Volume 11, Issue 2 (June 2024)
J. Food Qual. Hazards Control 2024, 11(2): 116-126 |
Back to browse issues page
Ethics code: 0000
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bekka-Hadji F. Evaluation the Ingredients and Influence of Thermal Treatment on Physicochemical Properties and Bioactive Compounds of Evernia prunastri from Algeria. J. Food Qual. Hazards Control 2024; 11 (2) :116-126
URL: http://jfqhc.ssu.ac.ir/article-1-1147-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1147-en.html
Département de Microbiologie Appliquée et Sciences Alimentaires, Faculté des Sciences de la Nature et de la Vie, Université de Jijel, Jijel 18000, Algeria, Laboratoire d’Ecologie Microbienne, Faculté des Sciences de la Nature et de la Vie, Université de Bejaia, Bejaia 06000, Algeria , fahima.bekka@univ-jijel.dz
Full-Text [PDF 574 kb]
(508 Downloads)
| Abstract (HTML) (1141 Views)
The principle of the method using Kjeldahl machine (Gerhardt, Germany) is based on the conversion of organic nitrogen in 1 g of lichen into ammonium sulfate (FLUKA, Germany) through the action of concentrated sulfuric acid in the presence of a catalyst. Distillation is then fulfilled in the form of ammonium in the presence of 35% sodium hydroxide (NaOH; VWR, France). Titration is accomplished with a 0.1 N H2S04 solution (Thiex et al., 2002).
Full-Text: (20 Views)
Evaluation the Ingredients and Influence of Thermal Treatment on Physicochemical Properties and Bioactive Compounds of Evernia prunastri from Algeria
F. Bekka-Hadji 1,2**
1. Département de Microbiologie Appliquée et Sciences Alimentaires, Faculté des Sciences de la Nature et de la Vie, Université de Jijel, Jijel 18000, Algeria
2. Laboratoire d’Ecologie Microbienne, Faculté des Sciences de la Nature et de la Vie, Université de Bejaia, Bejaia 06000, Algeria
HIGHLIGHTS
F. Bekka-Hadji 1,2**
1. Département de Microbiologie Appliquée et Sciences Alimentaires, Faculté des Sciences de la Nature et de la Vie, Université de Jijel, Jijel 18000, Algeria
2. Laboratoire d’Ecologie Microbienne, Faculté des Sciences de la Nature et de la Vie, Université de Bejaia, Bejaia 06000, Algeria
- The proximate composition of this lichen was rich in carbohydrates, ash, and fibers.
- Heating at 70 °C led to an increase in all studied parameters, except proteins.
- Heating at 100 °C led to a decrease in proteins, polyphenols, and flavonoids contents and an increase in sugars content.
| Article type Original article |
ABSTRACT Background: Lichen is a symbiotic organism with unique structure composed of a fungal partner and an algal partner. It has been utilized in folk foods and medicines. The objective of the present study was to analyze the ingredients and the impact of heating on various physicochemical and phytochemical characteristics of a 500 g sample of lichen Evernia prunastri from Jijel, Algeria collected in May 2021. Methods: The nutritional value of the lichen was determined by studying various parameters. The metal contents were analyzed using an Atomic Absorption Spectrophotometer. The effect of heating (70 and 100 °C) on the physicochemical parameters, and bioactive compounds of aqueous extracts obtained by maceration in distilled water was studied. Statistical analysis was performed using ANOVA. Results: A high percentage of carbohydrates (64.00%) were observed in the lichen material. Pectin, fats, and crude proteins levels were low. High ash content (10.85%) was obtained with the presence of metal elements. Preliminary phytochemical analysis indicated the presence of polyphenols and flavonoids with 51.13 mg Gallic Acid Equivalent (GAE)/100 g and 17.87 mg Quercetin Equivalent (QE)/100 g of lichen, respectively. The lichen sample subjected to 70 °C demonstrated an increase in the parameters examined, with the exception of proteins and vitamin C. Conversely, the lichen sample treated at 100 °C exhibited a reduction in vitamin C, protein, and bioactive compound content, as well as an increase in sugar content. Titratable acidity and pH parameters were unaffected. Conclusion: The appreciable quantities of carbohydrates, crude fibers, and other compounds in this lichen suggest a potential use for these active ingredients in the preparation of functional food. Heating to 70 °C produces a preparation enriched in bioactive substances and sugars. However, heating to 100 °C, exposes the various tissues to degradation, potentially leading to a decrease in nutrients. © 2024, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Algeria Heating Lichens Nutritive Value |
||
| Article history Received: 1 Mar 2024 Revised: 20 May 2024 Accepted: 27 Jun 2024 |
||
| Acronyms and abbreviations AAS=Atomic Absorption Spectrometry BSAE=Bovine Serum Albumin Equivalent EC=Electrical Conductivity GE=Glucose Equivalent L-AA=L-Ascorbic Acid TTA=Total Titratable Acidity |
To cite: Bekka-Hadji F. (2024). Evaluation the ingredients and influence of thermal treatment on physicochemical properties and bioactive compounds of Evernia prunastri from Algeria. Journal of Food Quality and Hazards Control. 11: 116-126.
Introduction
Lichens are distinguished by the symbiotic association of fungi (microbiont) and algae (photobiont) or fungi and cyanobacteria, and they differ in chemical and structural composition from vascular plants (Akbulut and Yildiz, 2010; Ren et al., 2023). Living plants, dead wood, and plant debris are used as substrates (Favero-Longo and Piervittori, 2010). The ability of lichens to survive harsh conditions may be directly associated with the production of certain unique metabolites (Ren et al., 2023). Lichen growth is adversely impacted as the concentration of sulfur dioxide in the air surpasses 30μg/m³ in spite of the high sensitivity to external surroundings. Lichens are also sensitive to fluorine, vehicle emissions, hydrocarbons, and dust, tending to accumulate heavy metals more easily (Zhao et al., 2021). The diversity of lichens continuously decreases with increasing stress related to air pollution (Sujetoviene and Sliumpaite, 2013). Lichens can be utilized as bioindicators of pollution by heavy metals, organic compounds, and radioactive elements present in the air. An epiphytic lichen Evernia prunastri was used as an indicator of excessive ambient air nitrogen deposition worldwide (Bukabayeva et al., 2023; Lackovičová et al., 2013).
Lichens are used as traditional remedies, rich in nutritional values, and modified as powerful medicines in various pharmacopoeias (Bekka-Hadji and Adjeroud-Abdellatif, 2023). For centuries, lichens have been used in the nutrition by individuals during famine times. They are consumed worldwide, as a more regular food source in North America (Bryoria fremontii), even as a delicacy in Japan (Umbilicaria esculenta), or as a dessert in Scandinavia and France (Cetraria islandica) (Ivanova and Ivanov, 2009). Similarly, three lichen species have been identified as used by Nepalese populations (Everniastrum cirrhatum, Parmotrema cetratum and E. nepalense) (Gautam et al., 2021). The most frequent medicinal use is that of the"Iceland moss," C. islandica, which dates back to the year 1673 (Podterob, 2008). The decoction of this lichen is consumed to improve digestion and strengthen the stomach (Gautam et al., 2021).
Lichens represent a less recognized nutritional source that could assist in reducing malnutrition problems in most developing countries (Kumar et al., 2010; Vinayaka et al., 2009). The primary metabolites in lichens are mainly the same as in other plants, while the secondary metabolites remain indistinct, some of which are unique and specific to lichens (Aoussaret al., 2021; Podterob, 2008). They can be phenolic compounds, dibenzofurans, depsides, depsidones, aliphatic acids, quinones, pulvinic acid derivatives, and compounds related to anthraquinones, among others (Poulsen-Silva et al., 2023). These metabolites are probably antibiotics (acids) or involved in photosynthesis (atranorin), act as light filters (parietin), facilitate the transfer of carbohydrates from the photobiont to the mycobiont, or have roles in degrading the mineral substrate (Podterob, 2008).
Currently, the search for novel active pharmaceutical molecules from natural sources, such as lichens, has gained considerable attention within the pharmaceutical industry (Poulsen-Silva et al., 2023). Research on the secondary metabolites and nutritional value of lichens at the international level is restricted (Gautam et al., 2021), and lichens in Algeria are underexplored. This study seeks to analyze the proximate composition and the influence of heating on certain physicochemical features and bioactive compounds of a lichen species, E. prunastri, from the Jijel region (Algeria). In order to enhance this aspect, the work consists of an experimental section presenting the equipment and techniques employed to study proximal composition (moisture, ash, protein content, fat, pectin, and fiber). A number of physico-chemical parameters (pH, titratable acidity, Electrical Conductivity (EC), protein, sugar, and vitamin C content) and phytochemical parameters (phenolic compounds, flavonoids) were monitored after heating at 70 and 100 °C.
Lichens are used as traditional remedies, rich in nutritional values, and modified as powerful medicines in various pharmacopoeias (Bekka-Hadji and Adjeroud-Abdellatif, 2023). For centuries, lichens have been used in the nutrition by individuals during famine times. They are consumed worldwide, as a more regular food source in North America (Bryoria fremontii), even as a delicacy in Japan (Umbilicaria esculenta), or as a dessert in Scandinavia and France (Cetraria islandica) (Ivanova and Ivanov, 2009). Similarly, three lichen species have been identified as used by Nepalese populations (Everniastrum cirrhatum, Parmotrema cetratum and E. nepalense) (Gautam et al., 2021). The most frequent medicinal use is that of the"Iceland moss," C. islandica, which dates back to the year 1673 (Podterob, 2008). The decoction of this lichen is consumed to improve digestion and strengthen the stomach (Gautam et al., 2021).
Lichens represent a less recognized nutritional source that could assist in reducing malnutrition problems in most developing countries (Kumar et al., 2010; Vinayaka et al., 2009). The primary metabolites in lichens are mainly the same as in other plants, while the secondary metabolites remain indistinct, some of which are unique and specific to lichens (Aoussaret al., 2021; Podterob, 2008). They can be phenolic compounds, dibenzofurans, depsides, depsidones, aliphatic acids, quinones, pulvinic acid derivatives, and compounds related to anthraquinones, among others (Poulsen-Silva et al., 2023). These metabolites are probably antibiotics (acids) or involved in photosynthesis (atranorin), act as light filters (parietin), facilitate the transfer of carbohydrates from the photobiont to the mycobiont, or have roles in degrading the mineral substrate (Podterob, 2008).
