized in producing the biscuit products (Table 1).
Proximate analysis of biscuits
The results for the proximate analysis of biscuits made from the three fermented and unfermented biscuits (2, 5, and 8) as well as the control are presented in Table 2. The biscuits’ ash content was between 3.16-3.71%, with the highest ash content in biscuits samples from the fermented mixes (2, 5, and 8) while the control biscuit had the least ash content. The ash contents for all the fermented and unfermented biscuit mixes had identical values with no significant differences (p>0.05). The moisture content analyzed from the biscuit was between 3.64 and 6.56%. It can be observed that all the unfermented biscuit sample mixes had significantly higher values (p<0.05) compared to the fermented biscuit sample mixes and the control biscuit. The biscuit fat content was between 17.339 and 21.161%, with the highest fat content in the sample used as control and the lowest recorded in the samples of fermented biscuits. The fat content was significantly lower (p<0.05) in all the fermented samples. All the fermented biscuits were produced using the different mixes as well as all the unfermented biscuit samples had similar fat content results (17.339±0.03 and 18.723±0.68%, respectively). The crude fiber analyzed from the biscuit was between 1.27-3.49%. This value was higher in all fermented biscuit sample mixes (2, 5, and 8) in comparison with the unfermented samples and the control, but with no significant differences (p>0.05). All the fermented biscuits which produced with the different mixes as well as all the unfermented biscuit samples had similar crude fiber content results (3.485±0.01 and 3.464±0.01%, respectively). The value of protein in the biscuits was between 9.07-12.81%. The protein content was higher (p<0.05) in the samples that were fermented in comparison with the samples not fermented. The highest protein content occurred in the control biscuit. The fermented and unfermented biscuit samples had protein contents of 11.918±0.08 and 9.074±0.09%, respectively. The biscuit carbohydrate value was between 55.62 and 59.93%. The values were higher in the unfermented samples compared to the fermented and the control samples. Approximately, the fermented and the unfermented biscuit samples had similar carbohydrate content (58.491±0.70 and 59.932±0.05%, respectively).
It was generally observed that apart from the control sample results, the mean values for all the fermented biscuit mixes and all the unfermented biscuit mixes for each proximate parameter had similar values with no significant differences (p>0.05) occurring (Table 2).
The modified criteria described by Zhang et al. (2013) were used. The experimental biscuit sample was dissociated in 0.2 M phosphate buffer with a pH of 6.6. An aliquot of 250 μl from this solution was mingled with 250 μl of the buffer and 250 μl of a 1% potassium ferricyanide mixture. The mixture was vortexed with a machine (LAB M, England) and then incubated for 20 m at 50 °C. At the end of incubation, 250 μl of 10% Trichloroacetic Acid (TCA) was added up. This was followed by the addition of 50 μl of a 0.1% FeCl3 solution dissociated in double distilled water, and ultimately, 200 μl of double distilled water was add up. The resulting mixture was stood for 10 m at 27 oC and thereafter, centrifuged at 1,000 rpm for 10 min. An aliquot of 200 μl from the supernatant was transferred to a clean-bottomed 96-well plate, and the absorbance was read at 700 nm.
-2,2’–Azino-Bis-(3-ethylbenzothiazoline-6-Sulfonic acid) (ABTS) radical scavenging assay
The ABTS activity was estimated following a modification of the criteria described by Sochor et al. (2013). The initial step involved preparing a stock solution consisting of 7 mM ABTS and 2.45 mM potassium persulfate. This stock solution was then mixed in the same proportions and left to react for 14 h at 27 oC in the absence of light. To create a working solution, the resulting mixture was diluted by combining 1 ml of the ABTS solution with 60 ml of methanol until the absorbance reached a specific value of 0.706±0.01 units at 734 nm with a spectrophotometer. A fresh ABTS solution was made ready for each test. In the assay itself, 1 ml of the biscuit sample was mixed with 1 ml of the ABTS solution, and the absorbance was measured at 734 nm after a 7 min incubation period with a spectrophotometer. The ABTS scavenging activity of the samples was then compared to that of Butylated Hydroxytoluene (BHT)/Trolox and ascorbic acid, and the % of inhibition was measured as the ABTS radical scavenging capacity.
ABTS radical scavenging effect (100%)=[(Acontrol-Asample/Acontrol)]×100
Where, Acontrol is the absorbance of ABTS radical in methanol; Asample is the absorbance of ABTS radical solution mixed with biscuit sample/standard.
Determination of color
The modified procedures outlined by Ntrallou et al. (2020) were used to determine the color of the composite biscuit and control product. The spectrophotometer (model PU8,740, Mettler Toledo) was employed which has glass cells of 2 mm wavelength to confine the spectra of absorption. Their L*, a*, b* values were measured with illuminant D65 and a 100 observer. The L* value represents the lightness, ranging from a complete opaque (0) to a complete transparent (100). The a* value indicates the level of preparedness, while the b* value represents the level of yellowness. The hue angle (H) was estimated with the formula: H=tan-1(b*/a*), and the chroma (C) was measured as C=[(a*)2+(b*)2]0.5.
Statistical analysis
Data collected from all samples were obtained in triplicates. The obtained results were presented in mean±SD format of interpretations. Analytical significance dissimilarity between the means samples were determined based on one-way analysis of variance (ANOVA) using IBM Statistical software known as Statistical Package for Social Science (SPSS) version 23.0 for Windows and Microsoft Office Excel 2010. The Least Significance Difference (LSD) test of means was a significant difference if p<0.05 at a 5% level of importance at a 95% confidence level.
Results
Experimental design
The different combinations of the botanicals were measured and used in formulating the respective fermented and unfermented composite mixes. Based on the proximate and the techno-functional results three composite mixes (2, 5, and 8) were further selected and utilized in producing the biscuit products (Table 1).
Proximate analysis of biscuits
The results for the proximate analysis of biscuits made from the three fermented and unfermented biscuits (2, 5, and 8) as well as the control are presented in Table 2. The biscuits’ ash content was between 3.16-3.71%, with the highest ash content in biscuits samples from the fermented mixes (2, 5, and 8) while the control biscuit had the least ash content. The ash contents for all the fermented and unfermented biscuit mixes had identical values with no significant differences (p>0.05). The moisture content analyzed from the biscuit was between 3.64 and 6.56%. It can be observed that all the unfermented biscuit sample mixes had significantly higher values (p<0.05) compared to the fermented biscuit sample mixes and the control biscuit. The biscuit fat content was between 17.339 and 21.161%, with the highest fat content in the sample used as control and the lowest recorded in the samples of fermented biscuits. The fat content was significantly lower (p<0.05) in all the fermented samples. All the fermented biscuits were produced using the different mixes as well as all the unfermented biscuit samples had similar fat content results (17.339±0.03 and 18.723±0.68%, respectively). The crude fiber analyzed from the biscuit was between 1.27-3.49%. This value was higher in all fermented biscuit sample mixes (2, 5, and 8) in comparison with the unfermented samples and the control, but with no significant differences (p>0.05). All the fermented biscuits which produced with the different mixes as well as all the unfermented biscuit samples had similar crude fiber content results (3.485±0.01 and 3.464±0.01%, respectively). The value of protein in the biscuits was between 9.07-12.81%. The protein content was higher (p<0.05) in the samples that were fermented in comparison with the samples not fermented. The highest protein content occurred in the control biscuit. The fermented and unfermented biscuit samples had protein contents of 11.918±0.08 and 9.074±0.09%, respectively. The biscuit carbohydrate value was between 55.62 and 59.93%. The values were higher in the unfermented samples compared to the fermented and the control samples. Approximately, the fermented and the unfermented biscuit samples had similar carbohydrate content (58.491±0.70 and 59.932±0.05%, respectively).
It was generally observed that apart from the control sample results, the mean values for all the fermented biscuit mixes and all the unfermented biscuit mixes for each proximate parameter had similar values with no significant differences (p>0.05) occurring (Table 2).
