Volume 11, Issue 2 (June 2024)
J. Food Qual. Hazards Control 2024, 11(2): 71-81 |
Back to browse issues page
Ethics code: 0000
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Guemra I, Adoui F, Sabba E, Ferhat R, Benatallah L. Influence of Soaking, Boiling, Roasting, and Germination on the Composition and Functional Properties of Algerian Chickpea Flour, and the Consumer Acceptability of Chickpea Cheese Analogue. J. Food Qual. Hazards Control 2024; 11 (2) :71-81
URL: http://jfqhc.ssu.ac.ir/article-1-1148-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1148-en.html
Génie Agro-Alimentaire Laboratory (GENIAAL), Institute of Nutrition Food and Agri-Food Technologies I.N.A.T.A-A., Mentouri Brothers University Constantine 1, Constantine, Algeria, Scientific and Technical Research Center in Physicochemical Analyses (CRAPC), Bousmail, Tipaza, Algeria , imane.guemra@doc.umc.edu.dz
Full-Text [PDF 563 kb]
(936 Downloads)
| Abstract (HTML) (1341 Views)
Full-Text: (10 Views)
Influence of Soaking, Boiling, Roasting, and Germination on the Composition and Functional Properties of Algerian Chickpea Flour, and the Consumer Acceptability of Chickpea Cheese Analogue
I. Guemra 1,2** , F. Adoui 1, E. Sabba 2,3, R. Ferhat 4, L. Benatallah 1
1. Génie Agro-Alimentaire Laboratory (GENIAAL), Institute of Nutrition Food and Agri-Food Technologies I.N.A.T.A-A., Mentouri Brothers University Constantine 1, Constantine, Algeria
2. Scientific and Technical Research Center in Physicochemical Analyses (CRAPC), Bousmail, Tipaza, Algeria
3. Bioqual Laboratory, Institute of Nutrition Food and Agri-Food Technologies I.N.A.T.A-A., Mentouri Brothers University Constantine 1, Constantine, Algeria
4. Food Science Laboratory (LSA), Batna 1 University, Batna, Algeria
HIGHLIGHTS
To cite: Guemra I., Adoui F., Sabba E., Ferhat R., Benatallah L. (2024). Influence of soaking, boiling, roasting, and germination on the composition and functional properties of Algerian chickpea flour, and the consumer acceptability of chickpea cheese Analogue. Journal of Food Quality and Hazards Control. 11: 71-81.
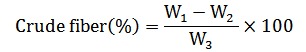
Where, W1 is the weight of the crucible with fiber; W2 is regarded as the weight of the crucible with ash; W3 is the weight of the sample.
The total carbohydrate content was determined by subtracting the sum of ash, moisture, fat, protein, and total dietary fiber content from 100.
Functional properties of chickpea flour
-Bulk Density (BD)
The BD was ascertained using a pre-weighed graduated cylinder (10 ml) filled with chickpea flour up to the 10 ml mark by continuous tapping, ensuring that there was no further change in volume. The graduated cylinder, now containing the flour, was re-weighed. The BD of the sample was then estimated in g/ml by quantifying the difference in weight (Benítez et al., 2011).
-Water Absorption Capacity (WAC)
The method of Shen et al. (2021) was utilized to analyze WAC. A mass of 0.6 g of chickpea flour was measured (W0) and thoroughly combined with 10 ml of distilled water within a centrifuge tube (W2). The mixture was then subjected to centrifugation at 3,000 g for 30 min. The tube containing the chickpea flour was reweighed after removing the supernatant (W1). The WAC was calculated as:
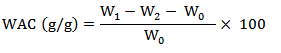
-Oil Absorption Capacity (OAC)
One g of accurately weighed chickpea flour (O0) was completely blended with 10 ml of corn oil in a centrifuge tube (O2). The mixture was kept at room temperature for 30 min and subsequently subjected to centrifugation at 3,000 g for 30 min. Afterward, the centrifuge tube was weighed after being inverted for 2 min to drain the supernatant and excess oil (O1). The OAC was calculated as:
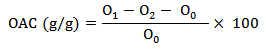
-Swelling
This parameter was defined in accordance with Benmeziane-Derradji et al. (2020); 10 ml of distilled water was added to 200 mg of chickpea flourin a graduated cylinder. The mixture was dispersed by moderate swirling and permitted to equilibrate at room temperature for 18 h. The swelling was calculated as follows:
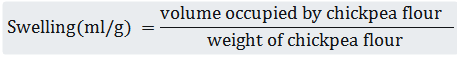
-Emulsifiying Capacity (EC)
Utilizing a homogenizer (Kinematica, Switzerland), 1.75 g of chickpea flour was blended with 25 ml of distilled water for 30 s. Subsequently, an additional 25 ml of maize oil were introduced into the solution, and the mixture was homogenized once more for 30 s. The emulsion was then centrifuged at 1,100 g for 5 min (Shen et al., 2021). The EC was determined as follows:
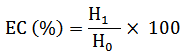
Where, H0 is the tube's overall emulsion height; and H1 is the tube's emulsified layer height.
-Foaming Capacity (FC)
For the measurement of FC in accordance with Schlegel et al. (2019), 100 ml of a 5% (w/w) chickpea flour solution was whipped at room temperature for 8 min with a hand mixer (CRRAFT model BT83, China). The increase in foam volume in a graduated cylinder was applied to measure foaming activity.
-Gelation
Based on Ouazib et al. (2015), suspensions of chickpea flour were generated at concentrations of 2, 4, 6, 8, 10, 12, 14, and 16% (w/v) using 5 ml of distilled water within test tubes. These test tubes were then heated in a water bath at 100 °C for 1 h. The test tubes were promptly subjected to cooling with cold tap water and then refrigerated at 4 °C for 2 h. The concentration at which the suspension exhibited no flow on tube inversion was identified as the minimum gelation concentration.
Preparation and characterization of chickpea cheese analogue
The chickpea cheese analogue was prepared with certain modifications according to Buckingham (2018), as detailed; 2.5 g of salt, 2.5 g of cheese flavour, 10 g of corn oil, 3 g of olive oil, 8 g of lemon juice, and 130 g of tap water were added to 100 g of treated and raw chickpea flour. The mixture was put on medium heat for 4 min with stirring continuous lyuntil a homogeneous paste was prepared, then transferred into glass containers and refrigerated for 24 h.
An analysis was conducted on a control sample of commercially accessible cheddar cheese in conjunction with chickpea cheese analogues.
-Color properties
The surface color of commercial cheddar cheese and chickpea cheese analogues was measured using a colorimeter (Chen Spec CS-10, China). Tristimulus values of the color namely L*, a*, and b* were recorded. The L* value is the lightness variable ranging from 100 for perfect white to zero for black, whereas the a*, and b* values are the chromaticity values, redness/greenness, and yellowness/blueness, respectively ( Ferawati et al., 2021).
-Texture profile
A texture analyzer (Shimadzu texture analyzer EZ-LX, Japan) was used to perform the analysis of the texture profile. Slight modifications of the method used by Le Tohic et al. (2018) have been applied. The cheese samples were cut into 20 mm cubes immediately after removal from the refrigerator (4 °C). Two compression-decompression cycles were executed between parallel plates utilizing a cylindrical probe at a constant rate of 3 mm/s to 50% of the sample's height. The analysis was fulfilled in triplicate.
-Sensory evaluation
The sensory evaluation or organoleptic characteristics were prosecuted by 70 panelists containing female and male students and stuff from the department of applied microbiology and food sciences, Jijel University, Algeria. Judges were requested to rate the taste, flavor, oral texture, color, and overall acceptability of coded chickpea cheese analogue samples and a commercial cheddar cheese, using the 9-point hedonic scale (1=dislike extremely, 2=dislike very much, 3=dislike moderately, 4=dislike slightly, 5=neither like nor dislike, 6=like slightly, 7=like moderately, 8=like very much, and 9=like extremely). All panelists had access to crackers and water to refresh their palates as required (Oyeyinka et al., 2019).
Statistical analysis
All measurements were implemented in triplicate. The data were analyzed by calculating the means±Standard Deviations (SD). XLSTAT 2014 software was utilized to perform statistical analysis using ANOVA, and mean values were compared with Tukey’s test at a significance level of 5%.
Results
Chemical analysis of chickpea flour
The results of the proximate composition of chickpea flours subjected to various treatments which are presented in Table 1.
The mean value of moisture ranged from 3.61 to 8.21%. A significant difference (p<0.05) was observed only after roasting treatment, with 3.61%.
Table 1: Proximate composition (as a percentage of wet weight) of raw and treated chickpea flour
Values were expressed as the average of triplicates±Standard Deviation (SD). Different letters in the same column indicate statistically significant differences (p<0.05).
Table 2: Functional properties of raw and treated chickpea flour
Values were expressed as the average of triplicates±Standard Deviation (SD). Different letters in the same column indicate statistically significant differences (p<0.05).
BD=Bulk Density; WAC=Water Absorption Capacity; OAC=Oil Absorption Capacity; EC=Emulsifiying Capacity; FC=Foaming Capacity.
Table 3: Color properties of chickpea cheese analogues
Values were expressed as the average of triplicates±Standard Deviation (SD). Different letters in the same column indicate statistically significant differences (p<0.05).
Table 4: Texture profile analysis of chickpea cheese analogues
Values were expressed as the average of triplicates±Standard Deviation (SD). Different letters in the same column indicate statistically significant differences (p<0.05).
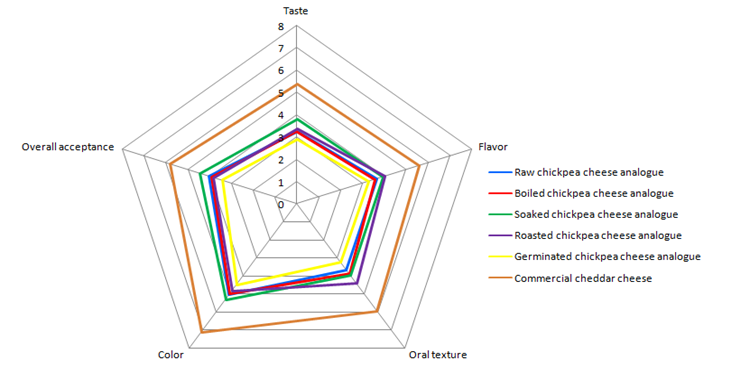
Figure 2: Sensory evaluation of chickpea cheese analogues
Conclusion
The effect of various treatments (boiling, soaking, roasting, as well as germination) on proximate composition, functional features of North Algerian chickpea flour and its suitability to develop a plant based cheese analogue was explored in this study.
In terms of proximate composition, among the various treatments utilized, exclusively moisture and lipid content were observed to be affected by the roasting and boiling treatments, respectively.
n contrast, each treatment proved different effects on the functional attributes of raw chickpea flour. They improved the EC while concurrently decreased the FC of raw chickpea, which was observed to have a substantial significance. This finding highlights a promising prospect for food technologists to incorporate raw chickpea flour into food preprations necessitating aeration, particularly for enhancing texture and characteristics of sourdough. In addition, the potential suitability for inclusion of treated chickpea flour in food formulations as an emulsifying agent might be evaluated.
Chickpea cheese analog failed to be appreciated by consumers in the sensory evaluation, This outcome prompts us to recommend alternative technological approaches, including fermentation or the incorporation of stabilizing and flavor-enhancing agents, for consideration in subsequent optimization investigations.
Author contributions
I.G. and F.A. designed the study; I.G. and R.F. conducted the experimental work; I.G and E.S. analyzed the data; I.G. wrote the manuscript; L.B. supervised the research. All authors read and approved the final manuscript.
Conflicts of interest
The authors declared no conflict of interest.
Funding
The study was funded by the Institute of Nutrition, Food, and Agri-food Technologies (INATAA), Mentouri Brothers University Constantine 1, and the Scientific and Technical Research Center in Physicochemical Analyses (CRAPC), as part of a doctoral thesis.
Ethical Consideration
Not applicable in this work.
Acknowledgements
The authors express their gratitude to BAISSISSE S. of Batna 1 University and Engineer BOUHALI S. from the Laboratory of Biology at the University of Jijel for their invaluable assistance. Moreover, appreciation is expanded to the INATAA Institute, particularly the GENIAAL laboratory, and the CRAPC Center, for their unwavering support.
References
Aguilar-Raymundo V.G., Vélez-Ruíz J.F. (2016). Characterization of two chickpea varieties and the effect of cooking on their physico-chemical and functional properties of flours. Journal of Food Research. 5: 2016. [DOI: 10.5539/jfr.v5n5p67]
Agume A.S.N., Njintang N.Y., Mbofung C.M.F. (2017). Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods. 6: 12. [DOI: 10.3390/foods6020012]
Alajaji S.A., El-Adawy T.A. (2006). Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. Journal of Food Composition and Analysis. 19: 806-812. [DOI: 10.1016/j.jfca.2006.03.015]
Association of Official Analytical Chemists (AOAC). (2005). Official method 978.10, fiber (crude) in animal feed and pet food, official methods of analysis of AOAC International. 18th edition. AOAC International, Gaithersburg, MD, USA.
Association of Official Analytical Chemists (AOAC). (1995). Official methods of analysis of AOAC international. 16th edition. Washington, DC. URL: https://search.worldcat.org/fr/title/Official-methods-of-analysis-of-AOAC-international/oclc/421897987.