Currently, the search for novel active pharmaceutical molecules from natural sources, such as lichens, has gained considerable attention within the pharmaceutical industry (Poulsen-Silva et al., 2023). Research on the secondary metabolites and nutritional value of lichens at the international level is restricted (Gautam et al., 2021), and lichens in Algeria are underexplored. This study seeks to analyze the proximate composition and the influence of heating on certain physicochemical features and bioactive compounds of a lichen species, E. prunastri, from the Jijel region (Algeria). In order to enhance this aspect, the work consists of an experimental section presenting the equipment and techniques employed to study proximal composition (moisture, ash, protein content, fat, pectin, and fiber). A number of physico-chemical parameters (pH, titratable acidity, Electrical Conductivity (EC), protein, sugar, and vitamin C content) and phytochemical parameters (phenolic compounds, flavonoids) were monitored after heating at 70 and 100 °C.
Materials and methods
Lichen preparation
Thalli from a sample of the lichen were collected in the Taksana region (Jijel, Northeast Algeria) in May 2021. The material was distinguished by Doctor S. Salem (Laboratory of Biotechnology, Environment, and Health, University of Jijel). Intact thalli of E. prunastri were dehydrated at 40 °C, ground using an electric grinder (Retsch Grindomix, Germany), and placed in containers for future use (proximate composition).
Determination of composition
-Moisture and volatile matter content
The procedure includes heating a quantity of lichen (2 g) in a drying oven (Memmert, Germany) at 105 °C until complete elimination of water and volatile matter and measuring the mass loss (Rozzamri et al., 2020).
Moisture (%)=(M-M2)/(M1-M0)×100
Where, M₀: weight of the empty crucible; M₁: weight of the crucible with the test sample prior to heating; M₂: weight of the crucible with the test sample after heating.
-Ash content
The method is based on the calcination of 2 g of lichen in a muffle furnace (Thermolyne, France) set at a controlled temperature of 550 °C until obtaining ashes of constant weight (AOAC, 2000).
Ash (%)=[(M1-M2)×100]/weight of test sample
Where, M1: weight of the crucible with the test sample; M2: weight of the crucible with the ash.
-Crude fibers content
For the determination of crude fiber content, 5 g of ground lichen were first treated with a 1.25% sulfuric acid (H2S04; Sharlab S.L., Spain) solution (10 ml), followed by a 1.25% potassium hydroxide (KOH; EMSURE, Germany) solution (10 ml). The applied solutions are close to the boiling point, and each treatment lasts 30±1 min. The residue is washed with hot water repeatedly and dried. Ultimately, the residue is incinerated for 1 h at 550 °C. The residue obtained from the incineration represents the fiber percentage (Thakur et al., 2021).
Crude fibers (%)=(P1-P2/P0)×100
P0: weight of the test sample; P1: weight of the crucible with the test sample prior to incineration; P2: weight of the crucible following incineration.
-Pectin content
A quantity of 5 g of ground lichen is added to a flask containing 100 ml of hydrochloric acid (HCl; Honeywell, Germany) solution (0.1 N) and heated to 90 °C for 45 min. A volume of the supernatant recovered after filtration is precipitated with 2 volumes of ethanol (Sigma, Germany). The obtained precipitate is washed with a volume of 6.6% alcohol and subsequently centrifuged at 6,000 t/30 min (Rezzoug et al., 2008). The obtained pectin is dried at 45 °C until a consistent weight is achieved (Constenla et al., 2002; Hosseini et al., 2016).
Pectin (%)=weight of dried pectin/weight of the test sample×100
-Crude proteins content (Kjeldahl Method)
The principle of the method using Kjeldahl machine (Gerhardt, Germany) is based on the conversion of organic nitrogen in 1 g of lichen into ammonium sulfate (FLUKA, Germany) through the action of concentrated sulfuric acid in the presence of a catalyst. Distillation is then fulfilled in the form of ammonium in the presence of 35% sodium hydroxide (NaOH; VWR, France). Titration is accomplished with a 0.1 N H2S04 solution (Thiex et al., 2002).
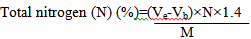
Where, Ve: volume of H2S04 required to titrate the sample solution (ml); Vb: volume of H2S04 needed titrate the blank (0 ml); M: weight of the test sample; N: normality of H2S04.
Crude proteins content (%)=Total N (%)×6.25
6.25: conversion factor according to the average nitrogen content of proteins
-Fats content (Soxhlet)
Approximately 20 g of crushed lichen was placed in a filter paper cartridge and inserted into the Soxhlet apparatus. A volume of 200 ml of petroleum ether (CHEM LAB, Belgium) was poured into the flask, and 50 ml into the extractor. The flask was heated for 6 h until the fat was exhausted. Following the elimination of the extraction solvent using a rotary evaporator, the weight of the flask containing the residue was determined (Rozzamri et al., 2020).
Fats (%)=[(weight of flask+fat)-(weight of flask)/weight of test sample]×100%
-Carbohydrates content and nutritive value
Carbohydrate content was mathematically determined by subtracting 100% from the percentages of moisture, ash, proteins, fats, crude fibers, and pectin (Tiencheu et al., 2021).
The nutritive value is calculated as follows:
Nutritive value (Kcal/g)=4×1 g of proteins+9×1 g of fats+4×1 g of carbohydrates (Kumar et al., 2010).
-Zinc (Zn), cadmium (Cd), and lead (Pb) content by Atomic Absorption Spectrometry (AAS)
Ashe obtained after incineration were moistened by slowly mixing 2 to 3 ml of water and 1 ml of concentrated HCl. The mixture was heated on a hot plate until the initial vapors were visible, followed by the addition of a few ml of water. In a 100 ml volumetric flask, rinse 3 or 4 times with warm water, then fill up to the mark (Shahbazi and Beheshti, 2019). This solution was examined for the presence of heavy metals (Zn, Cd, and Pb) using the SAA-6,200 AAS (Shimadzu Corporation, Kyoto, Japan). The contents in mg/100 g of dry weight of the dosed elements are calculated by reference to calibration curves established previously.
Lichen extraction
Aqueous extracts of lichen were prepared by macerating approximately 10 g of crushed lichen in 100 ml of distilled water for 24 h at room temperature. Similarly, aqueous extracts were prepared by heating in a water bath at 70 °C for 1 h (infusion) and at 100 °C for 1 h (decoction). The various extracts were filtered, and the recovered supernatants underwent different physicochemical and phytochemical analyses.
Physicochemical and phytochemical analyses
-pH
pH measurement consist of immersing the pH meter electrode (Hanna Instruments, Romania) in the aqueous extracts (Lackovičová et al., 2013).
-Total titratable acidity (TTA)
Volumes of 10 ml of the supernatants recovered after filtering the aqueous extracts were added to a few drops of phenolphthalein and titrated with a solution of NaOH (0.05 N) (Tiencheu et al., 2021).
TTA (%)=(C×V×0.064/sample weight)×100
C: concentration of NaOH solution (0.05 mol/L); V: volume (ml) of NaOH added to reach a pH of 8.1; 0.064: conventional factor established for citric acid (Debib et al., 2018).
-EC
Following homogenization, the conductivity meter electrode was immersed in the identical solutions utilized for pH measurement, and readings were collected directly on the meter display (Hanna Instruments, Romania) (Tiquia, 2010).
-Vitamin C assay
One ml of each aqueous extract was added to 10 ml of distilled water. The absorbance was evaluated using a UV-visible spectrophotometer (Analytikjena, Specord 50 plus, Gemany) at a wavelength of 265 nm. Ascorbic acid was used as a standard to establish the calibration curve (Witmer et al., 2016).
-Proteins assay by the Bradford method
For the assay, 1 ml of various aqueous extracts was mixed with 5 ml of the Bradford (Sigma, United Kingdom) reagent. Following 5 min of agitation, the mixture was incubated in the darkness for 30 min. Absorbance at 595 nm was recorded, and protein content was determined by referencing a calibration curve obtained from a Bovine Serum Albumin (BSA; Sigma, USA) solution. The results are reported in mg BSA Equivalent (BSAE)/100 g of lichen (Bradford, 1976).
-Qualitative and quantitative sugars content
The solution was observed for a red brick-colored reaction. In a test tube containing a mixture of 0.5 g of powder lichen and 5 ml of distilled water, 1 ml of boiling Fehling A (Riedel-de Haën, Germany) solution and 1 ml of boiling Fehling B solution were added. A blank was also prepared (all reagents without lichen) (Bekka-Hadji and Adjeroud-Abdellatif, 2023).
The presence of a reddish-brown precipitate was regarded positive for glycosides. One g of powder lichen was placed in each of two flasks. One of the flasks contained 5 ml of H2SO4 solution and 5 ml of distilled water were added, while the other flask received exclusively 5 ml of distilled water. After heating for 3 min, the recovered filtrate was made alkaline with 0.5 ml of NaOH solution and left to rest for 3min. A blank was also prepared (all reagents without lichen) (Bekka-Hadji and Adjeroud-Abdellatif, 2023).
Phenol in the presence of H2SO4 solution can be applied for the microcolorimetric quantitative determination of total sugars content and their methylated derivatives, oligosaccharides, and polysaccharides. A volume of 2 ml of various aqueous extracts was placed in test tubes containing 1 ml of phenol (Applichem Peareac, Germany) (5%). Five ml of H2SO4 solution was quickly added without permitting it to flow along the walls, and the mixture was immediately agitated. A steady yellow color developed, and the tubes were submerged in a water bath at 25-30 °C for 20 min. Absorbance was measured at 485 nm. The contents in mg of Glucose Equivalent (GE)/100 g of dry weight were determined with reference to a glucose calibration curve (Dubois et al., 1956).
-Total polyphenols content
One ml of the aqueous extract was introduced into 1 ml of Folin-Ciocalteu (Sigma, Switzerland) reagent diluted three times; 2 ml of 35% sodium carbonate solution was added to the mixture, well shaken, and diluted to 6 ml by adding 2 ml of distilled water. The mixture was incubated for 30 min in the darkness, and the blue color formed was measured at 700 nm using a spectrophotometer. The results are expressed in mg Gallic Acid Equivalent (GAE)/100 g of lichen (Turkmen et al., 2006).