Table 2: Proximate contents in the biscuits
| Sample | Ash content (%) |
Moisture content (%) |
Fat content (%) |
Crude fiber content (%) |
Protein content (%) |
Carbohydrate content (%) |
| CA | 3.163±0.03 b | 5.982±0.01 b | 21.161±0.08 a | 1.271±0.04 b | 12.805±0.25 a | 55.622±0.12 c |
| 2FA | 3.711±0.11 a | 3.639±0.05 c | 17.339±0.03 c | 3.485±0.01 a | 11.918±0.08 b | 58.491±0.70 b |
| 2UFA | 3.688±0.11 a | 6.562±0.03 a | 18.723±0.68 b | 3.464±0.01 a | 9.074±0.09 c | 59.932±0.05 a |
| 5FA | 3.711±0.11 a | 3.639±0.05 c | 17.339±0.03 c | 3.485±0.01 b | 11.918±0.08 b | 58.491±0.70 b |
| 5UFA | 3.688±0.11 a | 6.562±0.03 a | 18.723±0.68 b | 3.464±0.01 a | 9.074±0.09 c | 59.932±0.05 a |
| 8FA | 3.711±0.11 a | 3.639±0.05 c | 17.339±0.03 c | 3.485±0.01 a | 11.918±0.08 b | 58.491±0.70 b |
| 8UFA | 3.688±0.11 a | 6.562±0.03 a | 18.723±0.68 b | 3.464±0.01 a | 9.074±0.09 c | 59.932±0.05 a |
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
Antinutrient properties of the biscuits
The antinutrient composition of biscuits made from both fermented and unfermented biscuits (2, 5, and 8) along with a control is analyzed as follows (Table 3). The tannin content was highest in the unfermented sample of mix 5 (1.69 mg/g) and lowest in the control sample of mix 8 (0.720 mg/g). For flavonoid content, the highest was in the fermented mix 5 (23.85%), while the lowest was in the unfermented mix 2 (18.14%). The fermented mix 5 had the highest saponin content (23.50%), and the least was found in the control (12.50%). For oxalate content, the highest value was recorded in the unfermented mix 5 (4.59 mg/g), and the value was lowest in the control sample (0.90 mg/g). The values of the trypsin inhibitor range from 19.101 to 22.360%. The highest value was observed in unfermented mix 5, and the least was regarded in fermented mix 8. The produced biscuits with the unfermented composite had significantly higher (p<0.05) trypsin inhibitor levels compared with their fermented counterparts.
The antinutrient composition of biscuits made from both fermented and unfermented biscuits (2, 5, and 8) along with a control is analyzed as follows (Table 3). The tannin content was highest in the unfermented sample of mix 5 (1.69 mg/g) and lowest in the control sample of mix 8 (0.720 mg/g). For flavonoid content, the highest was in the fermented mix 5 (23.85%), while the lowest was in the unfermented mix 2 (18.14%). The fermented mix 5 had the highest saponin content (23.50%), and the least was found in the control (12.50%). For oxalate content, the highest value was recorded in the unfermented mix 5 (4.59 mg/g), and the value was lowest in the control sample (0.90 mg/g). The values of the trypsin inhibitor range from 19.101 to 22.360%. The highest value was observed in unfermented mix 5, and the least was regarded in fermented mix 8. The produced biscuits with the unfermented composite had significantly higher (p<0.05) trypsin inhibitor levels compared with their fermented counterparts.
Table 3: Phytochemical and antinutrient composition of biscuits
| Samples | Tannins (mg/g) |
Flavonoids (%) |
Saponins (%) |
Oxalates (mg/g) |
Trypsin inhibitor (%) |
| CA | 0.720±0.00 d | 12.592±0.76 e | 12.497±0.00 e | 0.902±0.00 e | 20.220±0.82 c |
| 2FA | 0.785±0.05 d | 23.162±0.36 a | 14.793±0.07 c | 3.599±0.00 b | 20.560±0.06 c |
| 2UFA | 1.015±0.01 c | 18.139±0.03 d | 13.635±0.04 d | 4.307±0.02 a | 21.120±0.85 b |
| 5FA | 1.461±0.02 b | 23.852±0.60 a | 23.495±0.03 a | 2.261±0.01 c | 19.405±0.01 d |
| 5UFA | 1.690±0.05 a | 19.087±0.09 c | 14.561±0.07 c | 4.585±0.14 a | 22.360±0.28 a |
| 8FA | 0.958±0.01 c | 23.277±0.03 a | 18.325±0.00 b | 1.646±0.04 d | 19.101±0.76 d |
| 8UFA | 1.409±0.01 b | 20.310±0.06 b | 14.031±0.04 c | 3.536±0.04 b | 21.960±0.28 ab |
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
Mineral composition of the biscuits
Na concentration ranged from 15.495 ppm in fermented mix 8 to 27.501 ppm in the control biscuit sample, with the control group having an intermediate value of 22.945 ppm. K concentration ranged from 19.398 ppm in unfermented mix 5 to 25.264 ppm in fermented mix 2, with the control group having a value of 20.601 ppm. Ca concentration ranged from 10.505 ppm in fermented mix 5 to 14.401 ppm in unfermented mix 8, with the control group having a value of 14.099 ppm. Mg concentration ranged from 9.200 ppm in control to 15.160 ppm in fermented mix 5. Fe concentration value of 0.087 ppm was recorded in the control, while the value of 0.218 ppm was recorded in the unfermented mix 2. Zn concentration ranged from 0.163 ppm in fermented mix 2 to 0.329 ppm in fermented mix 5, with the control group having a value of 0.194 ppm. P concentration ranged from 7.093 ppm in fermented mix 8 to 11.090 ppm in fermented mix 5,
with the control group having a value of 8.555 ppm (Table 4).
Na concentration ranged from 15.495 ppm in fermented mix 8 to 27.501 ppm in the control biscuit sample, with the control group having an intermediate value of 22.945 ppm. K concentration ranged from 19.398 ppm in unfermented mix 5 to 25.264 ppm in fermented mix 2, with the control group having a value of 20.601 ppm. Ca concentration ranged from 10.505 ppm in fermented mix 5 to 14.401 ppm in unfermented mix 8, with the control group having a value of 14.099 ppm. Mg concentration ranged from 9.200 ppm in control to 15.160 ppm in fermented mix 5. Fe concentration value of 0.087 ppm was recorded in the control, while the value of 0.218 ppm was recorded in the unfermented mix 2. Zn concentration ranged from 0.163 ppm in fermented mix 2 to 0.329 ppm in fermented mix 5, with the control group having a value of 0.194 ppm. P concentration ranged from 7.093 ppm in fermented mix 8 to 11.090 ppm in fermented mix 5,
with the control group having a value of 8.555 ppm (Table 4).
Table 4: Minerals analysis of biscuits (ppm)
| Sample | Sodium (Na) (ppm) |
Potassium (K) (ppm) |
Calcium (Ca) (ppm) |
Magnesium (Mg) (ppm) |
Iron (Fe) (ppm) |
Zinc (Zn) (ppm) |
Phosphorous (P) (ppm) |
| CA | 27.501±0.a | 20.601±0.0 d | 14.099±0 a | 9.200±0.0 e | 0.087±0 d | 0.194±0 c | 8.555±0.01 c |
| 2FA | 19.094±0.d | 25.264±0.5 a | 11.810±0 c | 9.997±0.0 d | 0.196±0 a | 0.163±0 d | 7.107±0.07 d |
| 2UFA | 21.993±0.c | 25.000±0.0 a | 13.209±0 b | 14.159±0 b | 0.218±0 a | 0.268±0 b | 8.595±0.03 c |
| 5FA | 17.105±0.e | 19.803±0.0 e | 10.505±0 d | 15.160±0 a | 0.100±0 c | 0.329±0 a | 11.090±0.0 a |
| 5UFA | 22.945±0.b | 19.398±0.0 f | 11.004±0 c | 12.800±0 c | 0.119±0 c | 0.284±0 b | 8.325±0.02 c |
| 8FA | 15.495±0.1f | 24.701±0.0 b | 13.300±0 b | 14.105±0 b | 0.167±0 b | 0.284±0 b | 7.093±0.01 d |
| 8UFA | 18.506±0.d | 21.500±0.0 c | 14.401±0 a | 15.004±0 a | 0.175±0 b | 0.200±0 c | 10.029±0.3 b |
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
Antioxidant composition of the biscuits
Table 5 unveiled the results which were observed in the fermented biscuits’ antioxidant effects and the unfermented flour blends mix (2, 5, and 8). The values illustrate that the fermented flour blends generally had higher antioxidant properties (such as Fe2+, DPPH) than the unfermented flour blends. The OH radical scavenging activity was highest in the fermented mix 5 with a value of 60.000% and least in unfermented mix 2. Significant differences (p≤0.05) failed to occur for ABTS radical scavenging activity in the flours except for unfermented mix 2. For DPPH radical scavenging activity, the highest value of 62.875% was recorded in fermented mix 2, while unfermented mix 8 had the lowest value. Fermented mix 8 had the highest Fe2+ chelation activity with a value of 40.242%, while the control had the lowest value of it. Regarding the FRAP assay, fermented mix 5 had the highest value, while the control sample had the lowest value.