Avanza M.V., Chaves M.G., Acevedo B.A., Añón M.C. (2012). Functional properties and microstructure of cowpea cultivated in north-east Argentina. LWT - Food Science and Technology. 49: 123-130. [DOI: 10.1016/j.lwt.2012.04.015]
Bachmann H.-P. (2001). Cheese analogues: a review. International Dairy Journal. 11: 505-515. [DOI: 10.1016/S0958-6946(01)00073-5]
Benítez V., Mollá E., Martín-Cabrejas M.A., Aguilera Y., López-Andréu F.J., Esteban R.M. (2011). Effect of sterilisation on dietary fibre and physicochemical properties of onion by-products. Food Chemistry. 127: 501-507. [DOI: 10.1016/j.foodchem.2011.01.031]
Benmeziane-Derradji F., Djermoune-Arkoub L., Ayat N.E.-H., Aoufi D. (2020). Impact of roasting on the physicochemical, functional properties, antioxidant content and microstructure changes of Algerian lentil (Lens culinaris) flour. Journal of Food Measurement and Characterization. 14: 2840-2853. [DOI: 10.1007/s11694-020-00529-7]
Bressani R. (1993). Grain quality of common beans. Food Reviews International. 9: 237-297. [DOI: 10.1080/87559129309540960]
Bubelová Z., Sumczynski D., Salek R.N. (2017). Effect of cooking and germination on antioxidant activity, total polyphenols and flavonoids, fiber content, and digestibility of lentils (Lens culinaris L.). Journal of Food Processing and Preservation. 42: e13388. [DOI: 10.1111/jfpp.13388]
Buckingham J. (2018). Super easy vegan cheese cookbook: 70 delicious plant-based cheeses. Rockridge Press, Emeryville, California.
Butt N.A., Ali T.M., Hasnain A. (2020). Development of rice starch‐based casein and fat mimetics and its application in imitation mozzarella cheese. Journal of Food Processing and Preservation. 44: e14928. [DOI: 10.1111/jfpp.14928]
Calles T., Del Castello R., Baratelli M., Xipsiti M., Navarro D.K. (2019). The international year of pulses: final report. FAO, Rome. URL: file:///C:/Users/admin/Downloads/ca2853en-3.pdf.
Chauhan A., Saxena D.C., Singh S. (2015). Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. LWT - Food Science and Technology. 63: 939-945. [DOI: 10.1016/j.lwt. 2015.03.115]
Chinma C.E., Adewuyi O., Abu J.O. (2009). Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus). Food Research International. 42: 1004-1009. [DOI: 10.1016/j.foodres.2009.04.024]
Chinma C.E., Anuonye J.C., Simon O.C., Ohiare R.O., Danbaba N. (2015). Effect of germination on the physicochemical and antioxidant characteristics of rice flour from three rice varieties from Nigeria. Food Chemistry. 185: 454-458. [DOI: 10.1016/j.foodchem.2015.04.010]
Cornejo F., Novillo G., Villacrés E., Rosell C.M. (2019). Evaluation of the physicochemical and nutritional changes in two amaranth species (Amaranthus quitensis and Amaranthus caudatus) after germination. Food Research International. 121: 933-939. [DOI: 10.1016/j.foodres.2019.01.022]
Du S.-K., Jiang H., Yu X., Jane J.-L. (2014). Physicochemical and functional properties of whole legume flour. LWT - Food Science and Technology. 55: 308-313. [DOI: 10.1016/j.lwt.2013.06.001]
El-Adawy T.A. (2002). Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods for Human Nutrition. 57: 83-97. [DOI: 10.1023/A:1013189620528]
Erba D., Angelino D., Marti A., Manini F., Faoro F., Morreale F., Pellegrini N., Casiraghi M.C. (2019). Effect of sprouting on nutritional quality of pulses. International Journal of Food Sciences and Nutrition. 70: 30-40. [DOI: 10.1080/09637486.2018.1478393]
Ferawati F., Hefni M., Östbring K., Witthöft C. (2021). The application of pulse flours in the development of plant-based cheese analogues: proximate composition, color, and texture properties. Foods. 10: 2208. [DOI: 10.3390/foods10092208]
Ferawati F., Hefni M., Witthöft C. (2019). Flours from swedish pulses: effects of treatment on functional properties and nutrient content. Food Science and Nutrition. 7: 4116-4126. [DOI: 10.1002/fsn3.1280]
Gerber P.J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J., Falcucci A., Tempio G. (2013). Tackling climate change through livestock – a global assessment of emissions and mitigation opportunities. Food and Agriculture Organization of the United Nations (FAO), Rome.
Grossmann L., McClements D.J. (2021). The science of plant-based foods: approaches to create nutritious and sustainable plant-based cheese analogs. Trends in Food Science and Technology. 118: 207-229. [DOI: 10.1016/j.tifs.2021.10.004]
Güzel D., Sayar S. (2012). Effect of cooking methods on selected physicochemical and nutritional properties of barlotto bean, chickpea, faba bean, and white kidney bean. Journal of Food Science and Technology. 49: 89-95. [DOI: 10.1007/s13197-011-0260-0]
Handa V., Kumar V., Panghal A., Suri S., Kaur J. (2017). Effect of soaking and germination on physicochemical and functional attributes of horsegram flour. Journal of Food Science and Technology. 54: 4229-4239. [DOI: 10.1007/s13197-017-2892-1]
Horwitz W., Latimer G.W., Association of Official Analytical Chemists International. (2006). Official methods of analysis of AOAC International, 18th edition. AOAC International, Gaithersburg, Maryland.
Iwe M.O., Michael N., Madu N.E., Obasi N.E., Onwuka G.I., Nwabueze T.U., Onuh J.O. (2017). Physicochemical and pasting properties high quality cassava flour (HQCF) and wheat flour blends. Agrotechnology. 6: 167. [DOI: 10.4172/2168-9881.1000167]
Jiang Z.-Q., Pulkkinen M., Wang Y.-J., Lampi A.-M., Stoddard F.L., Salovaara H., Piironen V., Sontag-Strohm T. (2016). Faba bean flavour and technological property improvement by thermal pre-treatments. LWT - Food Science and Technology. 68: 295-305. [DOI: 10.1016/j.lwt.2015.12.015]
Kaur M., Singh N. (2005). Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chemistry. 91: 403-411. [DOI: 10.1016/j. foodchem.2004.06.015]
Khattab R.Y., Arntfield S.D. (2009). Nutritional quality of legume seeds as affected by some physical treatments 2. antinutritional factors. LWT - Food Science and Technology. 42: 1113-1118. [DOI: 10.1016/j.lwt.2009.02.004]
Klang J.M., Tene S.T., Nguemguo Kalamo L.G., Boungo G.T., Ndomou Houketchang S.C., Kohole Foffe H.A., Womeni H.M. (2019). Effect of bleaching and variety on the physico-chemical, functional and rheological properties of three new Irish potatoes (Cipira, Pamela and Dosa) flours grown in the locality of Dschang (West region of Cameroon). Heliyon. 5: e02982. [DOI: 10.1016/j.heliyon.2019.e02982]
Ladjal Ettoumi Y., Chibane M. (2015). Some physicochemical and functional properties of pea, chickpea and lentil whole flours. International Food Research Journal. 22: 987-996.
Le Tohic C., O’Sullivan J.J., Drapala K.P., Chartrin V., Chan T., Morrison A.P., Kerry J.P., Kelly A.L. (2018). Effect of 3D printing on the structure and textural properties of processed cheese. Journal of Food Engineering. 220: 56-64. [DOI: 10.1016/j.jfoodeng.2017.02.003]
Martínez-Preciado A.H., Ponce-Simental J.A., Schorno A.L., Contreras-Pacheco M.L., Michel C.R., Rivera-Ortiz K.G., Soltero J.F.A. (2020). Characterization of nutritional and functional properties of “Blanco Sinaloa” chickpea (Cicer arietinum L.) variety, and study of the rheological behavior of hummus pastes. Journal of Food Science and Technology. 57: 1856-1865. [DOI: 10.1007/s13197-019-04220-8]
Ouazib M., Moussou N., Oomah B.D., Zaidi F., Wanasundara J.P.D. (2015). Effect of processing and germination on nutritional parameters and functional properties of chickpea (Cicer arietinum L.) from Algeria. Journal of Food Legumes. 28: 133-140.
Oyeyinka A.T., Odukoya J.O., Adebayo Y.S. (2019). Nutritional composition and consumer acceptability of cheese analog from soy and cashew nut milk. Journal of Food Processing and Preservation. 43: e14285. [DOI: 10.1111/jfpp.14285]
Prinyawiwatkul W., Mcwatters K.H., Beuchat L.R., Phillips R.D. (1997). Optimizing acceptability of chicken nuggets containing fermented cowpea and peanut flours. Journal of Food Science. 62: 889-905. [DOI: 10.1111/j.1365-2621.1997.tb15480.x]
Sabba E., Boudida Y., Boudjellal A. (2023). Evaluation of fatty acid and the composition of six different species of freshwater fish in the North of Algeria. Journal of Food Quality and Hazards Control. 10: 115-122. [DOI: 10.18502/jfqhc.10.3.13642]
Schlegel K., Leidigkeit A., Eisner P., Schweiggert-Weisz U. (2019). Technofunctional and sensory properties of fermented lupin protein isolates. Foods. 8: 678. [DOI: 10.3390/foods8120678]
Seleet F.L., Kassem J.M., Bayomim H.M., Abd-Rabou N.S., Ahmed N.S. (2014). Production of functional spreadable processed cheese analogue supplemented with chickpea. International Journal of Dairy Science. 9: 1-14. [DOI: 10.3923/ijds.2014.1.14]
Setia R., Dai Z., Nickerson M.T., Sopiwnyk E., Malcolmson L., Ai Y. (2019). Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Research International. 122: 263-272. [DOI: 10.1016/j.foodres. 2019.04.021]
Shen Y., Tang X., Li Y. (2021). Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chemistry. 339: 127823. [DOI: 10.1016/j.foodchem.2020.127823]
Shevkani K., Singh N., Kaur A., Rana J.C. (2015). Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocolloids. 43: 679-689. [DOI: 10.1016/j.foodhyd.2014.07.024]
Sofi S.A., Rafiq S., Singh J., Mir S.A., Sharma S., Bakshi P., McClements D.J., Mousavi Khaneghah A., Dar B.N. (2023). Impact of germination on structural, physicochemical, techno-functional, and digestion properties of desi chickpea (Cicer arietinum L.) flour. Food Chemistry. 405: 135011. [DOI: 10.1016/j.foodchem.2022.135011]
Sofi S.A., Singh J., Muzaffar K., Mir S.A., Dar B.N. (2020). Effect of germination time on physico-chemical, functional, pasting, rheology and electrophoretic characteristics of chickpea flour. Journal of Food Measurement and Characterization. 14: 2380-2392. [DOI: 10.1007/s11694-020-00485-2]
Suárez-Estrella D., Bresciani A., Iametti S., Marengo M., Pagani M.A., Marti A. (2020). Effect of sprouting on proteins and starch in quinoa (Chenopodium quinoa Willd.). Plant Foods for Human Nutrition. 75: 635-641. [DOI: 10.1007/s11130-020-00864-6]
Tonfack Djikeng F., Mouto Ndambwe C.M., Ngangoum E.S., Tiencheu B., Tambo Tene S., Achidi A.U., Womeni H.M. (2022). Effect of different processing methods on the proximate composition, mineral content and functional properties of snail (Archachatina marginata) meat. Journal of Agriculture and Food Research. 8: 100298. [DOI: 10.1016/j.jafr.2022.100298]
Wani S.A., Kumar P. (2014). Comparative study of chickpea and green pea flour based on chemical composition, functional and pasting properties. Journal of Food Research and Technology. 2: 124-129. [DOI: 10.13140/2.1.3470.4964]
Xu M., Jin Z., Simsek S., Hall C., Rao J., Chen B. (2019). Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chemistry. 295: 579-587. [DOI: 10.1016/j.foodchem.2019.05.167]
Xu Y., Thomas M., Bhardwaj H.L. (2014). Chemical composition, functional properties and microstructural characteristics of three kabuli chickpea (Cicer arietinum L.) as affected by different cooking methods. International Journal of Food Science & Technology. 49: 1215-1223. [DOI: 10.1111/ijfs.12419]
I. Guemra 1,2**
1. Génie Agro-Alimentaire Laboratory (GENIAAL), Institute of Nutrition Food and Agri-Food Technologies I.N.A.T.A-A., Mentouri Brothers University Constantine 1, Constantine, Algeria
2. Scientific and Technical Research Center in Physicochemical Analyses (CRAPC), Bousmail, Tipaza, Algeria
3. Bioqual Laboratory, Institute of Nutrition Food and Agri-Food Technologies I.N.A.T.A-A., Mentouri Brothers University Constantine 1, Constantine, Algeria
4. Food Science Laboratory (LSA), Batna 1 University, Batna, Algeria
- Moisture and lipid content were observed to be affected by roasting and boiling, respectively.
- Treatments improved emulsifying capacity, reduced foaming capacity of raw chickpea flour, notably high at 142.06%.