-Total flavonoid content
An aliquot of 1.5 ml of the aqueous lichen extracts was mixed with 1.5 ml of 2% aluminum trichloride (Thermo Scientific, Germany). After 15 min of incubation in the darkness, the absorbance was measured at 430 nm using a spectrophotometer. The results are expressed in mg Quercetin Equivalent (QE)/100 g of lichen (Djeridane et al., 2006).
Statistical Analysis
Means and Standard Deviations (SD) were calculated for each experimental parameter. Data obtained from various physicochemical and phytochemical parameters of aqueous extracts were analyzed by one-way analysis of variance (ANOVA) at p<0.05 and Tukey test using Origin PRO version 93 E software, 2016.
Results
Proximate composition of lichen
The proximate composition (%) of various components of a lichen species, E. prunastri, from Jijel, Algeria demonstrate a substantial amount of carbohydrates (64.00±0.47%), moisture (11.66±0.19%), ash (10.85±0.82%) and, fibers (10±0.56%) in the lichen studied. Based on these results, it is obvious that this lichen is a highly beneficial carbohydrate source, with an estimated nutritional value of 268.37 cal/100 g. The content of pectin (1.45±0.07%), protein (1.18±0.30%), and fat (0.85±0%) for this lichen were detected to be low. The high level of ash content suggests that this lichen could contain a significant quantity of minerals.
Zn, Cd, and Pb contents
The results of the determination of Zn, Cd, and Pb by AAS revealed that the content of Zn as an essential substance is 1.32±0.0480 mg/100 g. For non-essential or toxic elements, the obtained contents are 0.096±0.0021 and 0.0029±0.0001 mg/100 g for Cd and Pb, respectively.
Heating impact on physicochemical and phytochemical analyses of aqueous extracts of lichen
The effect of heating at 70 and 100 °C on several physicochemical and phytochemical characteristics of E. prunastri aqueous extracts was represented in Table 1.
Table 1: Impact of heating at 70 and 100 °C on physicochemical and phytochemical properties of Evernia prunastri aqueous extracts
| Room temperature (Mean±SD) |
70 °C (Mean±SD) |
100 °C (Mean±SD) |
p-value | |
| pH | 4.45±0.00 a | 4.51±0.06 a | 4.5±0.01 a | 0.2061 |
| TTA (% citric acid) | 0.48±0.00 a | 0.45±0.00 a | 0.47±0.05 a | 0.4548 |
| EC (μS/cm) | 521±5.00 a | 527.50±0.50 a | 560±2.00 b | <0.0001 |
| Proteins content (mg BSAE/100 g) | 8.81±0.06 a | 6.73±0.04 b | 1.48±0.00 c | <0.0001 |
| Total sugar content (mg GE/100 g) | 179.12±0.20 a | 381.57 ±0.83 b | 406.46±0.125 c | <0.0001 |
| Vitamin C content (mg/100 g) | 17.44±0.04 a | 19.81±0.01 b | 15.14±0.04 c | <0.0001 |
| Total polyphenols content (mg GAE/100 g) | 51.39±0.53 a | 75.45±0.25 b | 36.04±0.04 c | <0.0001 |
| Total flavonoids content (mg QE/100 g) | 17.87±0.07 a | 20.19±0.07 b | 8.54±0.01 c | <0.0001 |
BSAE=Bovine Serum Albumin Equivalent; EC=Electrical Conductivity; GAE=Gallic Acid Equivalent; QE=Quercetin Equivalent. The results are shown as mean±SD, and values in the same row with different superscript letter are significantly different (p<0.05).
-pH and TTA
The pH and TTA values of lichen samples macerated in distilled water at room temperature, 70 and 100 °C displayed no significant difference (p>0.05) and stabilization was observed (Table 1). This indicates that heating has no effect on these two parameters.
-EC
The result obtained for the EC of the aqueous extract of lichen macerated at 100 °C demonstrated a significant difference (p<0.05) compared to the other samples with a value reaching 560 μS/cm (Table 1).
-Vitamin C
According to the obtained results, the vitamin C content was 17.44 mg/100 g for the macerated lichen at room temperature (Table 1). Heating at 70 °C caused an increase in vitamin C content, while at a temperature of 100 °C, a decrease was observed with p-value<0.0001. Statistically significant differences (p<0.05) were detected.
-Proteins
Mean protein contents differed significantly (p<0.05) between the three aqueous extracts studied. Protein content was 8.81 mg BSAE/100 g for the aqueous extract macerated at room temperature, whereas a considerable decrease was observed for the aqueous extract macerated at 100 °C (1.48 mg BSAE/100 g) (Table 1).
-Sugars
The qualitative study of reducing sugars and glycosides yielded positive reactions for both tests. This recommends that this lichen contains both simple and complex sugars. Quantitative analyzes of the sugars of the macerated lichen at room temperature exhibited a sugar content of 179.12 mg GE/100 g. For lichen macerated at 100 °C, a significant increase (p<0.05) in the sugar content, reaching 406.46 mg GE/100 g, was detected (Table 1).
-Polyphenols and flavonoids
The polyphenols and flavonoids content of lichen macerated at 70 °C was significantly (p<0.05) higher than that of lichen macerated at room temperature. However, lichen macerated at 100 °C indicated a significant reduction in these metabolites (Table 1).
Discussion
Proximate substances of carbohydrates, proteins, fats, and antioxidant characteristics of secondary metabolites of phenols are naturally investigated in lichens (Muthu et al., 2021). Data reported by Zhao et al. (2021) indicate carbohydrates contents ranging from 53.2 to 79.08% and moisture contents ranging from 8.86 to 16.4% for all the studied lichens. The results obtained for E. prunastri are included in these ranges. Kumar et al. (2010), Vinayaka et al. (2009) suggest moisture contents of 16 and 16.4%, respectively, for the species Ramalina conduplicans and R. hosseis. For these identical species, carbohydrates contents are 61.1 and 59.9%, respectively, with nutritional values of 356.0 and 348.2 cal/100 g. The lowest carbohydrates content is obtained for the lichen species Peltigera aphthosa, and the highest is for the species Alectoria ochroleuca (Podterob, 2008). Lichens are poikilohydric organisms, whose internal water content tends to reflect external humidity conditions. As the water potential is low, their metabolic activity can be restored by absorbing water vapor or receiving liquid water from dew and fog (Di Nuzzo et al., 2022).
According to Svihus and Holand (2000), the crude fiber content in various lichen species varied between 1 and 24.2%, the content being higher in Cladina spp. and Stereocaulon paschale than in Cetraria and A. ochroleuca. In addition, Cladina rangiferina included higher crude fiber content than any of the other lichen species. The analysis of fiber content through hydrolysis and identification of monosaccharide composition verified that lichens predominantly consist of fiber. Results reported by Zhao et al. (2021) indicate crude fiber contents varied between 5.38 and 16.36% for all studied lichens. The fiber content for the species E. prunastri is within the reported range in the literature.
Even though fibers have virtually no nutritional value, they can have beneficial effects on intestinal health. Increasing dietary fiber intake can also reduce the risk of certain chronic diseases such as diabetes, obesity, and cardiovascular diseases (Zhao et al., 2021).
Pectin is an anionic biopolymer extracted from the cell walls of plant sources in a wide range from 0.1 to 30% (Moslemi, 2021). Pectin is a soluble dietary fiber with physiological effects on the gastrointestinal tract, such as delayed gastric emptying, reduced transit time and reduced glucose absorption. These effects are mainly due to it’s a capacity to form gels and retain water (Olano-Martin et al., 2002). Pectin is utilized in numerous food preparations as a gelling agent in jams and jellies, as a thickening and emulsifying agent in dairy products. Furthermore, it is applied in pharmacy and cosmetics for its gelling features (Rezzoug et al., 2008). Most lichens contain nutritional components such as Cladonia stellaris, which has 2.0% water-soluble carbohydrates and 78.4% hemicellulose but only 1.7% cellulose. Lichen polysaccharides isolated based on considerable quantity, such as α-glucans, β-glucans, and galactomannans, are typically derived from fungal origin (Akbulut and Yildiz, 2010).
The protein contents of several edible lichens range from 5.95 to 16.2%, and lipid contents are relatively low (1.3-6.5%) (Zhao et al., 2021).For the lichen species C. stellaris, the crude protein content is 3.1% (Gautam et al., 2021). Lichen proteins are considered as potential and biologically active substances with unique biochemical properties. The new candidate compounds for drugs (antibiotics and proteins with anti-prion activity), UVB protection, oxidative enzymes for the pulp industry, and antifreeze proteins for frozen foods have been investigated (Kondratiuk et al., 2015).
The most remarkable characteristic of lichen activity is the deposit of crystalline lipids in the thallus. The lipid crystals of lichens are perceived to have protective functions. They can shield algae from harmful short-wave radiation. Moreover, the lipids possess an antibiotic function, in particular to protect against the invasion of the thallus by gram-positive bacteria (Arakawa-Kobayashi and Kanaseki, 2004).
All human beings require a specific number of complex organic compounds as additional calories to meet the demands of their muscle activities. Carbohydrates, fats, and proteins constitute the major part of the diet, while minerals and vitamins comprise a relatively smaller portion (Vinayaka et al., 2009).
Heavy metal accumulation level fundamentally determines the success of lichens in the colonization of polluted sites (Rola, 2020). Lichens are known to naturally contain potentially toxic bitter acids, as well as concentrated heavy metals absorbed from the environment, particularly the atmosphere, which may pose serious health risks to consumers (Meli et al., 2018). Data reported by Zhao et al. (2021) indicate ash contents ranging from 4.01 to 12.1% for all studied lichens. Kumar et al. (2010); Vinayaka et al. (2009) obtained ash contents of 10 and 12.1%, respectively, for the Ramalina hosseis and R. conduplicans species.