Table 5 unveiled the results which were observed in the fermented biscuits’ antioxidant effects and the unfermented flour blends mix (2, 5, and 8). The values illustrate that the fermented flour blends generally had higher antioxidant properties (such as Fe2+, DPPH) than the unfermented flour blends. The OH radical scavenging activity was highest in the fermented mix 5 with a value of 60.000% and least in unfermented mix 2. Significant differences (p≤0.05) failed to occur for ABTS radical scavenging activity in the flours except for unfermented mix 2. For DPPH radical scavenging activity, the highest value of 62.875% was recorded in fermented mix 2, while unfermented mix 8 had the lowest value. Fermented mix 8 had the highest Fe2+ chelation activity with a value of 40.242%, while the control had the lowest value of it. Regarding the FRAP assay, fermented mix 5 had the highest value, while the control sample had the lowest value.
Table 5: Antioxidants Analysis of biscuits
| Samples | OH (%) | ABTS (mmol/g) | DPPH (%) | Fe2+ chelation (%) | FRAP (mg/g) |
| CA | 42.143±0.3 d | 0.025±0.00 a | 52.502±0.8 c | 2.980±0.23 f | 5.842±0.09 f |
| 2FA | 32.119±0.3 e | 0.026±0.00 a | 62.875±0.7 a | 21.950±1.32 c | 14.691±0.94 d |
| 2UFA | 11.825±0.1 g | 0.013±0.00 b | 34.503±0.1 f | 12.334±0.35 d | 6.545±0.01 e |
| 5FA | 60.000±0.4 a | 0.026±0.00 a | 55.608±0.4 b | 12.848±0.14 d | 26.309 ±0.76 a |
| 5UFA | 46.984±0.6 c | 0.025±0.00 a | 44.540±0.1 d | 8.020±2.24 e | 25.003 ±0.04 b |
| 8FA | 23.515±0.1 f | 0.021±0.00 a | 40.546±0.6 e | 40.242±0.22 a | 25.667±0.43 b |
| 8UFA | 57.849±0.3 b | 0.026±0.00 a | 33.287±0.6 g | 32.036±0.12 b | 24.399±0.09 c |
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
OH: Hydroxyl Radical; ABTS: 2,2’–Azino-Bis-(3-Ethylbenzothiazoline-6-Sulfonic acid); DPPH: 1,1-Diphenyl-2-Picrylhydrazyl; FRAP: Ferric Reducing Antioxidant Property
Color of the biscuits
Table 6 provides information about the color of biscuits made from fermented and unfermented biscuits (2, 5, and 8). The color values observed in the samples were between 0.205-2.325. The highest value was observed in fermented mix 5, and the lowest was observed in unfermented mix 2. The fermented mixes have higher color values compared to the unfermented mixes, with fermented mix 5 having the highest value among all samples.
Table 6 provides information about the color of biscuits made from fermented and unfermented biscuits (2, 5, and 8). The color values observed in the samples were between 0.205-2.325. The highest value was observed in fermented mix 5, and the lowest was observed in unfermented mix 2. The fermented mixes have higher color values compared to the unfermented mixes, with fermented mix 5 having the highest value among all samples.
Table 6: Color analysis of biscuits
| Samples | *Color value |
| CA | 0.803±0.00 c |
| 2FA | 0.305±0.01 e |
| 2UFA | 0.205±0.01 f |
| 5FA | 2.325±0.01 a |
| 5UFA | 1.206±0.01 b |
| 8FA | 0.695±0.01 d |
| 8UFA | 0.215±0.01 f |
Data presented as mean±SD, in each column, the values having different superscript of a-c in the same column are significantly different (p<0.05).
Key: CA=control A; FA=fermented sample; UFA=unfermented sample; 2, 5, 8=composite flour mixes
Discussion
The obtained results in the proximate of the analyzed biscuits recommend that fermentation has an effect being substantial on the nutritional component of the different biscuits. This finding correlates with the work reported earlier that changes in the nutrition content of food products will occur due to the fermentation process (Knez et al., 2023). In particular, the fermented biscuit products from different mixes had lower moisture content and higher ash content compared to the unfermented mixes. This can be linked to the reality that fermentation can decrease the water level of the flour and increase the mineral amount due to the action of microorganisms as found by N’zi et al. 2021. This increase observed in ash content is indicative of a higher mineral content, which can contribute to improved nutritional quality.
The fat content was identified to be decreased in values in the fermented biscuit sample in comparison with the samples unfermented. This correlates with the earlier report on fermented foods like maize (Mashau et al., 2021; Sanni and Adesulu, 2013.) It is assumed that the microorganisms involved in fermentation break down some of the fats into simpler components, resulting in a lower fat component.
The content of protein in the fermented samples was discovered to be higher compared to the unfermented samples. This finding is consistent with previous studies on fermented foods such as wheat flour (Charu et al., 2019) and beans (Garyfallia et al., 2020). It is thought that fermentation breaks down complex proteins into simpler components, increasing the overall protein content.
The antinutrient levels (tannins, oxalates) of the biscuits differ considerably from the control. The antinutrient composition of the biscuit samples varied depending on the type of biscuit used and whether or not it was fermented. Fermentation had a remarkable result on the antinutrient content of the biscuit samples. Overall, the fermented biscuits had higher saponin and flavonoid contents than the unfermented biscuits, while the unfermented biscuits had higher tannin content than the fermented biscuits. Moreover, the fermented biscuits had lower oxalate content compared to the unfermented biscuits. Observed in the biscuit samples were differences (p<0.05) that were significant between the samples in tannin, flavonoid, saponin, oxalate contents. The significant differences observed suggest that fermentation and flour type affected the antinutrient composition of the biscuits.
Among the different biscuits, the antinutrient composition also varied. For instance, unfermented biscuit mix 5 had the highest tannin content while fermented biscuit mix 5 had the highest flavonoid, saponin content. Fermented biscuit mix 8 had the lowest oxalate content. These findings are consistent with earlier reports that fermentation may positively affect the antinutrient composition of foods. For example, N’zi et al. (2021), detected that the fermentation of soybeans revealed a decreased value in tannin content while increasing the flavonoid, saponin content. The protein trypsin inhibitor hinders the action of trypsin, an enzyme responsible for protein digestion. This protein is often present in legumes and can cause digestive issues when consumed excessively. In this study, the fermented biscuit has lower trypsin inhibitor values than the unfermented mixes. Even though the values for each sample overlap with one another, the differences observed between the fermented and unfermented mixes are statistically significant (p<0.05). It is known that the fermentation process can decrease the levels of antinutrient factors such as trypsin inhibitors. Hence, the minor differences between the fermented and unfermented mixes imply that the fermentation process may have a limited impact on the digestibility of the biscuits (Knez et al., 2023; Sharma et al., 2020).
However, it’s essential to recognize that the trypsin inhibitor values for all samples are within safe consumption levels (5 to 25%), and the slight differences may not significantly infuence the nutritional value of the biscuits. Therefore, the antinutrient content of biscuits made from different biscuits varied depending on whether or not they were fermented. The findings clarified the importance of considering the effect of the preparation procedure on the antinutrient composition of foods.
The mineral analysis provides valuable information about the nutrient content of biscuits made from different types of composite biscuits. According to the data, the control sample has the highest concentration of Na, which is similar to the earlier report that uncovered high levels of Na in processed foods (Mente et al., 2014). The lower Na content in fermented mix 8 could be attributed to the fermentation process, which has been illustrated to have a decreased value of Na in samples of food (Walkers-Rooijackers et al., 2013).
The higher K content in fermented mix 2 and unfermented mix 2 is due to the report that has demonstrated high levels of k in whole grains and legumes (Drewnowski et al., 2015). The higher Ca content in unfermented mix 8 and control biscuit samples might be a result of the presence of Ca-rich ingredients such as milk and Ca-fortified flour (Lee et al., 2018). The higher Mg content in fermented mix 5 is in correlation with the earlier report that has shown that fermentation can increase the bioavailability of Mg in foods (Knez et al., 2023)
The higher Fe and Zn content in unfermented mix 2 and fermented mix 5 could be responsible for the availability of Fe and Zn-abundant ingredients such as beans and whole grains (Bouis and Saltzman, 2017). The higher P content in fermented mix 5 and unfermented mix 8 could be attributed to the presence of whole grains, which are known to be rich in phosphorous (Penn et al., 2023). The mineral analysis suggests that the fermentation process can have a positive impact on the nutrient content of biscuits, particularly in terms of reducing Na content and increasing the bioavailability of Mg.