- Sensory evaluation recommends fermentation and natural flavor enhancers to enhance consumer acceptance of chickpea cheese analogues.
| Article type Original article |
ABSTRACT Background: Chickpeas, rich in protein and fiber, are essential in a healthy diet, as the plant-based cheese industry responding to environmental demands. The objectives of current study were dual-folded: to scrutinize the impact of diverse treatments on the physicochemical and functional characteristics of chickpea flour, and to assess the suitability of this chickpea flour as a raw material for the formulation of a plant-based cheese analogue. Methods: Soaking at room temperature for 15 h, boiling for 20 min, roasting at 180 °C for 30 min, and germination for 24 h were utilized for a chickpea variety harvested from Constantine of Algeria in 2021. The effects of these treatments were investigated with regard to the chemical composition and functional features of chickpea flour. Additionally, The suitability of chickpea flour for the development of plant-based cheese analog was ascertained by analyzing its color properties, texture profile, and sensory evaluation. ANOVA (XLSTAT 2014) and Tukey’s pairwise comparison test at the 5% significance level (p<0.05) were applied to perform statistical analysis. Results: All used treatments resulted in significant enhancements (p<0.05) in crude fat content and Emulsifying Capacity, along with significant reductions in swelling and Foaming Capacity, which was notably high in raw chickpea flour with 142.06%. Moreover, roasting reduced significantly moister content and exerted a positive effect on Water Absorption Capacity. However, the remaining chemical composition parameters and functional characteristics failed to reveal significant changes following the applied treatments. In texture profile analysis, chickpea cheese analogs exhibited lower values of hardness and cohesiveness in comparison with the commercial cheese. The chickpea cheese analogues received lower scores compared to the commercial cheese based on the sensory evaluation. Conclusion: Each treatment manifested distinct impacts on the chemical composition and functional properties of raw chickpea flour. Chickpea cheese analogue failed to be well-received by consumers. © 2024, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Cicer Food Handling Food Analysis Cheese Algeria |
||
| Article history Received: 25 Nov 2023 Revised: 5 Mar 2024 Accept: 27 May 2024 |
||
| Acronyms and abbreviations BD=Bulk Density EC=Emulsifiying Capacity FC=Foaming Capacity OAC=Oil Absorption Capacity WAC=Water Absorption Capacity |
Introduction
According to studies, eating legumes including chickpeas promotes a healthy lifestyle containing high protein, carbohydrate, dietary fiber, vitamin, as well as mineral content, particularly as combined with cereals in the diet (Ferawati et al., 2019). The Food and Agriculture Organization (FAO) advocates for the consumption of pulses due to its positive nutritional profile, economic accessibility, and benefits for maintaining soil health (Calles et al., 2019). Furthermore, the functional features and characteristics of legumes flour play a significant role in the imparting of desirable traits and functionality to food items(Prinyawiwatkul et al., 1997). A requirement for using legume proteins as food ingredients, in addition to their sensory profile, is that they bear the right techno-functional characteristics, including emulsification, foaming, gelation, water, as well as oil binding properties (Schlegel et al., 2019). Pulse flour is considered as a versatile ingredient that finds application in diverse culinary contexts. Nonetheless, pulses also consist of certain antinutritional compounds, including protease inhibitors that impede protein digestion, oligosaccharides known to induce flatulence, and phytate, which can complex with essential minerals, thereby diminishing their bioavailability (Khattab and Arntfield, 2009; Jiang et al., 2016). Various treatments applied to legumes in food processing, including soaking, boiling, germination, and roasting, significantly increase the nutritional value by deactivating antinutritional factors, improve protein and starch digestibility, enhance mineral bioavailability, refine flavor and palatability, and modify functional features(Benmeziane-Derradji et al., 2020; Bubelová et al., 2017; Sofi et al., 2023). Various studies disclosed the effects of these treatments (Aguilar-Raymundo and Vélez-Ruíz, 2016; Erba et al., 2019; Ferawati et al., 2019; Handa et al., 2017). Dairy production generates roughly 20% of the greenhouse gases produced by livestock, accounting for 14.5% of all man-made greenhouse gas emissions. Therefore, decreasing dairy consumption and substituting it with plant-based dairy analogues could be a practical method to diminish greenhouse gas emissions (Gerber et al., 2013). Individuals with specific dietary considerations, including the persons affected by lactose intolerance or cow's milk allergies, or the ones with apprehensions regarding the presence of hormones in cow's milk, may detect it advantageous to contain plant-based alternatives to dairy products in their dietary regimens. The pulse-based cheese substitutes are valuable as potential meal options due to their high fiber content. These products possess the potential to serve as healthier alternatives within the current plant-based cheese market, therefore contributing to the enhanced consumption of pulses (Ferawati et al., 2021). There has been a recent rise in the invention of cheese alternatives obtained from plant-based components (Grossmann and McClements, 2021). However, scant research exists on the literature about Algerian chickpea, let alone plant-based cheese analogue. Therefore, the objectives of present study were twofold: (1) to investigate the impact of various treatments on the physicochemical and functional properties of chickpea flour, and (2) to evaluate the suitability of this chickpea flour as a raw material for the development of a plant-based cheese analogue.
Materials and methods
Source of material
A quantity of 10 kg Chickpea (Cicer arietinum L.) grains of the Kabuli FLIP 9,013 C variety were harvested from Constantine in Algeria in July 2021. These grains were generously supplied by the Cooperative of Cereals and Pulses (CCLS) in Constantine, Algeria. Corn oil, olive oil, lemon, salt, cheese flavoring, and cheddar cheese were procured from a local market situated in Jijel city, Algeria.
Preparation of chickpea flour
Four treatments were selected to be applicated on chickpea grains: soaking, boiling, roasting, and germination.
Materials and methods
Source of material
A quantity of 10 kg Chickpea (Cicer arietinum L.) grains of the Kabuli FLIP 9,013 C variety were harvested from Constantine in Algeria in July 2021. These grains were generously supplied by the Cooperative of Cereals and Pulses (CCLS) in Constantine, Algeria. Corn oil, olive oil, lemon, salt, cheese flavoring, and cheddar cheese were procured from a local market situated in Jijel city, Algeria.
Preparation of chickpea flour
Four treatments were selected to be applicated on chickpea grains: soaking, boiling, roasting, and germination.
- Soaking: the seeds were steeped in tap water (1:3 w/v) at room temperature for 15 h.
- Boiling: soaked seeds, as illustrated above, were boiled in tap water for 20 min (1:5 w/v).
Germination: soaked seeds, as modified above, were placed between two damp filter papers and left to germinate in the dark at room temperature for 24 h.- Roasting: chickpea seeds were cleaned and roasted in an oven (UN 110, MEMMERT GmbH+Co.KG, Germany) at 180 °C for 30 min.
The soaked, boiled, and germinated seeds were subsequently subjected to a drying process in an oven set to 45 °C for 16 h. Both raw and treated chickpea seeds were finely milled using a laboratory mill (Retsch GRINDOMIX GM 200, Germany) to achieve a particle size of 500 μm. The raw chickpea flour was regarded as the control sample. All samples were carefully stored at 4 °C until they were utilized in further analyses or experiments.
Chemical analysis of chickpea flour
Chickpea flour was analyzed with regard to moisture, ash, protein, fat, and total dietary fiber contents.
Moisture was regarded by drying in an oven at 105 °C, until constant weight was obtained according to Horwitz et al. (2006).
Ash content was assessed by combustion of the samples in a muffle furnace (Thermolyne, France) at 550 °C for 5 h (Horwitz et al., 2006).
Nitrogen content was measured with a Kjeldahl apparatus (Gerhardt, Germany), and subsequently converted into protein content by applying a conversion factor of 6.25 (AOAC, 1995).
The crude fat content was estimated based on the method of Sabba et al. (2023). One g of chickpea flour was homogenized with 20 ml of a chloroform (Sigma, Germany) and methanol (Sigma, Germany) solution (2/1; v/v). After filtration, a saline solution (sodium chloride (NaCl)) 0.58%) (VWR, France) was added to the filtrate and allowed to stand for 2 h to improve separation of the phases. The upper phase (methanol/water) was discarded, and the lower phase (chloroform/lipids) was collected. Subsequently, the solvent was distilled, and the residue was weighed.
The AOAC official method 978.10 (2005) was used to determine the crude total fiber content. Briefly, 2 g of chickpea flour was boiled for 30 min in 200 ml of 0.128 M of sulfuric acid (H2SO4; Scharlau, Spain) with periodic stirring. The resulting solution was filtered through a cotton cloth. The retentate was then transferred into 200 ml of 0.313 M of sodium hydroxide (NaOH; Loba chemie, India) and subjected to a further boiling step for 30 min. Following this, the sample underwent a filtration process and was thoroughly washed with hot water to completely eliminate all remaining traces of NaOH.
The fraction of total dietary fiber retained on the cloth was transferred to a crucible, dried at 130 °C for 2 h, weighed, and subsequently incinerated at 550 °C for 3 h. The total fiber content was calculated as follows:
Chemical analysis of chickpea flour
Chickpea flour was analyzed with regard to moisture, ash, protein, fat, and total dietary fiber contents.
Moisture was regarded by drying in an oven at 105 °C, until constant weight was obtained according to Horwitz et al. (2006).
Ash content was assessed by combustion of the samples in a muffle furnace (Thermolyne, France) at 550 °C for 5 h (Horwitz et al., 2006).
Nitrogen content was measured with a Kjeldahl apparatus (Gerhardt, Germany), and subsequently converted into protein content by applying a conversion factor of 6.25 (AOAC, 1995).
The crude fat content was estimated based on the method of Sabba et al. (2023). One g of chickpea flour was homogenized with 20 ml of a chloroform (Sigma, Germany) and methanol (Sigma, Germany) solution (2/1; v/v). After filtration, a saline solution (sodium chloride (NaCl)) 0.58%) (VWR, France) was added to the filtrate and allowed to stand for 2 h to improve separation of the phases. The upper phase (methanol/water) was discarded, and the lower phase (chloroform/lipids) was collected. Subsequently, the solvent was distilled, and the residue was weighed.
The AOAC official method 978.10 (2005) was used to determine the crude total fiber content. Briefly, 2 g of chickpea flour was boiled for 30 min in 200 ml of 0.128 M of sulfuric acid (H2SO4; Scharlau, Spain) with periodic stirring. The resulting solution was filtered through a cotton cloth. The retentate was then transferred into 200 ml of 0.313 M of sodium hydroxide (NaOH; Loba chemie, India) and subjected to a further boiling step for 30 min. Following this, the sample underwent a filtration process and was thoroughly washed with hot water to completely eliminate all remaining traces of NaOH.
The fraction of total dietary fiber retained on the cloth was transferred to a crucible, dried at 130 °C for 2 h, weighed, and subsequently incinerated at 550 °C for 3 h. The total fiber content was calculated as follows:
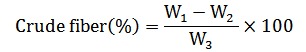
Where, W1 is the weight of the crucible with fiber; W2 is regarded as the weight of the crucible with ash; W3 is the weight of the sample.
The total carbohydrate content was determined by subtracting the sum of ash, moisture, fat, protein, and total dietary fiber content from 100.
Functional properties of chickpea flour
-Bulk Density (BD)
The BD was ascertained using a pre-weighed graduated cylinder (10 ml) filled with chickpea flour up to the 10 ml mark by continuous tapping, ensuring that there was no further change in volume. The graduated cylinder, now containing the flour, was re-weighed. The BD of the sample was then estimated in g/ml by quantifying the difference in weight (Benítez et al., 2011).
-Water Absorption Capacity (WAC)
The method of Shen et al. (2021) was utilized to analyze WAC. A mass of 0.6 g of chickpea flour was measured (W0) and thoroughly combined with 10 ml of distilled water within a centrifuge tube (W2). The mixture was then subjected to centrifugation at 3,000 g for 30 min. The tube containing the chickpea flour was reweighed after removing the supernatant (W1). The WAC was calculated as:
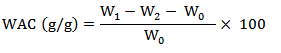
-Oil Absorption Capacity (OAC)
One g of accurately weighed chickpea flour (O0) was completely blended with 10 ml of corn oil in a centrifuge tube (O2). The mixture was kept at room temperature for 30 min and subsequently subjected to centrifugation at 3,000 g for 30 min. Afterward, the centrifuge tube was weighed after being inverted for 2 min to drain the supernatant and excess oil (O1). The OAC was calculated as:
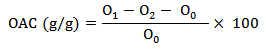
-Swelling
This parameter was defined in accordance with Benmeziane-Derradji et al. (2020); 10 ml of distilled water was added to 200 mg of chickpea flourin a graduated cylinder. The mixture was dispersed by moderate swirling and permitted to equilibrate at room temperature for 18 h. The swelling was calculated as follows:
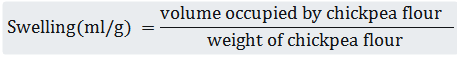
-Emulsifiying Capacity (EC)
Utilizing a homogenizer (Kinematica, Switzerland), 1.75 g of chickpea flour was blended with 25 ml of distilled water for 30 s. Subsequently, an additional 25 ml of maize oil were introduced into the solution, and the mixture was homogenized once more for 30 s. The emulsion was then centrifuged at 1,100 g for 5 min (Shen et al., 2021). The EC was determined as follows:
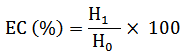
Where, H0 is the tube's overall emulsion height; and H1 is the tube's emulsified layer height.
-Foaming Capacity (FC)
For the measurement of FC in accordance with Schlegel et al. (2019), 100 ml of a 5% (w/w) chickpea flour solution was whipped at room temperature for 8 min with a hand mixer (CRRAFT model BT83, China). The increase in foam volume in a graduated cylinder was applied to measure foaming activity.
-Gelation
Based on Ouazib et al. (2015), suspensions of chickpea flour were generated at concentrations of 2, 4, 6, 8, 10, 12, 14, and 16% (w/v) using 5 ml of distilled water within test tubes. These test tubes were then heated in a water bath at 100 °C for 1 h. The test tubes were promptly subjected to cooling with cold tap water and then refrigerated at 4 °C for 2 h. The concentration at which the suspension exhibited no flow on tube inversion was identified as the minimum gelation concentration.
Preparation and characterization of chickpea cheese analogue
The chickpea cheese analogue was prepared with certain modifications according to Buckingham (2018), as detailed; 2.5 g of salt, 2.5 g of cheese flavour, 10 g of corn oil, 3 g of olive oil, 8 g of lemon juice, and 130 g of tap water were added to 100 g of treated and raw chickpea flour. The mixture was put on medium heat for 4 min with stirring continuous lyuntil a homogeneous paste was prepared, then transferred into glass containers and refrigerated for 24 h.
An analysis was conducted on a control sample of commercially accessible cheddar cheese in conjunction with chickpea cheese analogues.
-Color properties
The surface color of commercial cheddar cheese and chickpea cheese analogues was measured using a colorimeter (Chen Spec CS-10, China). Tristimulus values of the color namely L*, a*, and b* were recorded. The L* value is the lightness variable ranging from 100 for perfect white to zero for black, whereas the a*, and b* values are the chromaticity values, redness/greenness, and yellowness/blueness, respectively ( Ferawati et al., 2021).