According to Zhao et al. (2021), Zn contents vary from 2.2 to 8.34 mg/100 g for all examined lichens. Kumar et al. (2010) reported a Zn content of 3.28 mg/100 g for the R. hosseis species. Prashith Kekuda et al. (2011) detected a Zn content of 6.63 mg/100 g for the E. cirrhatum species (Bhadra wildlife sanctuary, Karnataka, India). Similarly, Stamenković et al. (2013), found Zn contents of 6.59 mg/100 g for the E. prunastri species from Cerjé and 3.02 mg/100 g for the E. prunastri species from Donje Vlase (Southeastern Serbia). Pb levels were 0.13 and less than 0.11 mg/100 g, respectively, while Cd was not detected in either species (Stamenković et al., 2013). The accumulated Pb content in the thallus of E. prunastri (Kaunas, central Lithuania) varies from 0.5 to 0.7 mg/100 g, and Cd accumulation was recorded exclusively in high-traffic areas, reaching 0.018 mg/100 g (Sujetoviene and Sliumpaite, 2013). According to Loppi et al. (1998), the E. prunastri species collected in Abruzzo (central Italy) demonstrates a Zn content of 1.24 mg/100 g, while Cd and Pb contents are 0.03 and 1.2 mg/100 g, respectively. These authors propose that Pb primarily originates from road traffic, whereas the common source of Cd and Zn is superphosphate fertilizers and pesticides. Meli et al. (2018) reported that the C. islandica species from Italy had a Zn content of 3.22 mg/100 g, Cd content less than 0.1 mg/100 g, and Pb content of 0.095 mg/100 g. Tabbabi and Karmous (2018) declared that all samples (L1-L27) belonging to three lichen species (Xanthoria parietina, Parmelia caperata, and Diploschistes gypsaceus) collected in Tunisia display Zn contents ranging from 0.2 to 8.1 mg/100 g, Cd contents lower than or equal to 0.1 and reaching 9.7 mg/100 g, and for Pb, contents are lower than or equal to 0.1 and reaching 21.7 mg/100 g. According to these authors, the two regions Zarzouna and Bizerte of Tunisia present the highest levels of lead. This can be attributed to the presence of an oil refining industry and lubricant recycling. Indeed, road traffic and the combustion of fossil fuels in factories can also result in the emission of Pb, Zn, and Cd (Tabbabi and Karmous, 2018). Identically, Cd levels in rural sites are lower than those in urban and industrial sites (Sujetoviene and Sliumpaite, 2013).
The pH scale defines the acidity or basicity of a “dilute aqueous solution, in which the solvent is water,” and the hydrogen ions can move about freely in the solution (Aydogdu et al., 2023). Although an increase in TTA reflects a decrease in pH, TTA determines the acidic taste of a product, while pH determines its susceptibility to microbes (Tiencheu et al., 2021).
EC measures the concentration of soluble ions or salinity that may arise from nitrogen mineralization and the production of organic acids. At high temperatures, some of lichen's complex organic compounds can degrade into smaller, ionized molecules, contributing to greater conductivity. Generally, the effects of salinity are most often negligible in extracts with EC readings of 2 μS/cm or less. Salinity in water extracts with EC values exceeding 10 μS/cm was responsible for phytotoxicity. The EC ranging from 1.2 to 3.4 μS/cm implies that the concentrations of soluble salts were in the range considered non-phytotoxic or marginally phytotoxic (Tiquia, 2010).
Vitamins detected in lichens contain ascorbic acid, biotin, folic acid, folinic acid, niacin, pantothenic acid, riboflavin, thiamine, and vitamin B12 (Akbulut and Yildiz, 2010). Vitamin C is affected by high temperatures. This effect is more pronounced with higher temperatures and longer processing times. From a chemical viewpoint, the term "vitamin C" is applied broadly to describe any compound proven to possess total or partial biological activity of L-Ascorbic Acid (L-AA). It includes esters of L-AA, such as ascorbyl palmitate (100% relative activity), synthetic forms including 6-deoxy-L-AA (33% relative activity), and the oxidized form of L-AA, namely dehydroascorbic acid (L-DHAA) (80% relative activity) (Giannakourou and Taoukis, 2021). The increase in vitamin C for the aqueous extract of this lichen heated at 70 °C can be explained by the presence of the dipalmitate form of ascorbic acid, which is hydrolyzed during the 70 °C treatment, releasing ascorbic acid as evidenced by spectrophotometry. During heating at 100 °C, a decrease in vitamin C content compared to the untreated extract was noted, explaining its denaturation at high temperatures. Vitamin C, a water-soluble compound, is a natural antioxidant present in various plant substances. During processing, this bioactive compound is subject to various degradation modes, with temperature and oxygen being recognized as the main factors responsible for this nutritional loss. Therefore, vitamin C is often utilized as an indicator of the overall deterioration in the quality of these products during processing and post-processing, storage, and transformation. Traditional preservation methods, such as heat treatment, drying, and freezing, are frequently associated with a substantial loss of vitamin C, presenting more realistic predictions (Giannakourou and Taoukis, 2021).Vitamin C plays an antioxidant role and possesses several health benefits. Ascorbic acid is applied not only to fortify food and losses during processing, but also contributes to the product stability and appearance. The consumption of vitamin C has also been reported to improve the rate of transformation of cholesterol, to prevent cancers and disorders associated with a lack of collagen (Tiencheu et al., 2021).
The protein content varies from 1165 mg of BSAE/100 g of sample to 2,578 mg of BSAE/100 g of sample. Similarly, the protein content varies from 3,163 mg GE/100 g sample to 6,460 mg GE/100 g sample. P. tinctorum had maximum total protein content compared to other lichens (Muthu et al., 2021). This reflects higher contents in comparison with the studied lichen. Research results shown that high temperatures cause protein degradation.
The phenolic content of a lichen extract is relative and seems to be linked to the species' nature, extraction conditions, and the applied extraction methods (Behera et al., 2005). Flavonoids are natural pigments present in the majority of plants, considered important micronutrients as they can play antioxidant roles or possess diverse biological characteristics (Yang et al., 2008). According to Bekka-Hadji and Adjeroud-Abdellatif (2023), polyphenols contents of methanolic extracts of E. prunastri species from Algerian range from 498.24 to 929.3 mg GAE/100 g. Similarly, flavonoid contents range from 38.49 to 56.34 QE/100 g. The ethanolic and methanolic extracts contained polyphenol contents approximately twice as high as those of the aqueous extracts (Lapornik et al., 2005).The heat treatment caused a reduction in phenolic compound contents (Rakić et al., 2007). The yields of phenolic compounds were higher at 60 °C than at 45 °C, and with apparent thermal degradation of the constituents beyond 20 h. The yield of phenols increased for water content of ethanol from 10 to 30% and remained constant for water content of 30 to 60%. While the phenol concentration of extracts decreased for water content of water above 50% (Spigno et al., 2007). Which confirms the best yield obtained for the polyphenols and flavonoids of lichen macerated at 70 °C. Research results shown that heating to 70 °C promotes their release, leading to more concentrated extracts, whereas heating to 100 °C favors their degradation.
As lichen powder is macerated at 100 °C, the obtained filtrate has a gelatinous appearance. According to Akbulut and Yildiz (2010), boiling C. islandica lichen with water leads to the formation of substantial quantity of a gelatinous product known as lichen starch or lichenin, composed of at least two fractions of various solubilities: a lichenan fraction insoluble in cold water but soluble in boiling water (lichenin), and an isolichenin fraction soluble in cold water. Turkmen et al. (2005) demonstrated that the cooking process causes alterations in the physical attributes and chemical composition. Boiling and cooking have an effect on ascorbic acid, phenolic compounds, lycopene, and antioxidant activity. This is reinforced by the results of lichen macerated at 100 °C where the contents of proteins, vitamin C, polyphenols, and flavonoids decreased.
Conclusion
This chemical analysis reveals that E. prunastri lichen from Jijel, Algeria, is a noteworthy source of carbohydrates, fiber, and potentially minerals, but is less rich in protein and fat. This information could prove valuable in grasping the nutritional benefits of lichens in human or animal nutrition, as well as exploring their use in various fields, such as medicine or the food industry. Heating at 70 and 100 °C had a negative or positive impact depending on the analyzed parameter. At a temperature of 70 °C, sugars, polyphenols, and flavonoids contents increased, whereas protein content had a reduction. However, heating to 100 °C led to a decline in protein, vitamin C, polyphenol, and flavonoid contents and an increase in sugar content. The potential health benefits of lichen extracts and their bioactive substances present further prospects for the development of drugs and functional foods.
Author contributions
Not applicable
Conflicts of interest
The author declared that there is no conflict of interest in the study.
Acknowledgements
The author would like to express his gratitude to the Department of Applied Microbiology and Food Science, and Biology Laboratory, Faculty of Natural and Life Sciences, University of Jijel (Algeria) and to the Algerian Ministry of Higher Education and Scientific Research.
Funding
This study was self-funded and did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
Ethical consideration
Not applicable.
References
Akbulut G., Yildiz A. (2010). An overview to lichens: the nutrient composition of some species. KafkasKafkas Üniversitesi Fen Bilimleri Enstitüsü Dergisi. 3: 79-86.
AOAC. (2000). Official methods of analysis. 17th edition. The Association of Official Analytical Chemists. Gaithersburg, MD, USA. URL: https://law.resource. org/ pub/us/cfr/ibr/002/aoac.methods.1.1990. pdf. Accessed 14 May 2022.
Aoussar N., Achmit M., Es-sadeqy Y., Vasiljević P., Rhallabi N., Ait Mhand R., Zerouali K., Manojlović N., Mellouki F. (2021). Phytochemical constituents, antioxidant and antistaphylococcal activities of Evernia prunastri (L.) Ach., Pseudevernia furfuracea (L.) Zopf. and Ramalina farinacea (L.) Ach. from Morocco. Archives of Microbiology. 203: 2887-2894. [DOI: 10.1007/s00203-021-02288-5]
Arakawa-Kobayashi S., Kanaseki T. (2004). A study of lipid secretion from the lichen symbionts, ascomycetous fungus Myelochroa leucotyliza and green alga Trebouxia sp. Journal of Structural Biology. 146: 401-415. [DOI: 10.1016/j.jsb.2004.01.016]
Aydogdu T., O’Mahony J.A., McCarthy N.A. (2023). pH, the fundamentals for milk and dairy processing:
a review. Dairy. 4: 395-340. [DOI: 10.3390/ dairy4030026]
Behera B.C., Verma N., Sonone A., Makhija U. (2005). Antioxydant and antibacterial activities of lichen usnea ghattensis in vitro. Biotechnology Letters. 27: 991-995. [DOI: 10.1007/s10529-005-7847-3]
Bekka-Hadji F., Adjeroud-Abdellatif N. (2023). Assessment of phytochemical, antioxidant and antibacterial activities of Evernia prunastri species collected from Algeria. Carpathian Journal of Food Science and Technology. 15: 99-112. [DOI: 10.34302/ crpjfst/2023.15.4.8]
Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72: 248-254. [DOI: 10.1016/ 0003-2697(76)90527-3]
Bukabayeva Z., Abiyev S., Silybayeva B., Assanova U., Sharipkhanova A., Sagdatkyzy B. (2023). Epiphytic and epigeal lichens as bioindicators of air pollution in the Burabay National Park, Kazakhstan. Biodiversitas Journal of Biological Diversity. 24: 2701-2709. [DOI: 10.13057/biodiv/d240523]
Constenla D., Ponce A.G., Lozano J.E. (2002). Effect of pomace drying on apple pectin. LWT - Food Science and Technology. 35: 216-221. [DOI: 10.1006/fstl. 2001.0841]
Debib A., Tir-Touil M.A., Meddah B., Hamaidi-Chergui F., Menadi S., Alsayadi M.S. (2018). Evaluation of antimicrobial and antioxidant activities of oily macerates of Algerian dried figs (Ficus carica L.). International Food Research Journal. 25: 351-356.