The fermented flour blends generally had higher antioxidant characteristics compared to the unfermented flour blends. The fermented mix 2 had significantly higher radical scavenging and ABTS radical scavenging activities, Fe2+ chelation properties, and FRAP assay values compared to the unfermented mix 2. The same trend was observed for fermented mix 5, which had higher OH and DPPH radical scavenging activities, Fe2+ chelation properties, and FRAP assay content compared to the unfermented mix 5. Interestingly, fermented mix 8 had lower OH and ABTS radical scavenging properties as compared to unfermented mix 8. However, it had significantly higher Fe2+ chelation activity and DPPH assay content compared to the unfermented mix 8. These suggest that the fermentation process positively affects the flour blends in the profile of the antioxidant, likely since the production of biologically active constituents during the fermentation process. This observation correlates with earlier reports that have shown that the fermentation process can improve the profile of antioxidants in food products (Zhao et al., 2021). Furthermore, the results highlight the potential of using a combination of sweet potato, pigeon pea, and maize flour blends to produce biscuits with high antioxidant properties. This is important as antioxidants are acknowledged to play a significant part in averting or reducing illnesses that are related to oxalate stress like cancer, cardiovascular disease, and diabetes (Luo et al., 2022).
Regarding color, fermented mix 5 had the highest value, indicating a more intense color than the other samples. This could be the fermentation process releasing more pigments from the ingredients or promoting the products in Maillard reaction formation, as it may contribute to the browning of food majorly during cooking. This may influence the sensory properties of the biscuits hence color serves as an attractant in most foods.
Conclusions
The research investigated that ternary flour mixes from sweet potato, maize, and the pigeon pea utilized for biscuit production can result in products with improved sensory evaluation, nutritional composition and functional activities. Fermentation of the botanical ingredients further enhanced the qualities of the final biscuit products, including reduced trypsin inhibitor activity and increased antioxidant properties. These findings bear implications for the food industry, particularly in regions where these botanicals are abundant and of comparative advantage. The use of regional raw materials that are readily accessible such as flours from maize, pigeon pea, and sweet potato blends could not simply make better the nourishment of baked foods, it could as well contribute to sustainable agricultural practices across all its value chains and promote the consumption of traditional crops. Further research and development are warranted to explore the commercial viability and scalability of these formulations for widespread adoption. The study further underscores the potential of incorporating composite flour blends and fermentation techniques in the production of biscuits, offering an innovative approach to enhance the nutritional, techno-functional, and physicochemical qualities of baked biscuit snack staples through the utilization of indigenous agricultural resources.
Acknowledgments
We appreciate Mrs. Okonudo D. and Mr. Aigbojie J.E. for their technical assistance during this research.
Funding
This research is funded by Tertiary Education Trust Fund (TETFUND) Bath Number 10 2022.
Author contribution
P.O.O. contributed in designing the work, literature search, and writing of the manuscript; F.C.A. designed the work and writing of the manuscript; C.B.E.-E. managed the analyses of data and the manuscript writing. All authors read and approved the final manuscript.
Conflict of interest
The authors declared no conflict of interest.
Ethical consideration
Not applicable in work please.
References
Asssociation of Official Analytical Chemists (AOAC). (2012). Official methods of analysis. 19th edition. Association of Official Analytical Chemists, Arlington, Virginia.
Asssociation of Official Analytical Chemists (AOAC). (2016). Official methods of analysis of AOAC international. 20th edition. AOAC International, Rockville, Maryland.
Azeez W. (2021). Nigeria spends $2bn on wheat importation yearly, says CBN. The Cable New and Views Unlimited. URL: https://www.thecable.ng/ nigeria-spends-2bn-on-wheat-importation-yearly-says-cbn.
Bouis H.E., Saltzman A. (2017). Improving nutrition through biofortification: a review of evidence from HarvestPlus, 2003 through 2016. Global Food Security. 12: 49-58. [DOI: 10.1016/j.gfs.2017.01.009]
Chandra S., Singh S., Kumari D. (2015). Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. Journal of Food Science and Technology. 52: 3681-3688. [DOI: 10.1007/s13197-014-1427-2]
Charu S., Vivek K., Medha S., Ajit Kumar S., Anit K. (2019). Study of physicochemical properties of fermented wheat flour (sheera). Journal of Agricultural Engineering and Food Technology. 6: 271-274.
Drewnowski A., Rehm C.D., Maillot M., Mendoza A., Monsivais P. (2015). The feasibility of meeting the WHO guidelines for sodium and potassium: a cross-national comparison study. BMJ Open. 5: e006625. [DOI: 10.1136/bmjopen-2014-006625]
Garyfallia K., Rosario M., Jole M., Jesus M.P., Ignacio F.-F. (2020). Natural fermentation of cowpea (Vigna unguiculata) flour improves the nutritive utilization of indispensable amino acids and phosphorus by growing rats. Nutrients. 12: 2186. [DOI: 10.3390/nu12082186]
Girgih A.T., Udenigwe C.C., Aluko R.E. (2011). In-vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. Journal of the American Oil Chemists' Society. 88: 381-389. [DOI: 10.1007/s11746-010-1686-7]
Govindappa M., Naga Sravya S., Poojashri M.N., Sadananda T.S., Chandrappa C.P., Gustavo Santoyo, Sharanappa P., Anil Kumar N.V. (2011). Antimicrobial, antioxidant and in vitro anti-inflammatory activity and phytochemical screening of water extract of Wedelia trilobata (L.) hitchc. Journal of Medicinal Plants Research. 5: 5718-5729.
Hasmadi M., Noorfarahzilah M., Noraidah H., Zainol M.K., Jahurul M.H.A. (2020). Functional properties of composite flour: a review. Food Research. 4: 1820-1831. [DOI: 10.26656/ fr.2017.4(6).419]
Joshi-Saha A., Reddy K.S. (2015). Repeat length variation in the 5’UTR of myo-inositol monophosphatase gene is related to phytic acid content and contributes to drought tolerance in chickpea (Cicer arietinum L.). Journal of Experimental Botany. 66: 5683-5690. [DOI: 10.1093/jxb/erv156]
Karamad D., Khosravi-Darani K., Hosseini H., Tavasoli S. (2019). Analytical procedures and methods validation for oxalate content estimation. Biointerface research in applied chemistry. 9: 4305-4310. [DOI: 10.33263/briac95.305310]
Kiin-Kabari D.B., Giami S.Y. (2015). Physico chemical properties and in-vitro protein digestibility of non-wheat cookies prepared from plantain flour and bambara groundnut protein concentrate. Journal of Food Research. 4: 78-86. [DOI: 10.5539/jfr.v4n2p78]
Knez E., Kadac-Czapska K., Grembecka M. (2023). Effect of fermentation on the nutritional quality of the selected vegetables and legumes and their health effects. Life. 13: 655. [DOI: 10.3390/life13030655]
Kwofie M.K., Bukari N., Adeboye O. (2020). Probiotics potential of yeast and lactic acid bacteria fermented foods and the impact of processing: a review of indigenous and continental food products. Advances in Microbiology. 10: 492-507. [DOI: 10.4236/aim. 2020.109037]
Lee S., Choi Y., Jeong H.S., Lee J., Sung J. (2018). Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Science and Biotechnology. 27: 333-342. [DOI: 10.1007/s10068-017-0281-1]
Luo M., Zhou L., Huang Z., Li B., Nice E.C., Xu J., Huang C. (2022). Antioxidant therapy in cancer: rationale and progress. Antioxidants. 11: 1128. [DOI: 10.3390/ antiox11061128]
Mashau M.E., Maliwichi L.L., Jideani A.I.O. (2021). Non-alcoholic fermentation of maize (zea mays) in sub-saharan Africa. Fermentation. 7: 158. [DOI: 10.3390/ fermentation7030158]
Matamane R.P., Pillai M.K., Magama S. (2020). DPPH radical scavenging activity of extracts from Urtica urens (Urticaceae). Journal of Medicinal Plants Research. 14: 232-238. [DOI: 10.5897/ JMPR2019.6880]
Mhada M., Metougui M.L., El Hazzam K., El Kacimi K., Yasri A. (2020). Variations of saponins, minerals and total phenolic compounds due to processing and cooking of quinoa (Chenopodium quinoa Willd.) seeds. Foods. 9: 660. [DOI: 10.3390/foods9050660]
Mente A., O'Donnell M.J., Rangarajan S., McQueen M.J., Poirier P., Wielgosz A., Morrison H., Li W., Wang X., Di C., Mony P., Devanath A., et al. (2014). Association of urinary sodium and potassium excretion with blood pressure. The New England Journal of Medicine. 371: 601-611. [DOI: 10.1056/ NEJMoa1311989]
Ntrallou K., Gika H., Tsochatzis E. (2020). Analytical and sample preparation techniques for the determination of food colorants in food matrices. Foods. 9: 58. [DOI: 10.3390/foods9010058]
N’zi K.P., Adingra K.M.-D., N’guessan K.F., Attchelouwa K.C., Tano K. (2021). Effect of spontaneous fermentation time on physicochemical, nutrient, anti-nutrient and microbiological composition of Lima bean (Phaseolus lunatus) flour. Journal of Applied Biosciences. 162: 16707-16725. [DOI: 10.35759/ JABs.162.3]
Okojie J. (2022). Wheat production drops despite FG’s funding, surging demand. Business Day. URL: https:// businessday.ng/ agriculture/article/wheat-production- drops-despite-fgs-funding-surging-demand/.