-Texture profile
A texture analyzer (Shimadzu texture analyzer EZ-LX, Japan) was used to perform the analysis of the texture profile. Slight modifications of the method used by Le Tohic et al. (2018) have been applied. The cheese samples were cut into 20 mm cubes immediately after removal from the refrigerator (4 °C). Two compression-decompression cycles were executed between parallel plates utilizing a cylindrical probe at a constant rate of 3 mm/s to 50% of the sample's height. The analysis was fulfilled in triplicate.
-Sensory evaluation
The sensory evaluation or organoleptic characteristics were prosecuted by 70 panelists containing female and male students and stuff from the department of applied microbiology and food sciences, Jijel University, Algeria. Judges were requested to rate the taste, flavor, oral texture, color, and overall acceptability of coded chickpea cheese analogue samples and a commercial cheddar cheese, using the 9-point hedonic scale (1=dislike extremely, 2=dislike very much, 3=dislike moderately, 4=dislike slightly, 5=neither like nor dislike, 6=like slightly, 7=like moderately, 8=like very much, and 9=like extremely). All panelists had access to crackers and water to refresh their palates as required (Oyeyinka et al., 2019).
Statistical analysis
All measurements were implemented in triplicate. The data were analyzed by calculating the means±Standard Deviations (SD). XLSTAT 2014 software was utilized to perform statistical analysis using ANOVA, and mean values were compared with Tukey’s test at a significance level of 5%.
Results
Chemical analysis of chickpea flour
The results of the proximate composition of chickpea flours subjected to various treatments which are presented in Table 1.
The mean value of moisture ranged from 3.61 to 8.21%. A significant difference (p<0.05) was observed only after roasting treatment, with 3.61%.
Table 1: Proximate composition (as a percentage of wet weight) of raw and treated chickpea flour
| Chickpea flour | Moisture (%) |
Ash (%) |
Crude protein (%) |
Crude fat (%) |
Crude fiber (%) |
Total carbohydrates (%) |
| Raw chickpea flour | 8.21±1.04 a | 2.33±0.34 a | 21.58±0.50 a | 2.98±0.32 b | 1.96±0.62 a | 62.91±1.52 a |
| Boiled chickpea flour | 6.75±0.71 a | 1.96±0.87 a | 20.70±2.67 a | 5.04±0.86 a | 2.45±0.24 a | 63.07±3.58 a |
| Soaked chickpea flour | 7.21±0.49 a | 2.44±0.38 a | 22.75±1.75 a | 2.98±0.53 ab | 2.19±0.06 a | 62.41±2.41 a |
| Roasted chickpea flour | 3.61±1.40 b | 3.07±1.08 a | 22.45±1.33 a | 3.46±0.99 ab | 2.17±0.21 a | 65.21±1.4 a |
| Germinated chickpea flour | 6.52±0.80 a | 2.98±0.57 a | 21.87±0.87 a | 3.32±0.54 ab | 2.30±0.17 a | 62.99±0.23 a |
| p-value | 0.0016 | 0.3404 | 0.5886 | 0.0239 | 0.4882 | 0.5689 |
No significant differences (p>0.05) were observed in ash content among the experimental samples. Boiled chickpea flour demonstrated the lowest ash content of 1.96%, as compared to raw chickpea flour, which yielded a mean value of 2.33%. Conversely, the roasted samples exhibited the highest ash content at 3.07%. A significant increase in ash content was noted following germination, with a recorded value of 2.98%.
The protein content mean ranged from 20.70% in roasted samples to 22.75% in soaked samples, with no significant difference observed (p>0.05).
The mean fat content varied between 2.98 and 5.04%. All treatments, except soaking, resulted in a substantial increase in fat content (p<0.05).
Crude fiber content ranged from 1.96 to 2.45%. All used treatments enhanced it but not significantly (p>0.05).
Total carbohydrate content revealed no significant changes (p>0.05), ranging from 62.41 to 65.21%.
Functional characteristics of chickpea flour
The results of the functional characteristics of chickpea flour are illustrated in Table 2.
The protein content mean ranged from 20.70% in roasted samples to 22.75% in soaked samples, with no significant difference observed (p>0.05).
The mean fat content varied between 2.98 and 5.04%. All treatments, except soaking, resulted in a substantial increase in fat content (p<0.05).
Crude fiber content ranged from 1.96 to 2.45%. All used treatments enhanced it but not significantly (p>0.05).
Total carbohydrate content revealed no significant changes (p>0.05), ranging from 62.41 to 65.21%.
Functional characteristics of chickpea flour
The results of the functional characteristics of chickpea flour are illustrated in Table 2.
Table 2: Functional properties of raw and treated chickpea flour
| Chickpea flour | BD (g/ml) |
WAC (%) |
OAC (%) |
Swelling (ml/g) |
EC (%) |
FC (%) |
Gelation (%) |
| Raw chickpea flour | 0.71±0.03 a | 283±58.33 b | 220±26.45 a | 7.66±1.75 a | 4.69±0.81 b | 142.06±11.74 a | 12±0.00 |
| Boiled chickpea flour | 0.67±0.02 a | 341±8.33 ab | 226.66±58.59 a | 3.5±0.86 b | 6.58±1.51 ab | 8.76±2.55 c | 16±0.00 |
| Soaked chickpea flour | 0.70±0.04 a | 261±19.24 b | 223.33±30.55 a | 4.33±1.04 b | 8.03±2.01 ab | 65.55±8.95 b | 8±0.00 |
| Roasted chickpea flour | 0.69±0.00 a | 433±88.19 a | 216.66±58.59 a | 5±0.86 ab | 9.63±2.00 a | 6.45±0.59 c | 14±0.00 |
| Germinated chickpea flour | 0.70±0,03 a | 302±9.62 b | 230±43.58 a | 3±0.86 b | 6.02±1.02 ab | 79.02±12.54 b | 6±0.00 |
| p-value | 0.7814 | 0.0107 | 0.9964 | 0.0040 | 0.0251 | <0.0001 | nd |
BD=Bulk Density; WAC=Water Absorption Capacity; OAC=Oil Absorption Capacity; EC=Emulsifiying Capacity; FC=Foaming Capacity.
-BD
No significant difference (p>0.05) was discerned after all the treatments utilized. BD ranged from 0.67 g/ml in boiled chickpea flour to 0.71 g/ml in raw chickpea flour.
-WAC and OAC
WAC was significantly (p<0.05) influenced by the treatments. Raw chickpea flour demonstrated an initial value of 283, which increased to 302, 341, and 433% after germination, boiling, and roasting, respectively. However, OAC disclose no significant variation, ranging from 216% in roasted chickpea flour to 230% in germinated chickpea flour.
-Swelling
According to Table 3, there was a significant decrease (p<0.05) in swelling capacity from 7.66 ml/g for raw chickpea flour to 3.5, 4.33, 5, and 3 ml/g after boiling, soaking, roasting, and germination treatment, respectively.
No significant difference (p>0.05) was discerned after all the treatments utilized. BD ranged from 0.67 g/ml in boiled chickpea flour to 0.71 g/ml in raw chickpea flour.
-WAC and OAC
WAC was significantly (p<0.05) influenced by the treatments. Raw chickpea flour demonstrated an initial value of 283, which increased to 302, 341, and 433% after germination, boiling, and roasting, respectively. However, OAC disclose no significant variation, ranging from 216% in roasted chickpea flour to 230% in germinated chickpea flour.
-Swelling
According to Table 3, there was a significant decrease (p<0.05) in swelling capacity from 7.66 ml/g for raw chickpea flour to 3.5, 4.33, 5, and 3 ml/g after boiling, soaking, roasting, and germination treatment, respectively.
Table 3: Color properties of chickpea cheese analogues
| Cheese | L* | a* | b* |
| Raw chickpea cheese analogue | 73.67±0.62 b | 1.42±0.15 d | 33.83±0.48 a |
| Boiled chickpea cheese analogue | 66.81±0.39 d | 4.98±0.21 b | 31.38±0.27 b |
| Soaked chickpea cheese analogue | 70.44±0.18 c | 1.24±0.06 d | 25.07±0.06 d |
| Roasted chickpea cheese analogue | 61.01±0.43 e | 8.40±0.15 a | 31.51±0.41 b |
| Germinated chickpea cheese analogue | 75.14±2.13 b | 2.35±0.64 c | 21.29±0.72 e |
| Commercial cheddar cheese | 85.61±0.32 a | -1.92±0.01 e | 26.41±0.16 c |
| p-value | <0.0001 | <0.0001 | <0.0001 |
-EC
All utilized treatments enhanced significantly (p<0.05) EC of chickpea flour. It ranged from 4.69% in sprouted chickpea flour to 6.02% in germinated chickpea flour, 6.58% in boiled chickpea flour, 8.03% in soaked chickpea flour, and 9.63% in roasted chickpea flour.
-FC
This work demonstrates that Algerian raw chickpea flour contains a very intriguing FC with 142.06%. Nevertheless, every administered treatment resulted in a significant decrease (p<0.05) in this FC by as much as 6.45 and 8.76% following roasting and boiling, respectively, and by as much as 79.02 and 65.55% after germination and soaking, respectively.
-Gelation
The minimum concentration required for gelation varied from 6% in germinated chickpea flour to 16% in boiled chickpea flour. The minimum concentration of gelation for raw chickpea flour was 12%.
Chickpea cheese analogue characteristics
-Color properties of chickpea cheese analogues
Color properties revealed remarkable significant differences (p<0.05) among samples (Table 3). The commercial cheddar cheese had the highest level of lightness (L*) with 85.61, followed by germinated, raw, and soaked chickpea cheese with 75.14, 73.67, and 70.44, respectively. The smallest values were observed in boiled chickpea cheese at 66.81 and roasted chickpea cheese at 61.01. A totally opposite ranking was acquired for redness (a*) which ranged between -1.92 and 8.40. All the cheese samples uncovered a positive yellowness (b*) value that ranged from 21.29 to 33.83.
-Texture profile analysis of chickpea cheese analogues
The various treatment applied in the production of chickpea flour significantly impacted (p<0.05) the hardness, adhesiveness, and springiness of chickpea cheese analogues (Table 4). The least adhesive sample was the roasted chickpea cheese analogue with -0.058 N followed by the commercial cheddar cheese -0.144 N, on the other hand the highest adhesiveness values were recorded for germinated and raw chickpea cheese analogue with -0.421 and -0.418 N, respectively. Raw chickpea cheese analogue manifested the highest values of hardness, cohesiveness, and springiness, followed by germinated and soaked chickpea cheese analogues, whereas boiled and roasted chickpea cheese analogues possessed the lowest values.
All utilized treatments enhanced significantly (p<0.05) EC of chickpea flour. It ranged from 4.69% in sprouted chickpea flour to 6.02% in germinated chickpea flour, 6.58% in boiled chickpea flour, 8.03% in soaked chickpea flour, and 9.63% in roasted chickpea flour.
-FC
This work demonstrates that Algerian raw chickpea flour contains a very intriguing FC with 142.06%. Nevertheless, every administered treatment resulted in a significant decrease (p<0.05) in this FC by as much as 6.45 and 8.76% following roasting and boiling, respectively, and by as much as 79.02 and 65.55% after germination and soaking, respectively.
-Gelation
The minimum concentration required for gelation varied from 6% in germinated chickpea flour to 16% in boiled chickpea flour. The minimum concentration of gelation for raw chickpea flour was 12%.
Chickpea cheese analogue characteristics
-Color properties of chickpea cheese analogues
Color properties revealed remarkable significant differences (p<0.05) among samples (Table 3). The commercial cheddar cheese had the highest level of lightness (L*) with 85.61, followed by germinated, raw, and soaked chickpea cheese with 75.14, 73.67, and 70.44, respectively. The smallest values were observed in boiled chickpea cheese at 66.81 and roasted chickpea cheese at 61.01. A totally opposite ranking was acquired for redness (a*) which ranged between -1.92 and 8.40. All the cheese samples uncovered a positive yellowness (b*) value that ranged from 21.29 to 33.83.
-Texture profile analysis of chickpea cheese analogues
The various treatment applied in the production of chickpea flour significantly impacted (p<0.05) the hardness, adhesiveness, and springiness of chickpea cheese analogues (Table 4). The least adhesive sample was the roasted chickpea cheese analogue with -0.058 N followed by the commercial cheddar cheese -0.144 N, on the other hand the highest adhesiveness values were recorded for germinated and raw chickpea cheese analogue with -0.421 and -0.418 N, respectively. Raw chickpea cheese analogue manifested the highest values of hardness, cohesiveness, and springiness, followed by germinated and soaked chickpea cheese analogues, whereas boiled and roasted chickpea cheese analogues possessed the lowest values.
Table 4: Texture profile analysis of chickpea cheese analogues
| Cheese | Hardness (N) | Adhesiveness (N) | cohesiveness | springiness |
| Raw chickpea cheese analogue | 21.648±2.12 b | -0.418±0.10 b | 0.390±0.08 b | 0.562±0.00 b |
| Boiled chickpea cheese analogue | 14.205±4.65 bc | -0.192±0.08 ab | 0.199±0.00 b | 0.264±0.02 d |
| Soaked chickpea cheese analogue | 15.419±2.26 bc | -0.264±0.00 ab | 0.316±0.05 b | 0.454±0.07 bcd |
| Roasted chickpea cheese analogue | 12.372 ±0.56 c | -0.058±0.00 a | 0.170±0.00 b | 0.349±0.12 cd |
| Germinated chickpea cheese analogue | 15.347±1.09 bc | -0.421±0.13 b | 0.349±0.05 b | 0.492±0.03 bc |
| Commercial cheddar cheese | 62.452±3.77 a | -0.144±0,08 a | 0.708±0.18 a | 0.872±0.08 a |
| p-value | <0.0001 | 0.0010 | 0.0001 | <0.0001 |
-Sensory evaluation
Overall, there was a notable discrepancy in all the parameters assessed, as indicated in Figure 2. The sample of commercial cheddar cheese received the highest rated sample in pleasantness in all evaluated organoleptic properties, while germinated chickpea cheese analogue was the least rated. Among the various chickpea cheese analogues, the soaked one had the highest overall acceptance at 4.47 followed by raw, boiled, and roasted types at 4.04, 3.9, and 3.83, respectively. The results for oral texture and flavor reflected that roasted chickpea cheese analogue had the best acceptability compared to other chickpea cheese analogues, scoring 4.39 and 4, respectively. Furthermore, soaked chickpea cheese analogue received the highest rating among the other chickpea cheese analogues, scoring 5.29 for color and 3.8 for taste evaluation.