Di Nuzzo L., Canali G., Giordani P., Nascimbene J., Benesperi R., Papini A., Bianchi E., Porada P. (2022). Life-stage dependent response of the epiphytic lichen Lobaria pulmonaria to climate. Frontiers in Forests and Global Change. 5: 903607. [DOI: 10.3389/ffgc. 2022.903607]
Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chemistry. 97: 654-660. [DOI: 10.1016/j.foodchem.2005.04.028]
Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 28: 350-356. [DOI: 10.1021/ac60111a017]
Favero-Longo S.E., Piervittori R. (2010). Lichen-plant interactions. Journal of Plant Interactions. 5: 163-177. [DOI: 10.1080/17429145.2010.492917]
Gautam A.K., Yadav D., Bhagyawant S.S., Singh P.K., Jin J.O. (2021). Lichen: a comprehensive review on lichens as a natural sources exploring nutritional and biopharmaceutical benefits. Progress in Nutrition. 23: e2021153. [DOI: 10.23751/pn.v23i3.9833]
Giannakourou M.C., Taoukis P.S. (2021). Effect of alternative preservation steps and storage on vitamin C stability in fruit and vegetable products: critical review and kinetic modelling approaches. Foods. 10: 2630. [DOI: 10.3390/foods10112630]
Hosseini S.S., Khodaiyan F., Yarmand M.S. (2016). Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydrate Polymers. 140: 59-65. [DOI: 10.1016/j.carbpol.2015.12.051]
Ivanova D.G., Ivanov D. (2009). Ethnobotanical use of lichens: lichens for food review. Scripta Scientifica Medica. 41: 11-16. [DOI: 10.14748/ssm.v41i1.456]
Kondratiuk A.S., Savchuk O.M., Hur J.S. (2015). Optimization of protein extraction for lichen thalli. Mycobiology. 43: 157-162. [DOI: 10.5941/MYCO. 2015.43.2.157]
Kumar S.V.P., Kekuda T.R.P.,Vinayaka K.S., Swathi D., Mallikarjun N., Nishanth B.C. (2010). Studies on proximate composition, antifungal and anthelmintic activity of a macrolichen Ramalina hossei H. Magn and G. Awasthi. International Journal of Biotechnology and Biochemistry. 6: 191-201.
Lackovičová A., Guttova A., Backor M., Pišút P. (2013). Response of Evernia prunastri to urban environmental conditions in central Europe after the decrease of air pollution. The Lichenologist. 45: 89-100. [DOI: 10.1017/S002428291200062X]
Lapornik B., Prošek M., Wondra A.G. (2005). Comparison of extracts prepared from plant by-products using different solvents and extraction time. Journal of Food Engineering. 71: 214-222. [DOI: 10.1016/j.jfoodeng. 2004.10.036]
Loppi S., Pacioni G., Olivieri N., Giacomo F.D. (1998). Accumulation of trace metals in the lichen Evernia prunastri transplanted at biomonitoring sites in central Italy. The Bryologist.101: 451-454. [DOI: 10.2307/ 3244187]
Meli M.A., Desideri D., Cantaluppi C., Ceccotto F., Feduzi L., Roselli C. (2018). Elemental and radiological characterization of commercial Cetraria islandica (L.) Acharius pharmaceutical and food supplementation products. Science of the Total Environment. 613-614: 1566-1572. [DOI: 10.1016/j.scitotenv.2017.08.320]
Moslemi M. (2021). Reviewing the recent advances in application of pectin for technical and health promotion purposes: from laboratory to market. Carbohydrate Polymers. 254: 117324. [DOI: 10.1016/j.carbpol.2020. 117324]
Muthu S., Murugan M., Rajendran K., Ponmurugan P. (2021). An assessment of proximate composition, antioxidant activities and LC/MS based phytochemical profiling of some lichen species collected fromwestern Ghats of southern part of India. Jordan Journal of Biological Sciences.14: 647-661. [DOI: 10.54319/jjbs/ 140404].
Olano-Martin E., Gibson G.R., Rastall R.A. (2002). Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. Journal of Applied Microbiology. 93: 505-511. [DOI: 10.1046/j.1365-2672.2002.01719.x.]
Podterob A.P. (2008). Chemical composition of lichens and their medical applications. Pharmaceutical Chemistry Journal. 42: 582-588. [DOI: 10.1007/ s11094-009-0183-5]
Poulsen-Silva E., Gordillo-Fuenzalida F., Atala C., Moreno A.A., Otero M.C. (2023). Bioactive lichen secondary metabolites and their presence in species from Chile. Metabolites. 13: 805. [DOI: 10.3390/metabo13070805]
Prashith Kekuda T.R., Vinayaka K.S., Swathi D., Suchitha Y., Venugopal T.M., Mallikarjun N. (2011). Mineral composition, total phenol content and antioxidant activity of a macrolichen Everniastrum cirrhatum (Fr.) Hale (Parmeliaceae). E-Journal of Chemistry. 8: 1886-1894. [DOI: 10.1155/2011/420673]
Rakić S., Petrović S., Kukić J., Jadranin M., Tešević V., Povrenović D., Šiler-Marinković S. (2007). Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chemistry. 104: 830-834. [DOI: 10.1016/j.foodchem. 2007.01.025]
Ren M., Jiang S., Wang Y., Pan X., Pan F., Wei X. (2023). Discovery and excavation of lichen bioactive natural products. Frontiers in Microbiology. 14: 1177123. [DOI: 10.3389/fmicb.2023.1177123]
Rezzoug S.A., Maache-Rezzoug Z., Sannier F., Allaf K. (2008). A thermomechanical preprocessing for pectin extraction from orange peel. Optimisation by response surface methodology. International Journal of Food Engineering. 4: 10. [DOI: 10.2202/1556-3758.1183]
Rola K. (2020). Insight into the pattern of heavy-metal accumulation in lichen thalli. Journal of Trace Elements in Medicine and Biology. 61: 126512. [DOI: 10.1016/j.jtemb.2020.126512]
Rozzamri A., Atiqah-Izyannie A.M., Mat Yusoff M., Ismail-Fitry M.R. (2020). Effects of leavening agents in batter system on quality of deep-fried chicken breast meat. Food Research. 4: 327-334. [DOI: 10.26656/ fr.2017.4(2).273]
Shahbazi K., Beheshti M. (2019). Comparison of three methods for measuring heavy metals in calcareous soils of Iran. SN Applied Sciences. 1: 1541. [DOI: 10.1007/s42452-019-1578-x]
Spigno G., Tramelli L., De Faveri D.M. (2007). Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. Journal of Food Engineering. 81: 200-208. [DOI: 10.1016/j.jfoodeng.2006.10.021]
Stamenković S.S., Mitrović T.LJ., Cvetković V.J., Krstić N.S., Baošić R.M., Marković M.S., Nikolić N.D., Marković V.L.J., Cvijan M.V. (2013). Biological indication of heavy metal pollution in the areas of DonjeVlase and Cerje (southeastern serbia) using epiphytic lichens. Archives of Biological Sciences. 65: 151-159. [DOI: 10.2298/ABS1301151S]
Sujetoviene G., Sliumpaite I. (2013). Response of EverniaE prunastri transplanted to an urban area in central Lithuania. Atmospheric Pollution Research. 4: 222-228. [DOI: 10.5094/APR.2013.023]
Svihus B., Holand Ø. (2000). Lichen polysaccharides and their relation to reindeer/caribou nutrition. Journal of Range Management. 53: 642-648. [DOI: 10.2307/ 4003160]
Tabbabi K., Karmous T. (2018). Use of lichens as bio-indicators in assessing the level of air pollution in the region of Bizerte study of pollutants attached to frond lichens by atomic spectroscopy and emission spectroscopy by plasma torch. Moroccan Journal of Chemistry. 6: 148-159. [DOI: 10.48317/IMIST.PRSM/ morjchem-v6i1.6064]
Thakur A., Singh S., Puri S. (2021). Nutritional evaluation, phytochemicals, antioxidant and antibacterial activity of Stellaria monosperma Buch.-Ham. Ex D. Don and Silene vulgaris (Moench) Garcke: wild edible plants of western Himalayas. Jordan Journal of Biological Sciences. 14: 83-90. [DOI: 10.54319/jjbs/140111]
Thiex N.J., Manson H., Anderson S., Persson J.Å. (2002). Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: collaborative study. Journal of AOAC International. 85: 309-317. [DOI: 10.1093/jaoac/85.2.309]
Tiencheu B., Njabi Nji D., Achidi A.U., Egbe A.C., Tenyang N., Ngongang E.F.T., Djikeng F.T., Fossi B.T. (2021). Nutritional, sensory, physico-chemical, phytochemical, microbiological and shelf-life studies of natural fruit juice formulated from orange (Citrus sinensis), lemon (Citrus limon), honey and ginger (Zingiberofficinale). Heliyon. 7: e07177. [DOI: 10. 1016/j.heliyon.2021.e07177]
Tiquia S.M. (2010). Reduction of compost phytotoxicity during the process of decomposition. Chemosphere. 79: 506-512. [DOI: 10.1016/j.chemosphere.2010. 02.040]
Turkmen N., Sari F., Velioglu Y.S. (2006). Effect of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chemistry. 99: 835-841. [DOI: 10. 1016/j.foodchem.2005.08.034]
Turkmen N., Sari F., Velioglu Y.S. (2005). The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chemistry. 93: 713-718. [DOI: 10.1016/j.foodchem.2004.12.038]
Vinayaka K.S., Praveen Kumar S.V., Prashith Kekuda T.R., Krishnamurthy Y.L., Mallikarjun N., Swathi D. (2009). Proximate composition, antioxidant, anthelmintic and insecticidal activity of a macrolichen Ramalina conduplicans Vain. (Ramalinaceae). European Journal of Applied Sciences.1: 40-46.