Oleghe P.O., Akharaiyi F.C., Ehis-Eriakha C.B., Oladebeye A.A., Johnson D.O. (2023). Microbiological and techno-functional assessment of unfermented and fermented gluten-free flour mixes. International Journal of Life Sciences Research. 11: 33-48. [DOI: 10.5281/zenodo.7984260].
Onabanjo O.O., Ighere D.A. (2014). Nutritional, functional and sensory properties of biscuit produced from wheat-sweet potato composite. Journal of Food Technology Research. 1: 111-121.
Penn C.J., Camberato J.J., Wiethorn M.A. (2023). How much phosphorus uptake is required for achieving maximum maize grain yield? part 2: impact of phosphorus uptake on grain quality and partitioning of nutrients. Agronomy. 13: 258. [DOI: 10.3390/ agronomy13010258]
Sanni A.I., Adesulu A.T. (2013). Microbiological and physico-chemical changes during fermentation of maize for masa production. African Journal of Microbiology Research. 34: 4355-4362. [[DOI: 10.5897/AJMR12.1362]
Sharma R., Garg P., Kumar P., Bhatia S.K., Kulshrestha S. (2020). Microbial fermentation and its role in quality improvement of fermented foods. Fermentation. 6: 106. [DOI: 10.3390/fermentation6040106]
Singh R., Verma P.K., Singh G. (2012). Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. Journal of Intercultural Ethnopharmacology. 1: 101-104. [DOI: 10.5455/jice.20120525014326]
Sochor J., Dobes J., Krystofova O., Ruttkay-Nedecky B., Babula P., Pohanka M., Jurikova T., Zitka O., Adam V., Klejdus B., Kizek R. (2013). Electrochemistry as a tool for studying antioxidant properties. International Journal of Electrochemical Science. 8: 8464-8489.
Squeo G., De Angelis D., Leardi R., Summo C., Caponio F. (2021). Background, applications and issues of the experimental designs for mixture in the food sector. Foods. 10: 1128. [DOI: 10.3390/foods10051128]
Szmigielski M., Wesołowska-Janczarek M., Szczepanik M. (2010). Determination of trypsin inhibitor activity of microwave-heated bean seeds using bromocresole purple index (BCPI). Polish Journal of Food and Nutrition Sciences. 60: 329-333.
Walkers-Rooijackers J.C.M., Thomas S.M., Nout M.J.R. (2013). Effects of sodium reduction scenarios on fermentation and quality of sauerkraut. LWT - Food Science and Technology. 54: 383-388. [DOI: 10.1016/j.lwt.2013.07.002]
Wong F.-C., Yong A.-L., Ting E.P.-S., Khoo S.-C., Ongc H.-C., Chai T.-T. (2014). Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iranian Journal of Pharmaceutical Research. 13: 1409-1415.
Zhao Y.-S., Eweys A.S., Zhang J.-Y., Zhu Y., Bai J., Darwesh O.M., Zhang H.-B., Xiao X. (2021). Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants. 10: 2004. [DOI: 10.3390/antiox10122004]
Zhang W., Chen H., Wang Z., Lan G., Zhang L. (2013). Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai. Journal of Food Science and Technology. 50: 1122-1129. [DOI: 10.1007/ s13197-011-0447-4]
The obtained results in the proximate of the analyzed biscuits recommend that fermentation has an effect being substantial on the nutritional component of the different biscuits. This finding correlates with the work reported earlier that changes in the nutrition content of food products will occur due to the fermentation process (Knez et al., 2023). In particular, the fermented biscuit products from different mixes had lower moisture content and higher ash content compared to the unfermented mixes. This can be linked to the reality that fermentation can decrease the water level of the flour and increase the mineral amount due to the action of microorganisms as found by N’zi et al. 2021. This increase observed in ash content is indicative of a higher mineral content, which can contribute to improved nutritional quality.
The fat content was identified to be decreased in values in the fermented biscuit sample in comparison with the samples unfermented. This correlates with the earlier report on fermented foods like maize (Mashau et al., 2021; Sanni and Adesulu, 2013.) It is assumed that the microorganisms involved in fermentation break down some of the fats into simpler components, resulting in a lower fat component.
The content of protein in the fermented samples was discovered to be higher compared to the unfermented samples. This finding is consistent with previous studies on fermented foods such as wheat flour (Charu et al., 2019) and beans (Garyfallia et al., 2020). It is thought that fermentation breaks down complex proteins into simpler components, increasing the overall protein content.
The antinutrient levels (tannins, oxalates) of the biscuits differ considerably from the control. The antinutrient composition of the biscuit samples varied depending on the type of biscuit used and whether or not it was fermented. Fermentation had a remarkable result on the antinutrient content of the biscuit samples. Overall, the fermented biscuits had higher saponin and flavonoid contents than the unfermented biscuits, while the unfermented biscuits had higher tannin content than the fermented biscuits. Moreover, the fermented biscuits had lower oxalate content compared to the unfermented biscuits. Observed in the biscuit samples were differences (p<0.05) that were significant between the samples in tannin, flavonoid, saponin, oxalate contents. The significant differences observed suggest that fermentation and flour type affected the antinutrient composition of the biscuits.
Among the different biscuits, the antinutrient composition also varied. For instance, unfermented biscuit mix 5 had the highest tannin content while fermented biscuit mix 5 had the highest flavonoid, saponin content. Fermented biscuit mix 8 had the lowest oxalate content. These findings are consistent with earlier reports that fermentation may positively affect the antinutrient composition of foods. For example, N’zi et al. (2021), detected that the fermentation of soybeans revealed a decreased value in tannin content while increasing the flavonoid, saponin content. The protein trypsin inhibitor hinders the action of trypsin, an enzyme responsible for protein digestion. This protein is often present in legumes and can cause digestive issues when consumed excessively. In this study, the fermented biscuit has lower trypsin inhibitor values than the unfermented mixes. Even though the values for each sample overlap with one another, the differences observed between the fermented and unfermented mixes are statistically significant (p<0.05). It is known that the fermentation process can decrease the levels of antinutrient factors such as trypsin inhibitors. Hence, the minor differences between the fermented and unfermented mixes imply that the fermentation process may have a limited impact on the digestibility of the biscuits (Knez et al., 2023; Sharma et al., 2020).
However, it’s essential to recognize that the trypsin inhibitor values for all samples are within safe consumption levels (5 to 25%), and the slight differences may not significantly infuence the nutritional value of the biscuits. Therefore, the antinutrient content of biscuits made from different biscuits varied depending on whether or not they were fermented. The findings clarified the importance of considering the effect of the preparation procedure on the antinutrient composition of foods.
The mineral analysis provides valuable information about the nutrient content of biscuits made from different types of composite biscuits. According to the data, the control sample has the highest concentration of Na, which is similar to the earlier report that uncovered high levels of Na in processed foods (Mente et al., 2014). The lower Na content in fermented mix 8 could be attributed to the fermentation process, which has been illustrated to have a decreased value of Na in samples of food (Walkers-Rooijackers et al., 2013).