Overall, there was a notable discrepancy in all the parameters assessed, as indicated in Figure 2. The sample of commercial cheddar cheese received the highest rated sample in pleasantness in all evaluated organoleptic properties, while germinated chickpea cheese analogue was the least rated. Among the various chickpea cheese analogues, the soaked one had the highest overall acceptance at 4.47 followed by raw, boiled, and roasted types at 4.04, 3.9, and 3.83, respectively. The results for oral texture and flavor reflected that roasted chickpea cheese analogue had the best acceptability compared to other chickpea cheese analogues, scoring 4.39 and 4, respectively. Furthermore, soaked chickpea cheese analogue received the highest rating among the other chickpea cheese analogues, scoring 5.29 for color and 3.8 for taste evaluation.

Figure 2: Sensory evaluation of chickpea cheese analogues
Discussion
Moisture levels serve as a key index of storage stability, with lower levels allowing for longer storage periods (Iwe et al., 2017). Sofi et al. (2023) reported comparable results (8.6%) for raw chickpea flour; nevertheless, they documented higher values for germinated chickpea flour (8.25%). The significant difference (p<0.05) observed following roasting treatment (3.61%) aligns with the the results of Benmeziane‑Derradji et al. (2020) for lentil flour and Agume et al. (2017) for soybean flour. This significant decrease in moisture could be attributed to the dehydration of the chickpea flour during roasting process.
The lowest ash content (1.96%) exhibited in boiled chickpea flour can be explained by a mineral loss during the boiling treatment. Conversely, the highest ash content detected in the roasted samples (3.07%) can be attributed to the removal of water during the roasting process, resulting in a higher proportion of ash in the final product. Xu et al. (2014) found that raw chickpeas yielded higher results at 3.40%, compared to boiled chickpeas which showed similar values at 1.68%. In contrast to previous studies (Erba et al., 2019; Setia et al., 2019; Sofi et al., 2020), the ash content exhibited a notable increase, reaching 2.98% following germination process. In congruence with extant investigations on rice (Chinma et al., 2015), amaranth (Chauhan et al., 2015; Cornejo et al., 2019), and tigernut (Chinma et al., 2009), our findings are consistent. This increase in ash content can be attributed to a decrease in dry matter, presumably resulting in a reduction in total soluble solids. It is plausible that hydrolytic enzymes failed significantly to enhance the production of total soluble solids during the germination phase (Chinma et al., 2015).
The protein content results, varying between 20.70 to 22.75%,confirm comparability with the findings presented by Sofi et al. (2020), who reported a value of 21.94%. Notably, disparate values have been documented in previous researches; for instance, Kaur and Singh (2005) reported higher results at 26.7%, Wani and Kumar (2014) at 24.61%, and Xu et al. (2019) at 24.36%. Conversely, lower protein content values have been observed in chickpea flour by Aguilar-Raymundo and Vélez-Ruíz (2016) with 18.06% and Erba et al. (2019) with 18.6%. These observed variations may be attributed to factors including seed variety, geographical location, harvest conditions, and the method utilized to measure protein levels
All treatments, with the exception of soaking, caused a notable rise (p<0.05) in fat content. These findings deviate from the values presented in a prior study by Alajaji and El-Adawy (2006) in which they observed a pivotal decline following treatments. Nonetheless, they demonstrate consistency with findings attributing the observed increase post-treatments to lipid solubilization induced by starch gelatinization. Moreover, these studies elucidate this phenomenon by highlighting that, in the case of raw chickpea flour, lipids are notably entrapped within the starch matrix (Aguilar-Raymundo and Vélez-Ruíz, 2016; Setia et al., 2019).
All interventions resulted in an increase in crude fiber content, however, the boost was not statistically significant. This elevation is hypothesized to stem from the formation of protein-fiber complexes, potentially arising from chemical alterations induced by the applied treatments (Bressani, 1993).
The results for the total carbohydrate content (62.41-65.21%) are surpassing the ones presented by El-Adawy (2002) with 59.23-62.34% and Kaur and Singh (2005) with 60.2%, while falling below values reported by Aguilar-Raymundo and Vélez-Ruíz (2016) with 68.67-72.61% as well as Güzel and Sayar (2012) with 67.97%. These observed disparities can be reasonably attributed to changes in the composition of other constituents. It is noteworthy that the determination of total carbohydrate content contains the deduction of the cumulative sum of other nutrients from 100.
BD was recognized for its crucial role in packaging requirements (Tonfack Djikeng et al., 2022). Our results (0.67 to 0.71 g/ml) were lower than those reported by Sofi et al. (2020) with 0.74 to 0.83 g/ml and Xu et al. (2014) with 0.94 to 1.29 g/ml.This implies that our chickpea flours necessitate a larger packaging space. The obtained low BD uncovers the potential use of this flour as a food ingredient in formulations with reduced concern concerning retrogradation (Benmeziane-Derradji et al., 2020).
Due to their effect on other functional and sensory aspects, WAC and OAC are considered as significant in food preparation. Ready-to-Eat (RTE) food products that require good viscosity, for instance dairy products (yogurt and cheese), sauces, and soups need flour that involves a high percentage of WAC as a functional ingredient (Benmeziane-Derradji et al., 2020).
A significant increase (p<0.05) in WAC was observed following germination, boiling, and roasting from 283 to 302, 341, and 433%, respectively. Sofi et al. (2020) illustrated an identical behavior following germination due to n rise in hydrophilic biopolymers. According to Avanza et al. (2012), in addition to the presence of hydrophilic carbohydrates, crude fibers swelling and starch gelatinization and heat treatment led to conformational alterations of proteins and consequently increased exposure of polar amino acids. However, soaking yielded a reduction of WAC which could be explained by a water saturation acquired during the soaking process.
In contrast, there was no notable distinction observed in OAC (p>0.05). Du et al. (2014) reported lower value with 110% and considered OAC as an important parameter, as it enhances mouth feel and preserves the flavor. OAC is affected by nonpolar amino acid side chain ratios on the hydrophobic protein molecule surface, starch content, as well as particles size (Wani and Kumar, 2014).
There was a significant decrease (p<0.05) in swelling capacity following boiling, soaking, roasting, and germination treatment. Based on the literature, these findings are not in line with those recorded by Handa et al. (2017) for horsegram flour and Klang et al. (2019) for potato flour, demonstrating a significant increase after germination and temperature treatment respectively, although they are in agreement with Agume et al. (2017) for soybean flour, attributingthe decrease by a destruction of the structure of proteins and starch responsible for this swelling capacity due to high temperature as well as the enzymatic hydrolysis of peptide and glycosidic bonds induced during soaking and germination.
EC is a highly intriguing functional attribute as it plays a role in achieving the preferred texture of a food matrix. According to Shen et al. (2021) the charge, size, shape, hydrophobicity, and composition of protein molecules play the most remrkable role in EC since they have the ability to absorb oil and water at the interfacial area. All applied treatments demonstrated a significant improvement in the EC of chickpea flour (p<0.05). This is clarified by the protein partial unfolding and dissociation which leads to exposure of the non-polar hydrophobic sites of these amphiphilic proteins.
FC is a desirable functional characteristic in food systems, permitting them to be applied for instance in baked foods, ice cream mixes, and whipped toppings. It is formed as the proteins are whipped and therefore form an interfacial film which maintains the gas bubbles in suspension and reduces coalescence (Shen et al., 2021; Shevkani et al., 2015). This study betrayes that our raw chickpea flour includes a highly intriguing FC with 142.06%, surpassing previous reports in the literature by Kaur and Singh, 2005, Martínez-Preciado et al. 2020, Wani and Kumar, 2014, and Xu et al. 2014. However, a significant reduction (p<0.05) was observed after roasting and boiling which could be explained by the protein denaturation from heat’s impact. Suárez-Estrella et al. (2020) described a comparable decrease in FC following germination in quinoa due to a comprehensive modification of the protein fraction during germination, including a decrease in albumins and globulins content in addition to an elimination of part of the saponins.
Gelation is a beneficial functional attribute in food applications and new product development that require gelling and thickening. It occurs as proteins and starch develop a three-dimensional network that resists waft underneath strain (Benmeziane-Derradji et al., 2020). Raw chickpea flour least gelation concentration (12%) was greater than the findings of Ladjal Ettoumi and Chibane (2015), and Agume et al. (2017), in which 8% concentration was enough to form a gel. As it is demonstrated in Table 2 both roasting and boiling treatment boosted the gelation of raw chickpea flour up to 14 and 16%, respectively, while soaking and germination reduced it up to 8 and 6%, respectively. Prinyawiwatkul et al. (1997) suggested that a combination of denatured proteins and pregelatinized starch in cowpeas, caused by heat, would necessitate higher flour concentrations for gel formation.. Additionally, low concentration required for gel formation in the case of soaked and germinated legume is due to the synergistic effects of protein and starch.
Significant differences in color features were observed among samples (Table 3). The commercial cheddar cheese had the most lightness (L*) with 85.61, followed by germinated, raw, and soaked chickpea cheese with 75.14, 73.67, and 70.44, respectively. Boiled and roasted chickpea cheese had the lowest values with 66.81 and 61.01, respectively. The redness score (a*) showed a completely different ranking, ranging from -1.92 to 8.40. Ferawati et al. (2021) explained a comparable behavior for pulse-based cheese analogues resulting from Maillard reactions genereted during heat treatments. Each of the cheese samples displayed a positive yellowness (b*) value, which varied between 21.29 to 33.83. The differences in color characteristics must be attributed to the effect of the treatments applied.
The various treatment applied in the production of chickpea flour had a significant effect on hardness, adhesiveness, and springiness of chickpea cheese analogues (Table 4). Adhesiveness plays an important role in cheese packaging, as excessively sticky cheese is undesirable during the packaging process (Butt et al., 2020). The least adhesive sample was the roasted chickpea cheese analogue followed by the commercial cheddar cheese, while the germinated and raw chickpea cheese analog had the highest adhesion levels. Raw chickpea cheese analogue contained the highest values of hardness, cohesiveness, and springiness, followed by germinated and soaked chickpea cheese analogues, whereas boiled and roasted chickpea cheese analogues had the lowest values. This is consequent of the denaturation of the great part of proteins in addition the gelatinization of the starch during the boiling and roasting process. Hence, ungelatinized starch and undenatured protein fractions in raw, soaked, and germinated chickpea flours continued to gelatinize, denature, and interact with each other during heating step in the production of chickpea cheese analogues, leading to the production of a firmer gel consistency (Ferawati et al., 2021). The commercial cheddar cheese revealed evidently the highest levels of hardness, cohesiveness, and springiness. This could be explained by the fact that chickpea proteins are unable to create dense gel networks in the way that casein can (Bachmann, 2001). Therefore, stabilizers including seaweed stabilizers could aid in enhancing the firmness of chickpea cheese analogues in future optimization studies.
Due to the lack of plant-based cheese analogues in the Algerian market, it is crucial to conduct a sensory analysis in order to improve formulation, select the optimal manufacturing techniques, and compare the product's features with those of competing products. In general, there was a significant difference in all the parameters assessed as indicated in Figure 2. The sample of commercial cheddar cheese received the highest rating in pleasantness in all the organoleptic properties tested, whereas the germinated chickpea cheese analogue obtained the lowest rating. Soaked chickpea cheese analogue was the most overall accepted among the other chickpea cheese analogues with 4.47 followed by raw, boiled, and roasted chickpea cheese analogues with 4.04, 3.9, and 3.83, respectively.
Moisture levels serve as a key index of storage stability, with lower levels allowing for longer storage periods (Iwe et al., 2017). Sofi et al. (2023) reported comparable results (8.6%) for raw chickpea flour; nevertheless, they documented higher values for germinated chickpea flour (8.25%). The significant difference (p<0.05) observed following roasting treatment (3.61%) aligns with the the results of Benmeziane‑Derradji et al. (2020) for lentil flour and Agume et al. (2017) for soybean flour. This significant decrease in moisture could be attributed to the dehydration of the chickpea flour during roasting process.
The lowest ash content (1.96%) exhibited in boiled chickpea flour can be explained by a mineral loss during the boiling treatment. Conversely, the highest ash content detected in the roasted samples (3.07%) can be attributed to the removal of water during the roasting process, resulting in a higher proportion of ash in the final product. Xu et al. (2014) found that raw chickpeas yielded higher results at 3.40%, compared to boiled chickpeas which showed similar values at 1.68%. In contrast to previous studies (Erba et al., 2019; Setia et al., 2019; Sofi et al., 2020), the ash content exhibited a notable increase, reaching 2.98% following germination process. In congruence with extant investigations on rice (Chinma et al., 2015), amaranth (Chauhan et al., 2015; Cornejo et al., 2019), and tigernut (Chinma et al., 2009), our findings are consistent. This increase in ash content can be attributed to a decrease in dry matter, presumably resulting in a reduction in total soluble solids. It is plausible that hydrolytic enzymes failed significantly to enhance the production of total soluble solids during the germination phase (Chinma et al., 2015).