Witmer J.R., Wetherell B.J., Wagner B.A., Du J., Cullen J.J., Buettner G.R. (2016). Direct spectrophotometric measurement of supra-physiological levels of ascorbate in plasma. Redox Biology. 8: 298-304. [DOI: 10.1016/ j.redox.2016.02.004]
Yang R.Y., Lin S., Kuo G. (2008). Content and distribution of flavonoids among 91 edible plant species. Asia of Pacific Journal of Clinical Nutrition. 17: 275-279.
Zhao Y., Wang M., Xu B. (2021). A comprehensive review on secondary metabolites and health-promoting effects of edible lichen. Journal of Functional Foods. 80: 1-17. [DOI: 10.1016/j.jff.2020.104283]
Aoussar N., Achmit M., Es-sadeqy Y., Vasiljević P., Rhallabi N., Ait Mhand R., Zerouali K., Manojlović N., Mellouki F. (2021). Phytochemical constituents, antioxidant and antistaphylococcal activities of Evernia prunastri (L.) Ach., Pseudevernia furfuracea (L.) Zopf. and Ramalina farinacea (L.) Ach. from Morocco. Archives of Microbiology. 203: 2887-2894. [DOI: 10.1007/s00203-021-02288-5]
Arakawa-Kobayashi S., Kanaseki T. (2004). A study of lipid secretion from the lichen symbionts, ascomycetous fungus Myelochroa leucotyliza and green alga Trebouxia sp. Journal of Structural Biology. 146: 401-415. [DOI: 10.1016/j.jsb.2004.01.016]
Aydogdu T., O’Mahony J.A., McCarthy N.A. (2023). pH, the fundamentals for milk and dairy processing:
a review. Dairy. 4: 395-340. [DOI: 10.3390/ dairy4030026]
Behera B.C., Verma N., Sonone A., Makhija U. (2005). Antioxydant and antibacterial activities of lichen usnea ghattensis in vitro. Biotechnology Letters. 27: 991-995. [DOI: 10.1007/s10529-005-7847-3]
Bekka-Hadji F., Adjeroud-Abdellatif N. (2023). Assessment of phytochemical, antioxidant and antibacterial activities of Evernia prunastri species collected from Algeria. Carpathian Journal of Food Science and Technology. 15: 99-112. [DOI: 10.34302/ crpjfst/2023.15.4.8]
Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72: 248-254. [DOI: 10.1016/ 0003-2697(76)90527-3]
Bukabayeva Z., Abiyev S., Silybayeva B., Assanova U., Sharipkhanova A., Sagdatkyzy B. (2023). Epiphytic and epigeal lichens as bioindicators of air pollution in the Burabay National Park, Kazakhstan. Biodiversitas Journal of Biological Diversity. 24: 2701-2709. [DOI: 10.13057/biodiv/d240523]
Constenla D., Ponce A.G., Lozano J.E. (2002). Effect of pomace drying on apple pectin. LWT - Food Science and Technology. 35: 216-221. [DOI: 10.1006/fstl. 2001.0841]
Debib A., Tir-Touil M.A., Meddah B., Hamaidi-Chergui F., Menadi S., Alsayadi M.S. (2018). Evaluation of antimicrobial and antioxidant activities of oily macerates of Algerian dried figs (Ficus carica L.). International Food Research Journal. 25: 351-356.
Di Nuzzo L., Canali G., Giordani P., Nascimbene J., Benesperi R., Papini A., Bianchi E., Porada P. (2022). Life-stage dependent response of the epiphytic lichen Lobaria pulmonaria to climate. Frontiers in Forests and Global Change. 5: 903607. [DOI: 10.3389/ffgc. 2022.903607]
Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chemistry. 97: 654-660. [DOI: 10.1016/j.foodchem.2005.04.028]
Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 28: 350-356. [DOI: 10.1021/ac60111a017]
Favero-Longo S.E., Piervittori R. (2010). Lichen-plant interactions. Journal of Plant Interactions. 5: 163-177. [DOI: 10.1080/17429145.2010.492917]
Gautam A.K., Yadav D., Bhagyawant S.S., Singh P.K., Jin J.O. (2021). Lichen: a comprehensive review on lichens as a natural sources exploring nutritional and biopharmaceutical benefits. Progress in Nutrition. 23: e2021153. [DOI: 10.23751/pn.v23i3.9833]
Giannakourou M.C., Taoukis P.S. (2021). Effect of alternative preservation steps and storage on vitamin C stability in fruit and vegetable products: critical review and kinetic modelling approaches. Foods. 10: 2630. [DOI: 10.3390/foods10112630]
Hosseini S.S., Khodaiyan F., Yarmand M.S. (2016). Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydrate Polymers. 140: 59-65. [DOI: 10.1016/j.carbpol.2015.12.051]
Ivanova D.G., Ivanov D. (2009). Ethnobotanical use of lichens: lichens for food review. Scripta Scientifica Medica. 41: 11-16. [DOI: 10.14748/ssm.v41i1.456]
Kondratiuk A.S., Savchuk O.M., Hur J.S. (2015). Optimization of protein extraction for lichen thalli. Mycobiology. 43: 157-162. [DOI: 10.5941/MYCO. 2015.43.2.157]
Kumar S.V.P., Kekuda T.R.P.,Vinayaka K.S., Swathi D., Mallikarjun N., Nishanth B.C. (2010). Studies on proximate composition, antifungal and anthelmintic activity of a macrolichen Ramalina hossei H. Magn and G. Awasthi. International Journal of Biotechnology and Biochemistry. 6: 191-201.
Lackovičová A., Guttova A., Backor M., Pišút P. (2013). Response of Evernia prunastri to urban environmental conditions in central Europe after the decrease of air pollution. The Lichenologist. 45: 89-100. [DOI: 10.1017/S002428291200062X]
Lapornik B., Prošek M., Wondra A.G. (2005). Comparison of extracts prepared from plant by-products using different solvents and extraction time. Journal of Food Engineering. 71: 214-222. [DOI: 10.1016/j.jfoodeng. 2004.10.036]
Loppi S., Pacioni G., Olivieri N., Giacomo F.D. (1998). Accumulation of trace metals in the lichen Evernia prunastri transplanted at biomonitoring sites in central Italy. The Bryologist.101: 451-454. [DOI: 10.2307/ 3244187]
Meli M.A., Desideri D., Cantaluppi C., Ceccotto F., Feduzi L., Roselli C. (2018). Elemental and radiological characterization of commercial Cetraria islandica (L.) Acharius pharmaceutical and food supplementation products. Science of the Total Environment. 613-614: 1566-1572. [DOI: 10.1016/j.scitotenv.2017.08.320]
Moslemi M. (2021). Reviewing the recent advances in application of pectin for technical and health promotion purposes: from laboratory to market. Carbohydrate Polymers. 254: 117324. [DOI: 10.1016/j.carbpol.2020. 117324]
Muthu S., Murugan M., Rajendran K., Ponmurugan P. (2021). An assessment of proximate composition, antioxidant activities and LC/MS based phytochemical profiling of some lichen species collected fromwestern Ghats of southern part of India. Jordan Journal of Biological Sciences.14: 647-661. [DOI: 10.54319/jjbs/ 140404].
Olano-Martin E., Gibson G.R., Rastall R.A. (2002). Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. Journal of Applied Microbiology. 93: 505-511. [DOI: 10.1046/j.1365-2672.2002.01719.x.]