The higher K content in fermented mix 2 and unfermented mix 2 is due to the report that has demonstrated high levels of k in whole grains and legumes (Drewnowski et al., 2015). The higher Ca content in unfermented mix 8 and control biscuit samples might be a result of the presence of Ca-rich ingredients such as milk and Ca-fortified flour (Lee et al., 2018). The higher Mg content in fermented mix 5 is in correlation with the earlier report that has shown that fermentation can increase the bioavailability of Mg in foods (Knez et al., 2023)
The higher Fe and Zn content in unfermented mix 2 and fermented mix 5 could be responsible for the availability of Fe and Zn-abundant ingredients such as beans and whole grains (Bouis and Saltzman, 2017). The higher P content in fermented mix 5 and unfermented mix 8 could be attributed to the presence of whole grains, which are known to be rich in phosphorous (Penn et al., 2023). The mineral analysis suggests that the fermentation process can have a positive impact on the nutrient content of biscuits, particularly in terms of reducing Na content and increasing the bioavailability of Mg.
The fermented flour blends generally had higher antioxidant characteristics compared to the unfermented flour blends. The fermented mix 2 had significantly higher radical scavenging and ABTS radical scavenging activities, Fe2+ chelation properties, and FRAP assay values compared to the unfermented mix 2. The same trend was observed for fermented mix 5, which had higher OH and DPPH radical scavenging activities, Fe2+ chelation properties, and FRAP assay content compared to the unfermented mix 5. Interestingly, fermented mix 8 had lower OH and ABTS radical scavenging properties as compared to unfermented mix 8. However, it had significantly higher Fe2+ chelation activity and DPPH assay content compared to the unfermented mix 8. These suggest that the fermentation process positively affects the flour blends in the profile of the antioxidant, likely since the production of biologically active constituents during the fermentation process. This observation correlates with earlier reports that have shown that the fermentation process can improve the profile of antioxidants in food products (Zhao et al., 2021). Furthermore, the results highlight the potential of using a combination of sweet potato, pigeon pea, and maize flour blends to produce biscuits with high antioxidant properties. This is important as antioxidants are acknowledged to play a significant part in averting or reducing illnesses that are related to oxalate stress like cancer, cardiovascular disease, and diabetes (Luo et al., 2022).
Regarding color, fermented mix 5 had the highest value, indicating a more intense color than the other samples. This could be the fermentation process releasing more pigments from the ingredients or promoting the products in Maillard reaction formation, as it may contribute to the browning of food majorly during cooking. This may influence the sensory properties of the biscuits hence color serves as an attractant in most foods.
Conclusions
The research investigated that ternary flour mixes from sweet potato, maize, and the pigeon pea utilized for biscuit production can result in products with improved sensory evaluation, nutritional composition and functional activities. Fermentation of the botanical ingredients further enhanced the qualities of the final biscuit products, including reduced trypsin inhibitor activity and increased antioxidant properties. These findings bear implications for the food industry, particularly in regions where these botanicals are abundant and of comparative advantage. The use of regional raw materials that are readily accessible such as flours from maize, pigeon pea, and sweet potato blends could not simply make better the nourishment of baked foods, it could as well contribute to sustainable agricultural practices across all its value chains and promote the consumption of traditional crops. Further research and development are warranted to explore the commercial viability and scalability of these formulations for widespread adoption. The study further underscores the potential of incorporating composite flour blends and fermentation techniques in the production of biscuits, offering an innovative approach to enhance the nutritional, techno-functional, and physicochemical qualities of baked biscuit snack staples through the utilization of indigenous agricultural resources.
Acknowledgments
We appreciate Mrs. Okonudo D. and Mr. Aigbojie J.E. for their technical assistance during this research.
Funding
This research is funded by Tertiary Education Trust Fund (TETFUND) Bath Number 10 2022.
Author contribution
P.O.O. contributed in designing the work, literature search, and writing of the manuscript; F.C.A. designed the work and writing of the manuscript; C.B.E.-E. managed the analyses of data and the manuscript writing. All authors read and approved the final manuscript.
Conflict of interest
The authors declared no conflict of interest.
Ethical consideration
Not applicable in work please.
References
Asssociation of Official Analytical Chemists (AOAC). (2012). Official methods of analysis. 19th edition. Association of Official Analytical Chemists, Arlington, Virginia.
Asssociation of Official Analytical Chemists (AOAC). (2016). Official methods of analysis of AOAC international. 20th edition. AOAC International, Rockville, Maryland.
Azeez W. (2021). Nigeria spends $2bn on wheat importation yearly, says CBN. The Cable New and Views Unlimited. URL: https://www.thecable.ng/ nigeria-spends-2bn-on-wheat-importation-yearly-says-cbn.
Bouis H.E., Saltzman A. (2017). Improving nutrition through biofortification: a review of evidence from HarvestPlus, 2003 through 2016. Global Food Security. 12: 49-58. [DOI: 10.1016/j.gfs.2017.01.009]
Chandra S., Singh S., Kumari D. (2015). Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. Journal of Food Science and Technology. 52: 3681-3688. [DOI: 10.1007/s13197-014-1427-2]
Charu S., Vivek K., Medha S., Ajit Kumar S., Anit K. (2019). Study of physicochemical properties of fermented wheat flour (sheera). Journal of Agricultural Engineering and Food Technology. 6: 271-274.
Drewnowski A., Rehm C.D., Maillot M., Mendoza A., Monsivais P. (2015). The feasibility of meeting the WHO guidelines for sodium and potassium: a cross-national comparison study. BMJ Open. 5: e006625. [DOI: 10.1136/bmjopen-2014-006625]
Garyfallia K., Rosario M., Jole M., Jesus M.P., Ignacio F.-F. (2020). Natural fermentation of cowpea (Vigna unguiculata) flour improves the nutritive utilization of indispensable amino acids and phosphorus by growing rats. Nutrients. 12: 2186. [DOI: 10.3390/nu12082186]
Girgih A.T., Udenigwe C.C., Aluko R.E. (2011). In-vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. Journal of the American Oil Chemists' Society. 88: 381-389. [DOI: 10.1007/s11746-010-1686-7]
Govindappa M., Naga Sravya S., Poojashri M.N., Sadananda T.S., Chandrappa C.P., Gustavo Santoyo, Sharanappa P., Anil Kumar N.V. (2011). Antimicrobial, antioxidant and in vitro anti-inflammatory activity and phytochemical screening of water extract of Wedelia trilobata (L.) hitchc. Journal of Medicinal Plants Research. 5: 5718-5729.
Hasmadi M., Noorfarahzilah M., Noraidah H., Zainol M.K., Jahurul M.H.A. (2020). Functional properties of composite flour: a review. Food Research. 4: 1820-1831. [DOI: 10.26656/ fr.2017.4(6).419]
Joshi-Saha A., Reddy K.S. (2015). Repeat length variation in the 5’UTR of myo-inositol monophosphatase gene is related to phytic acid content and contributes to drought tolerance in chickpea (Cicer arietinum L.). Journal of Experimental Botany. 66: 5683-5690. [DOI: 10.1093/jxb/erv156]
Karamad D., Khosravi-Darani K., Hosseini H., Tavasoli S. (2019). Analytical procedures and methods validation for oxalate content estimation. Biointerface research in applied chemistry. 9: 4305-4310. [DOI: 10.33263/briac95.305310]
Kiin-Kabari D.B., Giami S.Y. (2015). Physico chemical properties and in-vitro protein digestibility of non-wheat cookies prepared from plantain flour and bambara groundnut protein concentrate. Journal of Food Research. 4: 78-86. [DOI: 10.5539/jfr.v4n2p78]
Knez E., Kadac-Czapska K., Grembecka M. (2023). Effect of fermentation on the nutritional quality of the selected vegetables and legumes and their health effects. Life. 13: 655. [DOI: 10.3390/life13030655]
Kwofie M.K., Bukari N., Adeboye O. (2020). Probiotics potential of yeast and lactic acid bacteria fermented foods and the impact of processing: a review of indigenous and continental food products. Advances in Microbiology. 10: 492-507. [DOI: 10.4236/aim. 2020.109037]
Lee S., Choi Y., Jeong H.S., Lee J., Sung J. (2018). Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Science and Biotechnology. 27: 333-342. [DOI: 10.1007/s10068-017-0281-1]
Luo M., Zhou L., Huang Z., Li B., Nice E.C., Xu J., Huang C. (2022). Antioxidant therapy in cancer: rationale and progress. Antioxidants. 11: 1128. [DOI: 10.3390/ antiox11061128]
Mashau M.E., Maliwichi L.L., Jideani A.I.O. (2021). Non-alcoholic fermentation of maize (zea mays) in sub-saharan Africa. Fermentation. 7: 158. [DOI: 10.3390/ fermentation7030158]
Matamane R.P., Pillai M.K., Magama S. (2020). DPPH radical scavenging activity of extracts from Urtica urens (Urticaceae). Journal of Medicinal Plants Research. 14: 232-238. [DOI: 10.5897/ JMPR2019.6880]
Mhada M., Metougui M.L., El Hazzam K., El Kacimi K., Yasri A. (2020). Variations of saponins, minerals and total phenolic compounds due to processing and cooking of quinoa (Chenopodium quinoa Willd.) seeds. Foods. 9: 660. [DOI: 10.3390/foods9050660]
Mente A., O'Donnell M.J., Rangarajan S., McQueen M.J., Poirier P., Wielgosz A., Morrison H., Li W., Wang X., Di C., Mony P., Devanath A., et al. (2014). Association of urinary sodium and potassium excretion with blood pressure. The New England Journal of Medicine. 371: 601-611. [DOI: 10.1056/ NEJMoa1311989]
Ntrallou K., Gika H., Tsochatzis E. (2020). Analytical and sample preparation techniques for the determination of food colorants in food matrices. Foods. 9: 58. [DOI: 10.3390/foods9010058]
N’zi K.P., Adingra K.M.-D., N’guessan K.F., Attchelouwa K.C., Tano K. (2021). Effect of spontaneous fermentation time on physicochemical, nutrient, anti-nutrient and microbiological composition of Lima bean (Phaseolus lunatus) flour. Journal of Applied Biosciences. 162: 16707-16725. [DOI: 10.35759/ JABs.162.3]
Okojie J. (2022). Wheat production drops despite FG’s funding, surging demand. Business Day. URL: https:// businessday.ng/ agriculture/article/wheat-production- drops-despite-fgs-funding-surging-demand/.