The protein content results, varying between 20.70 to 22.75%,confirm comparability with the findings presented by Sofi et al. (2020), who reported a value of 21.94%. Notably, disparate values have been documented in previous researches; for instance, Kaur and Singh (2005) reported higher results at 26.7%, Wani and Kumar (2014) at 24.61%, and Xu et al. (2019) at 24.36%. Conversely, lower protein content values have been observed in chickpea flour by Aguilar-Raymundo and Vélez-Ruíz (2016) with 18.06% and Erba et al. (2019) with 18.6%. These observed variations may be attributed to factors including seed variety, geographical location, harvest conditions, and the method utilized to measure protein levels
All treatments, with the exception of soaking, caused a notable rise (p<0.05) in fat content. These findings deviate from the values presented in a prior study by Alajaji and El-Adawy (2006) in which they observed a pivotal decline following treatments. Nonetheless, they demonstrate consistency with findings attributing the observed increase post-treatments to lipid solubilization induced by starch gelatinization. Moreover, these studies elucidate this phenomenon by highlighting that, in the case of raw chickpea flour, lipids are notably entrapped within the starch matrix (Aguilar-Raymundo and Vélez-Ruíz, 2016; Setia et al., 2019).
All interventions resulted in an increase in crude fiber content, however, the boost was not statistically significant. This elevation is hypothesized to stem from the formation of protein-fiber complexes, potentially arising from chemical alterations induced by the applied treatments (Bressani, 1993).
The results for the total carbohydrate content (62.41-65.21%) are surpassing the ones presented by El-Adawy (2002) with 59.23-62.34% and Kaur and Singh (2005) with 60.2%, while falling below values reported by Aguilar-Raymundo and Vélez-Ruíz (2016) with 68.67-72.61% as well as Güzel and Sayar (2012) with 67.97%. These observed disparities can be reasonably attributed to changes in the composition of other constituents. It is noteworthy that the determination of total carbohydrate content contains the deduction of the cumulative sum of other nutrients from 100.
BD was recognized for its crucial role in packaging requirements (Tonfack Djikeng et al., 2022). Our results (0.67 to 0.71 g/ml) were lower than those reported by Sofi et al. (2020) with 0.74 to 0.83 g/ml and Xu et al. (2014) with 0.94 to 1.29 g/ml.This implies that our chickpea flours necessitate a larger packaging space. The obtained low BD uncovers the potential use of this flour as a food ingredient in formulations with reduced concern concerning retrogradation (Benmeziane-Derradji et al., 2020).
Due to their effect on other functional and sensory aspects, WAC and OAC are considered as significant in food preparation. Ready-to-Eat (RTE) food products that require good viscosity, for instance dairy products (yogurt and cheese), sauces, and soups need flour that involves a high percentage of WAC as a functional ingredient (Benmeziane-Derradji et al., 2020).
A significant increase (p<0.05) in WAC was observed following germination, boiling, and roasting from 283 to 302, 341, and 433%, respectively. Sofi et al. (2020) illustrated an identical behavior following germination due to n rise in hydrophilic biopolymers. According to Avanza et al. (2012), in addition to the presence of hydrophilic carbohydrates, crude fibers swelling and starch gelatinization and heat treatment led to conformational alterations of proteins and consequently increased exposure of polar amino acids. However, soaking yielded a reduction of WAC which could be explained by a water saturation acquired during the soaking process.
In contrast, there was no notable distinction observed in OAC (p>0.05). Du et al. (2014) reported lower value with 110% and considered OAC as an important parameter, as it enhances mouth feel and preserves the flavor. OAC is affected by nonpolar amino acid side chain ratios on the hydrophobic protein molecule surface, starch content, as well as particles size (Wani and Kumar, 2014).
There was a significant decrease (p<0.05) in swelling capacity following boiling, soaking, roasting, and germination treatment. Based on the literature, these findings are not in line with those recorded by Handa et al. (2017) for horsegram flour and Klang et al. (2019) for potato flour, demonstrating a significant increase after germination and temperature treatment respectively, although they are in agreement with Agume et al. (2017) for soybean flour, attributingthe decrease by a destruction of the structure of proteins and starch responsible for this swelling capacity due to high temperature as well as the enzymatic hydrolysis of peptide and glycosidic bonds induced during soaking and germination.
EC is a highly intriguing functional attribute as it plays a role in achieving the preferred texture of a food matrix. According to Shen et al. (2021) the charge, size, shape, hydrophobicity, and composition of protein molecules play the most remrkable role in EC since they have the ability to absorb oil and water at the interfacial area. All applied treatments demonstrated a significant improvement in the EC of chickpea flour (p<0.05). This is clarified by the protein partial unfolding and dissociation which leads to exposure of the non-polar hydrophobic sites of these amphiphilic proteins.
FC is a desirable functional characteristic in food systems, permitting them to be applied for instance in baked foods, ice cream mixes, and whipped toppings. It is formed as the proteins are whipped and therefore form an interfacial film which maintains the gas bubbles in suspension and reduces coalescence (Shen et al., 2021; Shevkani et al., 2015). This study betrayes that our raw chickpea flour includes a highly intriguing FC with 142.06%, surpassing previous reports in the literature by Kaur and Singh, 2005, Martínez-Preciado et al. 2020, Wani and Kumar, 2014, and Xu et al. 2014. However, a significant reduction (p<0.05) was observed after roasting and boiling which could be explained by the protein denaturation from heat’s impact. Suárez-Estrella et al. (2020) described a comparable decrease in FC following germination in quinoa due to a comprehensive modification of the protein fraction during germination, including a decrease in albumins and globulins content in addition to an elimination of part of the saponins.
Gelation is a beneficial functional attribute in food applications and new product development that require gelling and thickening. It occurs as proteins and starch develop a three-dimensional network that resists waft underneath strain (Benmeziane-Derradji et al., 2020). Raw chickpea flour least gelation concentration (12%) was greater than the findings of Ladjal Ettoumi and Chibane (2015), and Agume et al. (2017), in which 8% concentration was enough to form a gel. As it is demonstrated in Table 2 both roasting and boiling treatment boosted the gelation of raw chickpea flour up to 14 and 16%, respectively, while soaking and germination reduced it up to 8 and 6%, respectively. Prinyawiwatkul et al. (1997) suggested that a combination of denatured proteins and pregelatinized starch in cowpeas, caused by heat, would necessitate higher flour concentrations for gel formation.. Additionally, low concentration required for gel formation in the case of soaked and germinated legume is due to the synergistic effects of protein and starch.
Significant differences in color features were observed among samples (Table 3). The commercial cheddar cheese had the most lightness (L*) with 85.61, followed by germinated, raw, and soaked chickpea cheese with 75.14, 73.67, and 70.44, respectively. Boiled and roasted chickpea cheese had the lowest values with 66.81 and 61.01, respectively. The redness score (a*) showed a completely different ranking, ranging from -1.92 to 8.40. Ferawati et al. (2021) explained a comparable behavior for pulse-based cheese analogues resulting from Maillard reactions genereted during heat treatments. Each of the cheese samples displayed a positive yellowness (b*) value, which varied between 21.29 to 33.83. The differences in color characteristics must be attributed to the effect of the treatments applied.
The various treatment applied in the production of chickpea flour had a significant effect on hardness, adhesiveness, and springiness of chickpea cheese analogues (Table 4). Adhesiveness plays an important role in cheese packaging, as excessively sticky cheese is undesirable during the packaging process (Butt et al., 2020). The least adhesive sample was the roasted chickpea cheese analogue followed by the commercial cheddar cheese, while the germinated and raw chickpea cheese analog had the highest adhesion levels. Raw chickpea cheese analogue contained the highest values of hardness, cohesiveness, and springiness, followed by germinated and soaked chickpea cheese analogues, whereas boiled and roasted chickpea cheese analogues had the lowest values. This is consequent of the denaturation of the great part of proteins in addition the gelatinization of the starch during the boiling and roasting process. Hence, ungelatinized starch and undenatured protein fractions in raw, soaked, and germinated chickpea flours continued to gelatinize, denature, and interact with each other during heating step in the production of chickpea cheese analogues, leading to the production of a firmer gel consistency (Ferawati et al., 2021). The commercial cheddar cheese revealed evidently the highest levels of hardness, cohesiveness, and springiness. This could be explained by the fact that chickpea proteins are unable to create dense gel networks in the way that casein can (Bachmann, 2001). Therefore, stabilizers including seaweed stabilizers could aid in enhancing the firmness of chickpea cheese analogues in future optimization studies.
Due to the lack of plant-based cheese analogues in the Algerian market, it is crucial to conduct a sensory analysis in order to improve formulation, select the optimal manufacturing techniques, and compare the product's features with those of competing products. In general, there was a significant difference in all the parameters assessed as indicated in Figure 2. The sample of commercial cheddar cheese received the highest rating in pleasantness in all the organoleptic properties tested, whereas the germinated chickpea cheese analogue obtained the lowest rating. Soaked chickpea cheese analogue was the most overall accepted among the other chickpea cheese analogues with 4.47 followed by raw, boiled, and roasted chickpea cheese analogues with 4.04, 3.9, and 3.83, respectively.
The scores for oral texture and flavor reflected that roasted chickpea cheese analogue was the most preferred compared to other chickpea cheese analogues with 4.39 and 4, respectively. Additionally, the soaked chickpea cheese analogue was rated highest among the other chickpea cheese analogues while in color and taste evaluation with 5.29 and 3.8, respectively. These findings are relatively low compared to those reported by Seleet et al. (2014) for spreadable processed cheese analogue supplemented with chickpea and Oyeyinka et al. (2019) for cheese analog from soy and cashew nut milk.
Conclusion
The effect of various treatments (boiling, soaking, roasting, as well as germination) on proximate composition, functional features of North Algerian chickpea flour and its suitability to develop a plant based cheese analogue was explored in this study.
In terms of proximate composition, among the various treatments utilized, exclusively moisture and lipid content were observed to be affected by the roasting and boiling treatments, respectively.
n contrast, each treatment proved different effects on the functional attributes of raw chickpea flour. They improved the EC while concurrently decreased the FC of raw chickpea, which was observed to have a substantial significance. This finding highlights a promising prospect for food technologists to incorporate raw chickpea flour into food preprations necessitating aeration, particularly for enhancing texture and characteristics of sourdough. In addition, the potential suitability for inclusion of treated chickpea flour in food formulations as an emulsifying agent might be evaluated.
Chickpea cheese analog failed to be appreciated by consumers in the sensory evaluation, This outcome prompts us to recommend alternative technological approaches, including fermentation or the incorporation of stabilizing and flavor-enhancing agents, for consideration in subsequent optimization investigations.
Author contributions
I.G. and F.A. designed the study; I.G. and R.F. conducted the experimental work; I.G and E.S. analyzed the data; I.G. wrote the manuscript; L.B. supervised the research. All authors read and approved the final manuscript.
Conflicts of interest
The authors declared no conflict of interest.
Funding
The study was funded by the Institute of Nutrition, Food, and Agri-food Technologies (INATAA), Mentouri Brothers University Constantine 1, and the Scientific and Technical Research Center in Physicochemical Analyses (CRAPC), as part of a doctoral thesis.
Ethical Consideration
Not applicable in this work.
Acknowledgements
The authors express their gratitude to BAISSISSE S. of Batna 1 University and Engineer BOUHALI S. from the Laboratory of Biology at the University of Jijel for their invaluable assistance. Moreover, appreciation is expanded to the INATAA Institute, particularly the GENIAAL laboratory, and the CRAPC Center, for their unwavering support.
References
Aguilar-Raymundo V.G., Vélez-Ruíz J.F. (2016). Characterization of two chickpea varieties and the effect of cooking on their physico-chemical and functional properties of flours. Journal of Food Research. 5: 2016. [DOI: 10.5539/jfr.v5n5p67]
Agume A.S.N., Njintang N.Y., Mbofung C.M.F. (2017). Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods. 6: 12. [DOI: 10.3390/foods6020012]
Alajaji S.A., El-Adawy T.A. (2006). Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. Journal of Food Composition and Analysis. 19: 806-812. [DOI: 10.1016/j.jfca.2006.03.015]
Association of Official Analytical Chemists (AOAC). (2005). Official method 978.10, fiber (crude) in animal feed and pet food, official methods of analysis of AOAC International. 18th edition. AOAC International, Gaithersburg, MD, USA.
Association of Official Analytical Chemists (AOAC). (1995). Official methods of analysis of AOAC international. 16th edition. Washington, DC. URL: https://search.worldcat.org/fr/title/Official-methods-of-analysis-of-AOAC-international/oclc/421897987.
Avanza M.V., Chaves M.G., Acevedo B.A., Añón M.C. (2012). Functional properties and microstructure of cowpea cultivated in north-east Argentina. LWT - Food Science and Technology. 49: 123-130. [DOI: 10.1016/j.lwt.2012.04.015]
Bachmann H.-P. (2001). Cheese analogues: a review. International Dairy Journal. 11: 505-515. [DOI: 10.1016/S0958-6946(01)00073-5]
Benítez V., Mollá E., Martín-Cabrejas M.A., Aguilera Y., López-Andréu F.J., Esteban R.M. (2011). Effect of sterilisation on dietary fibre and physicochemical properties of onion by-products. Food Chemistry. 127: 501-507. [DOI: 10.1016/j.foodchem.2011.01.031]
Benmeziane-Derradji F., Djermoune-Arkoub L., Ayat N.E.-H., Aoufi D. (2020). Impact of roasting on the physicochemical, functional properties, antioxidant content and microstructure changes of Algerian lentil (Lens culinaris) flour. Journal of Food Measurement and Characterization. 14: 2840-2853. [DOI: 10.1007/s11694-020-00529-7]
Bressani R. (1993). Grain quality of common beans. Food Reviews International. 9: 237-297. [DOI: 10.1080/87559129309540960]
Bubelová Z., Sumczynski D., Salek R.N. (2017). Effect of cooking and germination on antioxidant activity, total polyphenols and flavonoids, fiber content, and digestibility of lentils (Lens culinaris L.). Journal of Food Processing and Preservation. 42: e13388. [DOI: 10.1111/jfpp.13388]
Buckingham J. (2018). Super easy vegan cheese cookbook: 70 delicious plant-based cheeses. Rockridge Press, Emeryville, California.