Podterob A.P. (2008). Chemical composition of lichens and their medical applications. Pharmaceutical Chemistry Journal. 42: 582-588. [DOI: 10.1007/ s11094-009-0183-5]
Poulsen-Silva E., Gordillo-Fuenzalida F., Atala C., Moreno A.A., Otero M.C. (2023). Bioactive lichen secondary metabolites and their presence in species from Chile. Metabolites. 13: 805. [DOI: 10.3390/metabo13070805]
Prashith Kekuda T.R., Vinayaka K.S., Swathi D., Suchitha Y., Venugopal T.M., Mallikarjun N. (2011). Mineral composition, total phenol content and antioxidant activity of a macrolichen Everniastrum cirrhatum (Fr.) Hale (Parmeliaceae). E-Journal of Chemistry. 8: 1886-1894. [DOI: 10.1155/2011/420673]
Rakić S., Petrović S., Kukić J., Jadranin M., Tešević V., Povrenović D., Šiler-Marinković S. (2007). Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chemistry. 104: 830-834. [DOI: 10.1016/j.foodchem. 2007.01.025]
Ren M., Jiang S., Wang Y., Pan X., Pan F., Wei X. (2023). Discovery and excavation of lichen bioactive natural products. Frontiers in Microbiology. 14: 1177123. [DOI: 10.3389/fmicb.2023.1177123]
Rezzoug S.A., Maache-Rezzoug Z., Sannier F., Allaf K. (2008). A thermomechanical preprocessing for pectin extraction from orange peel. Optimisation by response surface methodology. International Journal of Food Engineering. 4: 10. [DOI: 10.2202/1556-3758.1183]
Rola K. (2020). Insight into the pattern of heavy-metal accumulation in lichen thalli. Journal of Trace Elements in Medicine and Biology. 61: 126512. [DOI: 10.1016/j.jtemb.2020.126512]
Rozzamri A., Atiqah-Izyannie A.M., Mat Yusoff M., Ismail-Fitry M.R. (2020). Effects of leavening agents in batter system on quality of deep-fried chicken breast meat. Food Research. 4: 327-334. [DOI: 10.26656/ fr.2017.4(2).273]
Shahbazi K., Beheshti M. (2019). Comparison of three methods for measuring heavy metals in calcareous soils of Iran. SN Applied Sciences. 1: 1541. [DOI: 10.1007/s42452-019-1578-x]
Spigno G., Tramelli L., De Faveri D.M. (2007). Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. Journal of Food Engineering. 81: 200-208. [DOI: 10.1016/j.jfoodeng.2006.10.021]
Stamenković S.S., Mitrović T.LJ., Cvetković V.J., Krstić N.S., Baošić R.M., Marković M.S., Nikolić N.D., Marković V.L.J., Cvijan M.V. (2013). Biological indication of heavy metal pollution in the areas of DonjeVlase and Cerje (southeastern serbia) using epiphytic lichens. Archives of Biological Sciences. 65: 151-159. [DOI: 10.2298/ABS1301151S]
Sujetoviene G., Sliumpaite I. (2013). Response of EverniaE prunastri transplanted to an urban area in central Lithuania. Atmospheric Pollution Research. 4: 222-228. [DOI: 10.5094/APR.2013.023]
Svihus B., Holand Ø. (2000). Lichen polysaccharides and their relation to reindeer/caribou nutrition. Journal of Range Management. 53: 642-648. [DOI: 10.2307/ 4003160]
Tabbabi K., Karmous T. (2018). Use of lichens as bio-indicators in assessing the level of air pollution in the region of Bizerte study of pollutants attached to frond lichens by atomic spectroscopy and emission spectroscopy by plasma torch. Moroccan Journal of Chemistry. 6: 148-159. [DOI: 10.48317/IMIST.PRSM/ morjchem-v6i1.6064]
Thakur A., Singh S., Puri S. (2021). Nutritional evaluation, phytochemicals, antioxidant and antibacterial activity of Stellaria monosperma Buch.-Ham. Ex D. Don and Silene vulgaris (Moench) Garcke: wild edible plants of western Himalayas. Jordan Journal of Biological Sciences. 14: 83-90. [DOI: 10.54319/jjbs/140111]
Thiex N.J., Manson H., Anderson S., Persson J.Å. (2002). Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: collaborative study. Journal of AOAC International. 85: 309-317. [DOI: 10.1093/jaoac/85.2.309]
Tiencheu B., Njabi Nji D., Achidi A.U., Egbe A.C., Tenyang N., Ngongang E.F.T., Djikeng F.T., Fossi B.T. (2021). Nutritional, sensory, physico-chemical, phytochemical, microbiological and shelf-life studies of natural fruit juice formulated from orange (Citrus sinensis), lemon (Citrus limon), honey and ginger (Zingiberofficinale). Heliyon. 7: e07177. [DOI: 10. 1016/j.heliyon.2021.e07177]
Tiquia S.M. (2010). Reduction of compost phytotoxicity during the process of decomposition. Chemosphere. 79: 506-512. [DOI: 10.1016/j.chemosphere.2010. 02.040]
Turkmen N., Sari F., Velioglu Y.S. (2006). Effect of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chemistry. 99: 835-841. [DOI: 10. 1016/j.foodchem.2005.08.034]
Turkmen N., Sari F., Velioglu Y.S. (2005). The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chemistry. 93: 713-718. [DOI: 10.1016/j.foodchem.2004.12.038]
Vinayaka K.S., Praveen Kumar S.V., Prashith Kekuda T.R., Krishnamurthy Y.L., Mallikarjun N., Swathi D. (2009). Proximate composition, antioxidant, anthelmintic and insecticidal activity of a macrolichen Ramalina conduplicans Vain. (Ramalinaceae). European Journal of Applied Sciences.1: 40-46.
Witmer J.R., Wetherell B.J., Wagner B.A., Du J., Cullen J.J., Buettner G.R. (2016). Direct spectrophotometric measurement of supra-physiological levels of ascorbate in plasma. Redox Biology. 8: 298-304. [DOI: 10.1016/ j.redox.2016.02.004]
Yang R.Y., Lin S., Kuo G. (2008). Content and distribution of flavonoids among 91 edible plant species. Asia of Pacific Journal of Clinical Nutrition. 17: 275-279.
Zhao Y., Wang M., Xu B. (2021). A comprehensive review on secondary metabolites and health-promoting effects of edible lichen. Journal of Functional Foods. 80: 1-17. [DOI: 10.1016/j.jff.2020.104283]
* Corresponding author (F. Bekka-Hadji)
* E-mail: fahima.bekka@univ-jijel.dz
ORCID ID: https://orcid.org/0009-0007-7235-5125
* E-mail: fahima.bekka@univ-jijel.dz
ORCID ID: https://orcid.org/0009-0007-7235-5125
Type of Study: Original article |
Subject:
Special
Received: 24/03/01 | Accepted: 24/06/27 | Published: 24/06/30
Received: 24/03/01 | Accepted: 24/06/27 | Published: 24/06/30
References
1. Akbulut G., Yildiz A. (2010). An overview to lichens: the nutrient composition of some species. KafkasKafkas Üniversitesi Fen Bilimleri Enstitüsü Dergisi. 3: 79-86.
2. AOAC. (2000). Official methods of analysis. 17th edition. The Association of Official Analytical Chemists. Gaithersburg, MD, USA. URL: https://law.resource. org/ pub/us/cfr/ibr/002/aoac.methods.1.1990. pdf. Accessed 14 May 2022.
3. Aoussar N., Achmit M., Es-sadeqy Y., Vasiljević P., Rhallabi N., Ait Mhand R., Zerouali K., Manojlović N., Mellouki F. (2021). Phytochemical constituents, antioxidant and antistaphylococcal activities of Evernia prunastri (L.) Ach., Pseudevernia furfuracea (L.) Zopf. and Ramalina farinacea (L.) Ach. from Morocco. Archives of Microbiology. 203: 2887-2894. [DOI: 10.1007/s00203-021-02288-5] [DOI:10.1007/s00203-021-02288-5] [PMID]
4. Arakawa-Kobayashi S., Kanaseki T. (2004). A study of lipid secretion from the lichen symbionts, ascomycetous fungus Myelochroa leucotyliza and green alga Trebouxia sp. Journal of Structural Biology. 146: 401-415. [DOI: 10.1016/j.jsb.2004.01.016] [DOI:10.1016/j.jsb.2004.01.016] [PMID]
5. Aydogdu T., O'Mahony J.A., McCarthy N.A. (2023). pH, the fundamentals for milk and dairy processing: a review. Dairy. 4: 395-340. [DOI: 10.3390/ dairy4030026] [DOI:10.3390/dairy4030026]
6. Behera B.C., Verma N., Sonone A., Makhija U. (2005). Antioxydant and antibacterial activities of lichen usnea ghattensis in vitro. Biotechnology Letters. 27: 991-995. [DOI: 10.1007/s10529-005-7847-3] [DOI:10.1007/s10529-005-7847-3] [PMID]
7. Bekka-Hadji F., Adjeroud-Abdellatif N. (2023). Assessment of phytochemical, antioxidant and antibacterial activities of Evernia prunastri species collected from Algeria. Carpathian Journal of Food Science and Technology. 15: 99-112. [DOI: 10.34302/ crpjfst/2023.15.4.8] [DOI:10.34302/crpjfst/2023.15.4.8]
8. Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72: 248-254. [DOI: 10.1016/ 0003-2697(76)90527-3] [DOI:10.1016/0003-2697(76)90527-3] [PMID]
9. Bukabayeva Z., Abiyev S., Silybayeva B., Assanova U., Sharipkhanova A., Sagdatkyzy B. (2023). Epiphytic and epigeal lichens as bioindicators of air pollution in the Burabay National Park, Kazakhstan. Biodiversitas Journal of Biological Diversity. 24: 2701-2709. [DOI: 10.13057/biodiv/d240523] [DOI:10.13057/biodiv/d240523]
10. Constenla D., Ponce A.G., Lozano J.E. (2002). Effect of pomace drying on apple pectin. LWT - Food Science and Technology. 35: 216-221. [DOI: 10.1006/fstl. 2001.0841] [DOI:10.1006/fstl.2001.0841]
11. Debib A., Tir-Touil M.A., Meddah B., Hamaidi-Chergui F., Menadi S., Alsayadi M.S. (2018). Evaluation of antimicrobial and antioxidant activities of oily macerates of Algerian dried figs (Ficus carica L.). International Food Research Journal. 25: 351-356.
12. Di Nuzzo L., Canali G., Giordani P., Nascimbene J., Benesperi R., Papini A., Bianchi E., Porada P. (2022). Life-stage dependent response of the epiphytic lichen Lobaria pulmonaria to climate. Frontiers in Forests and Global Change. 5: 903607. [DOI: 10.3389/ffgc. 2022.903607] [DOI:10.3389/ffgc.2022.903607]
13. Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chemistry. 97: 654-660. [DOI: 10.1016/j.foodchem.2005.04.028] [DOI:10.1016/j.foodchem.2005.04.028]
14. Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 28: 350-356. [DOI: 10.1021/ac60111a017] [DOI:10.1021/ac60111a017]
15. Favero-Longo S.E., Piervittori R. (2010). Lichen-plant interactions. Journal of Plant Interactions. 5: 163-177. [DOI: 10.1080/17429145.2010.492917] [DOI:10.1080/17429145.2010.492917]
16. Gautam A.K., Yadav D., Bhagyawant S.S., Singh P.K., Jin J.O. (2021). Lichen: a comprehensive review on lichens as a natural sources exploring nutritional and biopharmaceutical benefits. Progress in Nutrition. 23: e2021153. [DOI: 10.23751/pn.v23i3.9833]
17. Giannakourou M.C., Taoukis P.S. (2021). Effect of alternative preservation steps and storage on vitamin C stability in fruit and vegetable products: critical review and kinetic modelling approaches. Foods. 10: 2630. [DOI: 10.3390/foods10112630] [DOI:10.3390/foods10112630] [PMID] [PMCID]
18. Hosseini S.S., Khodaiyan F., Yarmand M.S. (2016). Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydrate Polymers. 140: 59-65. [DOI: 10.1016/j.carbpol.2015.12.051] [DOI:10.1016/j.carbpol.2015.12.051] [PMID]
19. Ivanova D.G., Ivanov D. (2009). Ethnobotanical use of lichens: lichens for food review. Scripta Scientifica Medica. 41: 11-16. [DOI: 10.14748/ssm.v41i1.456] [DOI:10.14748/ssm.v41i1.456]
20. Kondratiuk A.S., Savchuk O.M., Hur J.S. (2015). Optimization of protein extraction for lichen thalli. Mycobiology. 43: 157-162. [DOI: 10.5941/MYCO. 2015.43.2.157] [DOI:10.5941/MYCO.2015.43.2.157] [PMID] [PMCID]
21. Kumar S.V.P., Kekuda T.R.P.,Vinayaka K.S., Swathi D., Mallikarjun N., Nishanth B.C. (2010). Studies on proximate composition, antifungal and anthelmintic activity of a macrolichen Ramalina hossei H. Magn and G. Awasthi. International Journal of Biotechnology and Biochemistry. 6: 191-201.