Oleghe P.O., Akharaiyi F.C., Ehis-Eriakha C.B., Oladebeye A.A., Johnson D.O. (2023). Microbiological and techno-functional assessment of unfermented and fermented gluten-free flour mixes. International Journal of Life Sciences Research. 11: 33-48. [DOI: 10.5281/zenodo.7984260].
Onabanjo O.O., Ighere D.A. (2014). Nutritional, functional and sensory properties of biscuit produced from wheat-sweet potato composite. Journal of Food Technology Research. 1: 111-121.
Penn C.J., Camberato J.J., Wiethorn M.A. (2023). How much phosphorus uptake is required for achieving maximum maize grain yield? part 2: impact of phosphorus uptake on grain quality and partitioning of nutrients. Agronomy. 13: 258. [DOI: 10.3390/ agronomy13010258]
Sanni A.I., Adesulu A.T. (2013). Microbiological and physico-chemical changes during fermentation of maize for masa production. African Journal of Microbiology Research. 34: 4355-4362. [[DOI: 10.5897/AJMR12.1362]
Sharma R., Garg P., Kumar P., Bhatia S.K., Kulshrestha S. (2020). Microbial fermentation and its role in quality improvement of fermented foods. Fermentation. 6: 106. [DOI: 10.3390/fermentation6040106]
Singh R., Verma P.K., Singh G. (2012). Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. Journal of Intercultural Ethnopharmacology. 1: 101-104. [DOI: 10.5455/jice.20120525014326]
Sochor J., Dobes J., Krystofova O., Ruttkay-Nedecky B., Babula P., Pohanka M., Jurikova T., Zitka O., Adam V., Klejdus B., Kizek R. (2013). Electrochemistry as a tool for studying antioxidant properties. International Journal of Electrochemical Science. 8: 8464-8489.
Squeo G., De Angelis D., Leardi R., Summo C., Caponio F. (2021). Background, applications and issues of the experimental designs for mixture in the food sector. Foods. 10: 1128. [DOI: 10.3390/foods10051128]
Szmigielski M., Wesołowska-Janczarek M., Szczepanik M. (2010). Determination of trypsin inhibitor activity of microwave-heated bean seeds using bromocresole purple index (BCPI). Polish Journal of Food and Nutrition Sciences. 60: 329-333.
Walkers-Rooijackers J.C.M., Thomas S.M., Nout M.J.R. (2013). Effects of sodium reduction scenarios on fermentation and quality of sauerkraut. LWT - Food Science and Technology. 54: 383-388. [DOI: 10.1016/j.lwt.2013.07.002]
Wong F.-C., Yong A.-L., Ting E.P.-S., Khoo S.-C., Ongc H.-C., Chai T.-T. (2014). Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iranian Journal of Pharmaceutical Research. 13: 1409-1415.
Zhao Y.-S., Eweys A.S., Zhang J.-Y., Zhu Y., Bai J., Darwesh O.M., Zhang H.-B., Xiao X. (2021). Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants. 10: 2004. [DOI: 10.3390/antiox10122004]
Zhang W., Chen H., Wang Z., Lan G., Zhang L. (2013). Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai. Journal of Food Science and Technology. 50: 1122-1129. [DOI: 10.1007/ s13197-011-0447-4]
* Corresponding author (F.C. Akharaiyi)
* E-mail: akharaiyi.fred@edouniversity.edu.ng
ORCID ID: https://orcid.org/0000-0001-5605-5543
* E-mail: akharaiyi.fred@edouniversity.edu.ng
ORCID ID: https://orcid.org/0000-0001-5605-5543
Type of Study: Original article |
Subject:
Special
Received: 23/07/04 | Accepted: 24/01/25 | Published: 24/03/26
Received: 23/07/04 | Accepted: 24/01/25 | Published: 24/03/26
References
1. Asssociation of Official Analytical Chemists (AOAC). (2012). Official methods of analysis. 19th edition. Association of Official Analytical Chemists, Arlington, Virginia.
2. Asssociation of Official Analytical Chemists (AOAC). (2016). Official methods of analysis of AOAC international. 20th edition. AOAC International, Rockville, Maryland.
3. Azeez W. (2021). Nigeria spends $2bn on wheat importation yearly, says CBN. The Cable New and Views Unlimited. URL: https://www.thecable.ng/ nigeria-spends-2bn-on-wheat-importation-yearly-says-cbn.
4. Bouis H.E., Saltzman A. (2017). Improving nutrition through biofortification: a review of evidence from HarvestPlus, 2003 through 2016. Global Food Security. 12: 49-58. [DOI: 10.1016/j.gfs.2017.01.009] [DOI:10.1016/j.gfs.2017.01.009] [PMID] [PMCID]
5. Chandra S., Singh S., Kumari D. (2015). Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. Journal of Food Science and Technology. 52: 3681-3688. [DOI: 10.1007/s13197-014-1427-2] [DOI:10.1007/s13197-014-1427-2]
6. Charu S., Vivek K., Medha S., Ajit Kumar S., Anit K. (2019). Study of physicochemical properties of fermented wheat flour (sheera). Journal of Agricultural Engineering and Food Technology. 6: 271-274.
7. Drewnowski A., Rehm C.D., Maillot M., Mendoza A., Monsivais P. (2015). The feasibility of meeting the WHO guidelines for sodium and potassium: a cross-national comparison study. BMJ Open. 5: e006625. [DOI: 10.1136/bmjopen-2014-006625] [DOI:10.1136/bmjopen-2014-006625] [PMID] [PMCID]
8. Garyfallia K., Rosario M., Jole M., Jesus M.P., Ignacio F.-F. (2020). Natural fermentation of cowpea (Vigna unguiculata) flour improves the nutritive utilization of indispensable amino acids and phosphorus by growing rats. Nutrients. 12: 2186. [DOI: 10.3390/nu12082186] [DOI:10.3390/nu12082186] [PMID] [PMCID]
9. Girgih A.T., Udenigwe C.C., Aluko R.E. (2011). In-vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. Journal of the American Oil Chemists' Society. 88: 381-389. [DOI: 10.1007/s11746-010-1686-7] [DOI:10.1007/s11746-010-1686-7]
10. Govindappa M., Naga Sravya S., Poojashri M.N., Sadananda T.S., Chandrappa C.P., Gustavo Santoyo, Sharanappa P., Anil Kumar N.V. (2011). Antimicrobial, antioxidant and in vitro anti-inflammatory activity and phytochemical screening of water extract of Wedelia trilobata (L.) hitchc. Journal of Medicinal Plants Research. 5: 5718-5729.