Butt N.A., Ali T.M., Hasnain A. (2020). Development of rice starch‐based casein and fat mimetics and its application in imitation mozzarella cheese. Journal of Food Processing and Preservation. 44: e14928. [DOI: 10.1111/jfpp.14928]
Calles T., Del Castello R., Baratelli M., Xipsiti M., Navarro D.K. (2019). The international year of pulses: final report. FAO, Rome. URL: file:///C:/Users/admin/Downloads/ca2853en-3.pdf.
Chauhan A., Saxena D.C., Singh S. (2015). Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. LWT - Food Science and Technology. 63: 939-945. [DOI: 10.1016/j.lwt. 2015.03.115]
Chinma C.E., Adewuyi O., Abu J.O. (2009). Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus). Food Research International. 42: 1004-1009. [DOI: 10.1016/j.foodres.2009.04.024]
Chinma C.E., Anuonye J.C., Simon O.C., Ohiare R.O., Danbaba N. (2015). Effect of germination on the physicochemical and antioxidant characteristics of rice flour from three rice varieties from Nigeria. Food Chemistry. 185: 454-458. [DOI: 10.1016/j.foodchem.2015.04.010]
Cornejo F., Novillo G., Villacrés E., Rosell C.M. (2019). Evaluation of the physicochemical and nutritional changes in two amaranth species (Amaranthus quitensis and Amaranthus caudatus) after germination. Food Research International. 121: 933-939. [DOI: 10.1016/j.foodres.2019.01.022]
Du S.-K., Jiang H., Yu X., Jane J.-L. (2014). Physicochemical and functional properties of whole legume flour. LWT - Food Science and Technology. 55: 308-313. [DOI: 10.1016/j.lwt.2013.06.001]
El-Adawy T.A. (2002). Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods for Human Nutrition. 57: 83-97. [DOI: 10.1023/A:1013189620528]
Erba D., Angelino D., Marti A., Manini F., Faoro F., Morreale F., Pellegrini N., Casiraghi M.C. (2019). Effect of sprouting on nutritional quality of pulses. International Journal of Food Sciences and Nutrition. 70: 30-40. [DOI: 10.1080/09637486.2018.1478393]
Ferawati F., Hefni M., Östbring K., Witthöft C. (2021). The application of pulse flours in the development of plant-based cheese analogues: proximate composition, color, and texture properties. Foods. 10: 2208. [DOI: 10.3390/foods10092208]
Ferawati F., Hefni M., Witthöft C. (2019). Flours from swedish pulses: effects of treatment on functional properties and nutrient content. Food Science and Nutrition. 7: 4116-4126. [DOI: 10.1002/fsn3.1280]
Gerber P.J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J., Falcucci A., Tempio G. (2013). Tackling climate change through livestock – a global assessment of emissions and mitigation opportunities. Food and Agriculture Organization of the United Nations (FAO), Rome.
Grossmann L., McClements D.J. (2021). The science of plant-based foods: approaches to create nutritious and sustainable plant-based cheese analogs. Trends in Food Science and Technology. 118: 207-229. [DOI: 10.1016/j.tifs.2021.10.004]
Güzel D., Sayar S. (2012). Effect of cooking methods on selected physicochemical and nutritional properties of barlotto bean, chickpea, faba bean, and white kidney bean. Journal of Food Science and Technology. 49: 89-95. [DOI: 10.1007/s13197-011-0260-0]
Handa V., Kumar V., Panghal A., Suri S., Kaur J. (2017). Effect of soaking and germination on physicochemical and functional attributes of horsegram flour. Journal of Food Science and Technology. 54: 4229-4239. [DOI: 10.1007/s13197-017-2892-1]
Horwitz W., Latimer G.W., Association of Official Analytical Chemists International. (2006). Official methods of analysis of AOAC International, 18th edition. AOAC International, Gaithersburg, Maryland.
Iwe M.O., Michael N., Madu N.E., Obasi N.E., Onwuka G.I., Nwabueze T.U., Onuh J.O. (2017). Physicochemical and pasting properties high quality cassava flour (HQCF) and wheat flour blends. Agrotechnology. 6: 167. [DOI: 10.4172/2168-9881.1000167]
Jiang Z.-Q., Pulkkinen M., Wang Y.-J., Lampi A.-M., Stoddard F.L., Salovaara H., Piironen V., Sontag-Strohm T. (2016). Faba bean flavour and technological property improvement by thermal pre-treatments. LWT - Food Science and Technology. 68: 295-305. [DOI: 10.1016/j.lwt.2015.12.015]
Kaur M., Singh N. (2005). Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chemistry. 91: 403-411. [DOI: 10.1016/j. foodchem.2004.06.015]
Khattab R.Y., Arntfield S.D. (2009). Nutritional quality of legume seeds as affected by some physical treatments 2. antinutritional factors. LWT - Food Science and Technology. 42: 1113-1118. [DOI: 10.1016/j.lwt.2009.02.004]
Klang J.M., Tene S.T., Nguemguo Kalamo L.G., Boungo G.T., Ndomou Houketchang S.C., Kohole Foffe H.A., Womeni H.M. (2019). Effect of bleaching and variety on the physico-chemical, functional and rheological properties of three new Irish potatoes (Cipira, Pamela and Dosa) flours grown in the locality of Dschang (West region of Cameroon). Heliyon. 5: e02982. [DOI: 10.1016/j.heliyon.2019.e02982]
Ladjal Ettoumi Y., Chibane M. (2015). Some physicochemical and functional properties of pea, chickpea and lentil whole flours. International Food Research Journal. 22: 987-996.
Le Tohic C., O’Sullivan J.J., Drapala K.P., Chartrin V., Chan T., Morrison A.P., Kerry J.P., Kelly A.L. (2018). Effect of 3D printing on the structure and textural properties of processed cheese. Journal of Food Engineering. 220: 56-64. [DOI: 10.1016/j.jfoodeng.2017.02.003]
Martínez-Preciado A.H., Ponce-Simental J.A., Schorno A.L., Contreras-Pacheco M.L., Michel C.R., Rivera-Ortiz K.G., Soltero J.F.A. (2020). Characterization of nutritional and functional properties of “Blanco Sinaloa” chickpea (Cicer arietinum L.) variety, and study of the rheological behavior of hummus pastes. Journal of Food Science and Technology. 57: 1856-1865. [DOI: 10.1007/s13197-019-04220-8]
Ouazib M., Moussou N., Oomah B.D., Zaidi F., Wanasundara J.P.D. (2015). Effect of processing and germination on nutritional parameters and functional properties of chickpea (Cicer arietinum L.) from Algeria. Journal of Food Legumes. 28: 133-140.
Oyeyinka A.T., Odukoya J.O., Adebayo Y.S. (2019). Nutritional composition and consumer acceptability of cheese analog from soy and cashew nut milk. Journal of Food Processing and Preservation. 43: e14285. [DOI: 10.1111/jfpp.14285]
Prinyawiwatkul W., Mcwatters K.H., Beuchat L.R., Phillips R.D. (1997). Optimizing acceptability of chicken nuggets containing fermented cowpea and peanut flours. Journal of Food Science. 62: 889-905. [DOI: 10.1111/j.1365-2621.1997.tb15480.x]
Sabba E., Boudida Y., Boudjellal A. (2023). Evaluation of fatty acid and the composition of six different species of freshwater fish in the North of Algeria. Journal of Food Quality and Hazards Control. 10: 115-122. [DOI: 10.18502/jfqhc.10.3.13642]
Schlegel K., Leidigkeit A., Eisner P., Schweiggert-Weisz U. (2019). Technofunctional and sensory properties of fermented lupin protein isolates. Foods. 8: 678. [DOI: 10.3390/foods8120678]
Seleet F.L., Kassem J.M., Bayomim H.M., Abd-Rabou N.S., Ahmed N.S. (2014). Production of functional spreadable processed cheese analogue supplemented with chickpea. International Journal of Dairy Science. 9: 1-14. [DOI: 10.3923/ijds.2014.1.14]
Setia R., Dai Z., Nickerson M.T., Sopiwnyk E., Malcolmson L., Ai Y. (2019). Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Research International. 122: 263-272. [DOI: 10.1016/j.foodres. 2019.04.021]
Shen Y., Tang X., Li Y. (2021). Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chemistry. 339: 127823. [DOI: 10.1016/j.foodchem.2020.127823]
Shevkani K., Singh N., Kaur A., Rana J.C. (2015). Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocolloids. 43: 679-689. [DOI: 10.1016/j.foodhyd.2014.07.024]
Sofi S.A., Rafiq S., Singh J., Mir S.A., Sharma S., Bakshi P., McClements D.J., Mousavi Khaneghah A., Dar B.N. (2023). Impact of germination on structural, physicochemical, techno-functional, and digestion properties of desi chickpea (Cicer arietinum L.) flour. Food Chemistry. 405: 135011. [DOI: 10.1016/j.foodchem.2022.135011]
Sofi S.A., Singh J., Muzaffar K., Mir S.A., Dar B.N. (2020). Effect of germination time on physico-chemical, functional, pasting, rheology and electrophoretic characteristics of chickpea flour. Journal of Food Measurement and Characterization. 14: 2380-2392. [DOI: 10.1007/s11694-020-00485-2]
Suárez-Estrella D., Bresciani A., Iametti S., Marengo M., Pagani M.A., Marti A. (2020). Effect of sprouting on proteins and starch in quinoa (Chenopodium quinoa Willd.). Plant Foods for Human Nutrition. 75: 635-641. [DOI: 10.1007/s11130-020-00864-6]
Tonfack Djikeng F., Mouto Ndambwe C.M., Ngangoum E.S., Tiencheu B., Tambo Tene S., Achidi A.U., Womeni H.M. (2022). Effect of different processing methods on the proximate composition, mineral content and functional properties of snail (Archachatina marginata) meat. Journal of Agriculture and Food Research. 8: 100298. [DOI: 10.1016/j.jafr.2022.100298]
Wani S.A., Kumar P. (2014). Comparative study of chickpea and green pea flour based on chemical composition, functional and pasting properties. Journal of Food Research and Technology. 2: 124-129. [DOI: 10.13140/2.1.3470.4964]
Xu M., Jin Z., Simsek S., Hall C., Rao J., Chen B. (2019). Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chemistry. 295: 579-587. [DOI: 10.1016/j.foodchem.2019.05.167]
Xu Y., Thomas M., Bhardwaj H.L. (2014). Chemical composition, functional properties and microstructural characteristics of three kabuli chickpea (Cicer arietinum L.) as affected by different cooking methods. International Journal of Food Science & Technology. 49: 1215-1223. [DOI: 10.1111/ijfs.12419]
* Corresponding author (I. Guemra)
* E-mail: imane.guemra@doc.umc.edu.dz
ORCID ID: https://orcid.org/0009-0007-8043-4165
* E-mail: imane.guemra@doc.umc.edu.dz
ORCID ID: https://orcid.org/0009-0007-8043-4165
Type of Study: Original article |
Subject:
Special
Received: 23/11/25 | Accepted: 24/05/27 | Published: 24/06/30
Received: 23/11/25 | Accepted: 24/05/27 | Published: 24/06/30
References
1. Aguilar-Raymundo V.G., Vélez-Ruíz J.F. (2016). Characterization of two chickpea varieties and the effect of cooking on their physico-chemical and functional properties of flours. Journal of Food Research. 5: 2016. [DOI: 10.5539/jfr.v5n5p67] [DOI:10.5539/jfr.v5n5p67]
2. Agume A.S.N., Njintang N.Y., Mbofung C.M.F. (2017). Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods. 6: 12. [DOI: 10.3390/foods6020012] [DOI:10.3390/foods6020012] [PMID] [PMCID]
3. Alajaji S.A., El-Adawy T.A. (2006). Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. Journal of Food Composition and Analysis. 19: 806-812. [DOI: 10.1016/j.jfca.2006.03.015] [DOI:10.1016/j.jfca.2006.03.015]
4. Association of Official Analytical Chemists (AOAC). (2005). Official method 978.10, fiber (crude) in animal feed and pet food, official methods of analysis of AOAC International. 18th edition. AOAC International, Gaithersburg, MD, USA.
5. Association of Official Analytical Chemists (AOAC). (1995). Official methods of analysis of AOAC international. 16th edition. Washington, DC. URL: https://search.worldcat.org/fr/title/Official-methods-of-analysis-of-AOAC-international/oclc/421897987.
6. Avanza M.V., Chaves M.G., Acevedo B.A., Añón M.C. (2012). Functional properties and microstructure of cowpea cultivated in north-east Argentina. LWT - Food Science and Technology. 49: 123-130. [DOI: 10.1016/j.lwt.2012.04.015] [DOI:10.1016/j.lwt.2012.04.015]
7. Bachmann H.-P. (2001). Cheese analogues: a review. International Dairy Journal. 11: 505-515. [DOI: 10.1016/S0958-6946(01)00073-5] [DOI:10.1016/S0958-6946(01)00073-5]
8. Benítez V., Mollá E., Martín-Cabrejas M.A., Aguilera Y., López-Andréu F.J., Esteban R.M. (2011). Effect of sterilisation on dietary fibre and physicochemical properties of onion by-products. Food Chemistry. 127: 501-507. [DOI: 10.1016/j.foodchem.2011.01.031] [DOI:10.1016/j.foodchem.2011.01.031] [PMID]
9. Benmeziane-Derradji F., Djermoune-Arkoub L., Ayat N.E.-H., Aoufi D. (2020). Impact of roasting on the physicochemical, functional properties, antioxidant content and microstructure changes of Algerian lentil (Lens culinaris) flour. Journal of Food Measurement and Characterization. 14: 2840-2853. [DOI: 10.1007/s11694-020-00529-7] [DOI:10.1007/s11694-020-00529-7]
10. Bressani R. (1993). Grain quality of common beans. Food Reviews International. 9: 237-297. [DOI: 10.1080/87559129309540960] [DOI:10.1080/87559129309540960]
11. Bubelová Z., Sumczynski D., Salek R.N. (2017). Effect of cooking and germination on antioxidant activity, total polyphenols and flavonoids, fiber content, and digestibility of lentils (Lens culinaris L.). Journal of Food Processing and Preservation. 42: e13388. [DOI: 10.1111/jfpp.13388] [DOI:10.1111/jfpp.13388]
12. Buckingham J. (2018). Super easy vegan cheese cookbook: 70 delicious plant-based cheeses. Rockridge Press, Emeryville, California.