22. Lackovičová A., Guttova A., Backor M., Pišút P. (2013). Response of Evernia prunastri to urban environmental conditions in central Europe after the decrease of air pollution. The Lichenologist. 45: 89-100. [DOI: 10.1017/S002428291200062X] [DOI:10.1017/S002428291200062X]
23. Lapornik B., Prošek M., Wondra A.G. (2005). Comparison of extracts prepared from plant by-products using different solvents and extraction time. Journal of Food Engineering. 71: 214-222. [DOI: 10.1016/j.jfoodeng. 2004.10.036] [DOI:10.1016/j.jfoodeng.2004.10.036]
24. Loppi S., Pacioni G., Olivieri N., Giacomo F.D. (1998). Accumulation of trace metals in the lichen Evernia prunastri transplanted at biomonitoring sites in central Italy. The Bryologist.101: 451-454. [DOI: 10.2307/ 3244187] [DOI:10.2307/3244187]
25. Meli M.A., Desideri D., Cantaluppi C., Ceccotto F., Feduzi L., Roselli C. (2018). Elemental and radiological characterization of commercial Cetraria islandica (L.) Acharius pharmaceutical and food supplementation products. Science of the Total Environment. 613-614: 1566-1572. [DOI: 10.1016/j.scitotenv.2017.08.320] [DOI:10.1016/j.scitotenv.2017.08.320] [PMID]
26. Moslemi M. (2021). Reviewing the recent advances in application of pectin for technical and health promotion purposes: from laboratory to market. Carbohydrate Polymers. 254: 117324. [DOI: 10.1016/j.carbpol.2020. 117324] [DOI:10.1016/j.carbpol.2020.117324] [PMID]
27. Muthu S., Murugan M., Rajendran K., Ponmurugan P. (2021). An assessment of proximate composition, antioxidant activities and LC/MS based phytochemical profiling of some lichen species collected fromwestern Ghats of southern part of India. Jordan Journal of Biological Sciences.14: 647-661. [DOI: 10.54319/jjbs/ 140404]. [DOI:10.54319/jjbs/140404]
28. Olano-Martin E., Gibson G.R., Rastall R.A. (2002). Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. Journal of Applied Microbiology. 93: 505-511. [DOI: 10.1046/j.1365-2672.2002.01719.x.] [DOI:10.1046/j.1365-2672.2002.01719.x] [PMID]
29. Podterob A.P. (2008). Chemical composition of lichens and their medical applications. Pharmaceutical Chemistry Journal. 42: 582-588. [DOI: 10.1007/ s11094-009-0183-5] [DOI:10.1007/s11094-009-0183-5]
30. Poulsen-Silva E., Gordillo-Fuenzalida F., Atala C., Moreno A.A., Otero M.C. (2023). Bioactive lichen secondary metabolites and their presence in species from Chile. Metabolites. 13: 805. [DOI: 10.3390/metabo13070805] [DOI:10.3390/metabo13070805] [PMID] [PMCID]
31. Prashith Kekuda T.R., Vinayaka K.S., Swathi D., Suchitha Y., Venugopal T.M., Mallikarjun N. (2011). Mineral composition, total phenol content and antioxidant activity of a macrolichen Everniastrum cirrhatum (Fr.) Hale (Parmeliaceae). E-Journal of Chemistry. 8: 1886-1894. [DOI: 10.1155/2011/420673] [DOI:10.1155/2011/420673]
32. Rakić S., Petrović S., Kukić J., Jadranin M., Tešević V., Povrenović D., Šiler-Marinković S. (2007). Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chemistry. 104: 830-834. [DOI: 10.1016/j.foodchem. 2007.01.025] [DOI:10.1016/j.foodchem.2007.01.025]
33. Ren M., Jiang S., Wang Y., Pan X., Pan F., Wei X. (2023). Discovery and excavation of lichen bioactive natural products. Frontiers in Microbiology. 14: 1177123. [DOI: 10.3389/fmicb.2023.1177123] [DOI:10.3389/fmicb.2023.1177123] [PMID] [PMCID]
34. Rezzoug S.A., Maache-Rezzoug Z., Sannier F., Allaf K. (2008). A thermomechanical preprocessing for pectin extraction from orange peel. Optimisation by response surface methodology. International Journal of Food Engineering. 4: 10. [DOI: 10.2202/1556-3758.1183] [DOI:10.2202/1556-3758.1183]
35. Rola K. (2020). Insight into the pattern of heavy-metal accumulation in lichen thalli. Journal of Trace Elements in Medicine and Biology. 61: 126512. [DOI: 10.1016/j.jtemb.2020.126512] [DOI:10.1016/j.jtemb.2020.126512] [PMID]
36. Rozzamri A., Atiqah-Izyannie A.M., Mat Yusoff M., Ismail-Fitry M.R. (2020). Effects of leavening agents in batter system on quality of deep-fried chicken breast meat. Food Research. 4: 327-334. [DOI: 10.26656/ fr.2017.4(2).273] [DOI:10.26656/fr.2017.4(2).273]
37. Shahbazi K., Beheshti M. (2019). Comparison of three methods for measuring heavy metals in calcareous soils of Iran. SN Applied Sciences. 1: 1541. [DOI: 10.1007/s42452-019-1578-x] [DOI:10.1007/s42452-019-1578-x]
38. Spigno G., Tramelli L., De Faveri D.M. (2007). Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. Journal of Food Engineering. 81: 200-208. [DOI: 10.1016/j.jfoodeng.2006.10.021] [DOI:10.1016/j.jfoodeng.2006.10.021]
39. Stamenković S.S., Mitrović T.LJ., Cvetković V.J., Krstić N.S., Baošić R.M., Marković M.S., Nikolić N.D., Marković V.L.J., Cvijan M.V. (2013). Biological indication of heavy metal pollution in the areas of DonjeVlase and Cerje (southeastern serbia) using epiphytic lichens. Archives of Biological Sciences. 65: 151-159. [DOI: 10.2298/ABS1301151S] [DOI:10.2298/ABS1301151S]
40. Sujetoviene G., Sliumpaite I. (2013). Response of EverniaE prunastri transplanted to an urban area in central Lithuania. Atmospheric Pollution Research. 4: 222-228. [DOI: 10.5094/APR.2013.023] [DOI:10.5094/APR.2013.023]
41. Svihus B., Holand Ø. (2000). Lichen polysaccharides and their relation to reindeer/caribou nutrition. Journal of Range Management. 53: 642-648. [DOI: 10.2307/ 4003160] [DOI:10.2307/4003160]
42. Tabbabi K., Karmous T. (2018). Use of lichens as bio-indicators in assessing the level of air pollution in the region of Bizerte study of pollutants attached to frond lichens by atomic spectroscopy and emission spectroscopy by plasma torch. Moroccan Journal of Chemistry. 6: 148-159. [DOI: 10.48317/IMIST.PRSM/ morjchem-v6i1.6064]
43. Thakur A., Singh S., Puri S. (2021). Nutritional evaluation, phytochemicals, antioxidant and antibacterial activity of Stellaria monosperma Buch.-Ham. Ex D. Don and Silene vulgaris (Moench) Garcke: wild edible plants of western Himalayas. Jordan Journal of Biological Sciences. 14: 83-90. [DOI: 10.54319/jjbs/140111] [DOI:10.54319/jjbs/140111]
44. Thiex N.J., Manson H., Anderson S., Persson J.Å. (2002). Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: collaborative study. Journal of AOAC International. 85: 309-317. [DOI: 10.1093/jaoac/85.2.309] [DOI:10.1093/jaoac/85.2.309] [PMID]
45. Tiencheu B., Njabi Nji D., Achidi A.U., Egbe A.C., Tenyang N., Ngongang E.F.T., Djikeng F.T., Fossi B.T. (2021). Nutritional, sensory, physico-chemical, phytochemical, microbiological and shelf-life studies of natural fruit juice formulated from orange (Citrus sinensis), lemon (Citrus limon), honey and ginger (Zingiberofficinale). Heliyon. 7: e07177. [DOI: 10. 1016/j.heliyon.2021.e07177] [DOI:10.1016/j.heliyon.2021.e07177] [PMID] [PMCID]
46. Tiquia S.M. (2010). Reduction of compost phytotoxicity during the process of decomposition. Chemosphere. 79: 506-512. [DOI: 10.1016/j.chemosphere.2010. 02.040] [DOI:10.1016/j.chemosphere.2010.02.040] [PMID]
47. Turkmen N., Sari F., Velioglu Y.S. (2006). Effect of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chemistry. 99: 835-841. [DOI: 10. 1016/j.foodchem.2005.08.034] [DOI:10.1016/j.foodchem.2005.08.034]
48. Turkmen N., Sari F., Velioglu Y.S. (2005). The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chemistry. 93: 713-718. [DOI: 10.1016/j.foodchem.2004.12.038] [DOI:10.1016/j.foodchem.2004.12.038]
49. Vinayaka K.S., Praveen Kumar S.V., Prashith Kekuda T.R., Krishnamurthy Y.L., Mallikarjun N., Swathi D. (2009). Proximate composition, antioxidant, anthelmintic and insecticidal activity of a macrolichen Ramalina conduplicans Vain. (Ramalinaceae). European Journal of Applied Sciences.1: 40-46.
50. Witmer J.R., Wetherell B.J., Wagner B.A., Du J., Cullen J.J., Buettner G.R. (2016). Direct spectrophotometric measurement of supra-physiological levels of ascorbate in plasma. Redox Biology. 8: 298-304. [DOI: 10.1016/ j.redox.2016.02.004] [DOI:10.1016/j.redox.2016.02.004] [PMID] [PMCID]
51. Yang R.Y., Lin S., Kuo G. (2008). Content and distribution of flavonoids among 91 edible plant species. Asia of Pacific Journal of Clinical Nutrition. 17: 275-279.
52. Zhao Y., Wang M., Xu B. (2021). A comprehensive review on secondary metabolites and health-promoting effects of edible lichen. Journal of Functional Foods. 80: 1-17. [DOI: 10.1016/j.jff.2020.104283] [DOI:10.1016/j.jff.2020.104283]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