11. Hasmadi M., Noorfarahzilah M., Noraidah H., Zainol M.K., Jahurul M.H.A. (2020). Functional properties of composite flour: a review. Food Research. 4: 1820-1831. [DOI: 10.26656/ fr.2017.4(6).419] [DOI:10.26656/fr.2017.4(6).419]
12. Joshi-Saha A., Reddy K.S. (2015). Repeat length variation in the 5'UTR of myo-inositol monophosphatase gene is related to phytic acid content and contributes to drought tolerance in chickpea (Cicer arietinum L.). Journal of Experimental Botany. 66: 5683-5690. [DOI: 10.1093/jxb/erv156] [DOI:10.1093/jxb/erv156] [PMID]
13. Karamad D., Khosravi-Darani K., Hosseini H., Tavasoli S. (2019). Analytical procedures and methods validation for oxalate content estimation. Biointerface research in applied chemistry. 9: 4305-4310. [DOI: 10.33263/briac95.305310] [DOI:10.33263/BRIAC95.305310] [PMID] [PMCID]
14. Kiin-Kabari D.B., Giami S.Y. (2015). Physico chemical properties and in-vitro protein digestibility of non-wheat cookies prepared from plantain flour and bambara groundnut protein concentrate. Journal of Food Research. 4: 78-86. [DOI: 10.5539/jfr.v4n2p78] [DOI:10.5539/jfr.v4n2p78]
15. Knez E., Kadac-Czapska K., Grembecka M. (2023). Effect of fermentation on the nutritional quality of the selected vegetables and legumes and their health effects. Life. 13: 655. [DOI: 10.3390/life13030655] [DOI:10.3390/life13030655] [PMID] [PMCID]
16. Kwofie M.K., Bukari N., Adeboye O. (2020). Probiotics potential of yeast and lactic acid bacteria fermented foods and the impact of processing: a review of indigenous and continental food products. Advances in Microbiology. 10: 492-507. [DOI: 10.4236/aim. 2020.109037] [DOI:10.4236/aim.2020.109037]
17. Lee S., Choi Y., Jeong H.S., Lee J., Sung J. (2018). Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Science and Biotechnology. 27: 333-342. [DOI: 10.1007/s10068-017-0281-1] [DOI:10.1007/s10068-017-0281-1]
18. Luo M., Zhou L., Huang Z., Li B., Nice E.C., Xu J., Huang C. (2022). Antioxidant therapy in cancer: rationale and progress. Antioxidants. 11: 1128. [DOI: 10.3390/ antiox11061128] [DOI:10.3390/antiox11061128]
19. Mashau M.E., Maliwichi L.L., Jideani A.I.O. (2021). Non-alcoholic fermentation of maize (zea mays) in sub-saharan Africa. Fermentation. 7: 158. [DOI: 10.3390/ fermentation7030158] [DOI:10.3390/fermentation7030158]
20. Matamane R.P., Pillai M.K., Magama S. (2020). DPPH radical scavenging activity of extracts from Urtica urens (Urticaceae). Journal of Medicinal Plants Research. 14: 232-238. [DOI: 10.5897/ JMPR2019.6880] [DOI:10.5897/JMPR2019.6880]
21. Mhada M., Metougui M.L., El Hazzam K., El Kacimi K., Yasri A. (2020). Variations of saponins, minerals and total phenolic compounds due to processing and cooking of quinoa (Chenopodium quinoa Willd.) seeds. Foods. 9: 660. [DOI: 10.3390/foods9050660] [DOI:10.3390/foods9050660] [PMID] [PMCID]
22. Mente A., O'Donnell M.J., Rangarajan S., McQueen M.J., Poirier P., Wielgosz A., Morrison H., Li W., Wang X., Di C., Mony P., Devanath A., et al. (2014). Association of urinary sodium and potassium excretion with blood pressure. The New England Journal of Medicine. 371: 601-611. [DOI: 10.1056/ NEJMoa1311989] [DOI:10.1056/NEJMoa1311989]
23. Ntrallou K., Gika H., Tsochatzis E. (2020). Analytical and sample preparation techniques for the determination of food colorants in food matrices. Foods. 9: 58. [DOI: 10.3390/foods9010058] [DOI:10.3390/foods9010058] [PMID] [PMCID]
24. N'zi K.P., Adingra K.M.-D., N'guessan K.F., Attchelouwa K.C., Tano K. (2021). Effect of spontaneous fermentation time on physicochemical, nutrient, anti-nutrient and microbiological composition of Lima bean (Phaseolus lunatus) flour. Journal of Applied Biosciences. 162: 16707-16725. [DOI: 10.35759/ JABs.162.3] [DOI:10.35759/JABs.162.3]
25. Okojie J. (2022). Wheat production drops despite FG's funding, surging demand. Business Day. URL: https:// businessday.ng/ agriculture/article/wheat-production- drops-despite-fgs-funding-surging-demand/.
26. Oleghe P.O., Akharaiyi F.C., Ehis-Eriakha C.B., Oladebeye A.A., Johnson D.O. (2023). Microbiological and techno-functional assessment of unfermented and fermented gluten-free flour mixes. International Journal of Life Sciences Research. 11: 33-48. [DOI: 10.5281/zenodo.7984260].
27. Onabanjo O.O., Ighere D.A. (2014). Nutritional, functional and sensory properties of biscuit produced from wheat-sweet potato composite. Journal of Food Technology Research. 1: 111-121. [DOI:10.18488/journal.58/2014.1.2/58.2.111.121]
28. Penn C.J., Camberato J.J., Wiethorn M.A. (2023). How much phosphorus uptake is required for achieving maximum maize grain yield? part 2: impact of phosphorus uptake on grain quality and partitioning of nutrients. Agronomy. 13: 258. [DOI: 10.3390/ agronomy13010258] [DOI:10.3390/agronomy13010258]
29. Sanni A.I., Adesulu A.T. (2013). Microbiological and physico-chemical changes during fermentation of maize for masa production. African Journal of Microbiology Research. 34: 4355-4362. [[DOI: 10.5897/AJMR12.1362]
30. Sharma R., Garg P., Kumar P., Bhatia S.K., Kulshrestha S. (2020). Microbial fermentation and its role in quality improvement of fermented foods. Fermentation. 6: 106. [DOI: 10.3390/fermentation6040106] [DOI:10.3390/fermentation6040106]
31. Singh R., Verma P.K., Singh G. (2012). Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. Journal of Intercultural Ethnopharmacology. 1: 101-104. [DOI: 10.5455/jice.20120525014326] [DOI:10.5455/jice.20120525014326]
32. Sochor J., Dobes J., Krystofova O., Ruttkay-Nedecky B., Babula P., Pohanka M., Jurikova T., Zitka O., Adam V., Klejdus B., Kizek R. (2013). Electrochemistry as a tool for studying antioxidant properties. International Journal of Electrochemical Science. 8: 8464-8489. [DOI:10.1016/S1452-3981(23)12902-6]
33. Squeo G., De Angelis D., Leardi R., Summo C., Caponio F. (2021). Background, applications and issues of the experimental designs for mixture in the food sector. Foods. 10: 1128. [DOI: 10.3390/foods10051128] [DOI:10.3390/foods10051128] [PMID] [PMCID]
34. Szmigielski M., Wesołowska-Janczarek M., Szczepanik M. (2010). Determination of trypsin inhibitor activity of microwave-heated bean seeds using bromocresole purple index (BCPI). Polish Journal of Food and Nutrition Sciences. 60: 329-333.
35. Walkers-Rooijackers J.C.M., Thomas S.M., Nout M.J.R. (2013). Effects of sodium reduction scenarios on fermentation and quality of sauerkraut. LWT - Food Science and Technology. 54: 383-388. [DOI: 10.1016/j.lwt.2013.07.002] [DOI:10.1016/j.lwt.2013.07.002]
36. Wong F.-C., Yong A.-L., Ting E.P.-S., Khoo S.-C., Ongc H.-C., Chai T.-T. (2014). Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iranian Journal of Pharmaceutical Research. 13: 1409-1415.
37. Zhao Y.-S., Eweys A.S., Zhang J.-Y., Zhu Y., Bai J., Darwesh O.M., Zhang H.-B., Xiao X. (2021). Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants. 10: 2004. [DOI: 10.3390/antiox10122004] [DOI:10.3390/antiox10122004] [PMID] [PMCID]
38. Zhang W., Chen H., Wang Z., Lan G., Zhang L. (2013). Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai. Journal of Food Science and Technology. 50: 1122-1129. [DOI: 10.1007/ s13197-011-0447-4] [DOI:10.1007/s13197-011-0447-4]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