13. Butt N.A., Ali T.M., Hasnain A. (2020). Development of rice starch‐based casein and fat mimetics and its application in imitation mozzarella cheese. Journal of Food Processing and Preservation. 44: e14928. [DOI: 10.1111/jfpp.14928] [DOI:10.1111/jfpp.14928]
14. Calles T., Del Castello R., Baratelli M., Xipsiti M., Navarro D.K. (2019). The international year of pulses: final report. FAO, Rome. URL: file:///C:/Users/admin/Downloads/ca2853en-3.pdf. [DOI:10.1007/s12665-019-8106-6]
15. Chauhan A., Saxena D.C., Singh S. (2015). Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. LWT - Food Science and Technology. 63: 939-945. [DOI: 10.1016/j.lwt. 2015.03.115] [DOI:10.1016/j.lwt.2015.03.115]
16. Chinma C.E., Adewuyi O., Abu J.O. (2009). Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus). Food Research International. 42: 1004-1009. [DOI: 10.1016/j.foodres.2009.04.024] [DOI:10.1016/j.foodres.2009.04.024]
17. Chinma C.E., Anuonye J.C., Simon O.C., Ohiare R.O., Danbaba N. (2015). Effect of germination on the physicochemical and antioxidant characteristics of rice flour from three rice varieties from Nigeria. Food Chemistry. 185: 454-458. [DOI: 10.1016/j.foodchem.2015.04.010] [DOI:10.1016/j.foodchem.2015.04.010] [PMID]
18. Cornejo F., Novillo G., Villacrés E., Rosell C.M. (2019). Evaluation of the physicochemical and nutritional changes in two amaranth species (Amaranthus quitensis and Amaranthus caudatus) after germination. Food Research International. 121: 933-939. [DOI: 10.1016/j.foodres.2019.01.022] [DOI:10.1016/j.foodres.2019.01.022] [PMID]
19. Du S.-K., Jiang H., Yu X., Jane J.-L. (2014). Physicochemical and functional properties of whole legume flour. LWT - Food Science and Technology. 55: 308-313. [DOI: 10.1016/j.lwt.2013.06.001] [DOI:10.1016/j.lwt.2013.06.001]
20. El-Adawy T.A. (2002). Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods for Human Nutrition. 57: 83-97. [DOI: 10.1023/A:1013189620528] [DOI:10.1023/A:1013189620528] [PMID]
21. Erba D., Angelino D., Marti A., Manini F., Faoro F., Morreale F., Pellegrini N., Casiraghi M.C. (2019). Effect of sprouting on nutritional quality of pulses. International Journal of Food Sciences and Nutrition. 70: 30-40. [DOI: 10.1080/09637486.2018.1478393] [DOI:10.1080/09637486.2018.1478393] [PMID]
22. Ferawati F., Hefni M., Östbring K., Witthöft C. (2021). The application of pulse flours in the development of plant-based cheese analogues: proximate composition, color, and texture properties. Foods. 10: 2208. [DOI: 10.3390/foods10092208] [DOI:10.3390/foods10092208] [PMID] [PMCID]
23. Ferawati F., Hefni M., Witthöft C. (2019). Flours from swedish pulses: effects of treatment on functional properties and nutrient content. Food Science and Nutrition. 7: 4116-4126. [DOI: 10.1002/fsn3.1280] [DOI:10.1002/fsn3.1280] [PMID] [PMCID]
24. Gerber P.J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J., Falcucci A., Tempio G. (2013). Tackling climate change through livestock - a global assessment of emissions and mitigation opportunities. Food and Agriculture Organization of the United Nations (FAO), Rome.
25. Grossmann L., McClements D.J. (2021). The science of plant-based foods: approaches to create nutritious and sustainable plant-based cheese analogs. Trends in Food Science and Technology. 118: 207-229. [DOI: 10.1016/j.tifs.2021.10.004] [DOI:10.1016/j.tifs.2021.10.004]
26. Güzel D., Sayar S. (2012). Effect of cooking methods on selected physicochemical and nutritional properties of barlotto bean, chickpea, faba bean, and white kidney bean. Journal of Food Science and Technology. 49: 89-95. [DOI: 10.1007/s13197-011-0260-0] [DOI:10.1007/s13197-011-0260-0] [PMID] [PMCID]
27. Handa V., Kumar V., Panghal A., Suri S., Kaur J. (2017). Effect of soaking and germination on physicochemical and functional attributes of horsegram flour. Journal of Food Science and Technology. 54: 4229-4239. [DOI: 10.1007/s13197-017-2892-1] [DOI:10.1007/s13197-017-2892-1] [PMID] [PMCID]
28. Horwitz W., Latimer G.W., Association of Official Analytical Chemists International. (2006). Official methods of analysis of AOAC International, 18th edition. AOAC International, Gaithersburg, Maryland.
29. Iwe M.O., Michael N., Madu N.E., Obasi N.E., Onwuka G.I., Nwabueze T.U., Onuh J.O. (2017). Physicochemical and pasting properties high quality cassava flour (HQCF) and wheat flour blends. Agrotechnology. 6: 167. [DOI: 10.4172/2168-9881.1000167] [DOI:10.4172/2168-9881.1000167]
30. Jiang Z.-Q., Pulkkinen M., Wang Y.-J., Lampi A.-M., Stoddard F.L., Salovaara H., Piironen V., Sontag-Strohm T. (2016). Faba bean flavour and technological property improvement by thermal pre-treatments. LWT - Food Science and Technology. 68: 295-305. [DOI: 10.1016/j.lwt.2015.12.015] [DOI:10.1016/j.lwt.2015.12.015]
31. Kaur M., Singh N. (2005). Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chemistry. 91: 403-411. [DOI: 10.1016/j. foodchem.2004.06.015] [DOI:10.1016/j.foodchem.2004.06.015]
32. Khattab R.Y., Arntfield S.D. (2009). Nutritional quality of legume seeds as affected by some physical treatments 2. antinutritional factors. LWT - Food Science and Technology. 42: 1113-1118. [DOI: 10.1016/j.lwt.2009.02.004] [DOI:10.1016/j.lwt.2009.02.004]
33. Klang J.M., Tene S.T., Nguemguo Kalamo L.G., Boungo G.T., Ndomou Houketchang S.C., Kohole Foffe H.A., Womeni H.M. (2019). Effect of bleaching and variety on the physico-chemical, functional and rheological properties of three new Irish potatoes (Cipira, Pamela and Dosa) flours grown in the locality of Dschang (West region of Cameroon). Heliyon. 5: e02982. [DOI: 10.1016/j.heliyon.2019.e02982] [DOI:10.1016/j.heliyon.2019.e02982] [PMID] [PMCID]
34. Ladjal Ettoumi Y., Chibane M. (2015). Some physicochemical and functional properties of pea, chickpea and lentil whole flours. International Food Research Journal. 22: 987-996.
35. Le Tohic C., O'Sullivan J.J., Drapala K.P., Chartrin V., Chan T., Morrison A.P., Kerry J.P., Kelly A.L. (2018). Effect of 3D printing on the structure and textural properties of processed cheese. Journal of Food Engineering. 220: 56-64. [DOI: 10.1016/j.jfoodeng.2017.02.003] [DOI:10.1016/j.jfoodeng.2017.02.003]
36. Martínez-Preciado A.H., Ponce-Simental J.A., Schorno A.L., Contreras-Pacheco M.L., Michel C.R., Rivera-Ortiz K.G., Soltero J.F.A. (2020). Characterization of nutritional and functional properties of "Blanco Sinaloa" chickpea (Cicer arietinum L.) variety, and study of the rheological behavior of hummus pastes. Journal of Food Science and Technology. 57: 1856-1865. [DOI: 10.1007/s13197-019-04220-8] [DOI:10.1007/s13197-019-04220-8] [PMID] [PMCID]
37. Ouazib M., Moussou N., Oomah B.D., Zaidi F., Wanasundara J.P.D. (2015). Effect of processing and germination on nutritional parameters and functional properties of chickpea (Cicer arietinum L.) from Algeria. Journal of Food Legumes. 28: 133-140.
38. Oyeyinka A.T., Odukoya J.O., Adebayo Y.S. (2019). Nutritional composition and consumer acceptability of cheese analog from soy and cashew nut milk. Journal of Food Processing and Preservation. 43: e14285. [DOI: 10.1111/jfpp.14285] [DOI:10.1111/jfpp.14285]
39. Prinyawiwatkul W., Mcwatters K.H., Beuchat L.R., Phillips R.D. (1997). Optimizing acceptability of chicken nuggets containing fermented cowpea and peanut flours. Journal of Food Science. 62: 889-905. [DOI: 10.1111/j.1365-2621.1997.tb15480.x] [DOI:10.1111/j.1365-2621.1997.tb15480.x]
40. Sabba E., Boudida Y., Boudjellal A. (2023). Evaluation of fatty acid and the composition of six different species of freshwater fish in the North of Algeria. Journal of Food Quality and Hazards Control. 10: 115-122. [DOI: 10.18502/jfqhc.10.3.13642] [DOI:10.18502/jfqhc.10.3.13642]
41. Schlegel K., Leidigkeit A., Eisner P., Schweiggert-Weisz U. (2019). Technofunctional and sensory properties of fermented lupin protein isolates. Foods. 8: 678. [DOI: 10.3390/foods8120678] [DOI:10.3390/foods8120678] [PMID] [PMCID]
42. Seleet F.L., Kassem J.M., Bayomim H.M., Abd-Rabou N.S., Ahmed N.S. (2014). Production of functional spreadable processed cheese analogue supplemented with chickpea. International Journal of Dairy Science. 9: 1-14. [DOI: 10.3923/ijds.2014.1.14] [DOI:10.3923/ijds.2014.1.14]
43. Setia R., Dai Z., Nickerson M.T., Sopiwnyk E., Malcolmson L., Ai Y. (2019). Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Research International. 122: 263-272. [DOI: 10.1016/j.foodres. 2019.04.021] [DOI:10.1016/j.foodres.2019.04.021] [PMID]
44. Shen Y., Tang X., Li Y. (2021). Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chemistry. 339: 127823. [DOI: 10.1016/j.foodchem.2020.127823] [DOI:10.1016/j.foodchem.2020.127823] [PMID]
45. Shevkani K., Singh N., Kaur A., Rana J.C. (2015). Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocolloids. 43: 679-689. [DOI: 10.1016/j.foodhyd.2014.07.024] [DOI:10.1016/j.foodhyd.2014.07.024]
46. Sofi S.A., Rafiq S., Singh J., Mir S.A., Sharma S., Bakshi P., McClements D.J., Mousavi Khaneghah A., Dar B.N. (2023). Impact of germination on structural, physicochemical, techno-functional, and digestion properties of desi chickpea (Cicer arietinum L.) flour. Food Chemistry. 405: 135011. [DOI: 10.1016/j.foodchem.2022.135011] [DOI:10.1016/j.foodchem.2022.135011] [PMID]
47. Sofi S.A., Singh J., Muzaffar K., Mir S.A., Dar B.N. (2020). Effect of germination time on physico-chemical, functional, pasting, rheology and electrophoretic characteristics of chickpea flour. Journal of Food Measurement and Characterization. 14: 2380-2392. [DOI: 10.1007/s11694-020-00485-2] [DOI:10.1007/s11694-020-00485-2]
48. Suárez-Estrella D., Bresciani A., Iametti S., Marengo M., Pagani M.A., Marti A. (2020). Effect of sprouting on proteins and starch in quinoa (Chenopodium quinoa Willd.). Plant Foods for Human Nutrition. 75: 635-641. [DOI: 10.1007/s11130-020-00864-6] [DOI:10.1007/s11130-020-00864-6] [PMID]
49. Tonfack Djikeng F., Mouto Ndambwe C.M., Ngangoum E.S., Tiencheu B., Tambo Tene S., Achidi A.U., Womeni H.M. (2022). Effect of different processing methods on the proximate composition, mineral content and functional properties of snail (Archachatina marginata) meat. Journal of Agriculture and Food Research. 8: 100298. [DOI: 10.1016/j.jafr.2022.100298] [DOI:10.1016/j.jafr.2022.100298]
50. Wani S.A., Kumar P. (2014). Comparative study of chickpea and green pea flour based on chemical composition, functional and pasting properties. Journal of Food Research and Technology. 2: 124-129. [DOI: 10.13140/2.1.3470.4964]
51. Xu M., Jin Z., Simsek S., Hall C., Rao J., Chen B. (2019). Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chemistry. 295: 579-587. [DOI: 10.1016/j.foodchem.2019.05.167] [DOI:10.1016/j.foodchem.2019.05.167] [PMID]
52. Xu Y., Thomas M., Bhardwaj H.L. (2014). Chemical composition, functional properties and microstructural characteristics of three kabuli chickpea (Cicer arietinum L.) as affected by different cooking methods. International Journal of Food Science & Technology. 49: 1215-1223. [DOI: 10.1111/ijfs.12419] [DOI:10.1111/ijfs.12419]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







