Volume 12, Issue 3 (September 2025)
J. Food Qual. Hazards Control 2025, 12(3): 183-191 |
Back to browse issues page
Ethics code: Not applicable.
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shakeri F, Bahmani Z, Mirmoghtadaie L. Effect of Deamination and Agar Addition on Physical Properties of Gelatin Extracted from Thunnus tonggol Skin. J. Food Qual. Hazards Control 2025; 12 (3) :183-191
URL: http://jfqhc.ssu.ac.ir/article-1-1175-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1175-en.html
National Fish Processing Research Center, Iranian Fisheries Science Research Institute, Agricultural Research, Education and Extension Organization, Bandar Anzali, Iran , zabihbahmani@gmail.com
Full-Text [PDF 725 kb]
(264 Downloads)
| Abstract (HTML) (371 Views)
Full-Text: (46 Views)
Effect of Deamination and Agar Addition on Physical Properties of Gelatin Extracted from Thunnus tonggol Skin
F. Shakeri 1, Z. Bahmani 2** , L. Mirmoghtadaie 1
1. Department of Food Science and Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2. National Fish Processing Research Center, Iranian Fisheries Science Research Institute, Agricultural Research, Education and Extension Organization, Bandar Anzali, Iran
HIGHLIGHTS
Table 1: pH and Isoelectric Point (IEP) of control, deaminated gelatin, and gelatin/agar mix gel samples (n=3)
Table 2: Gel strength and melting point of fish waste gelatin in different control, deaminated gelatin, and gelatin/agar mix gel samples (n=3)
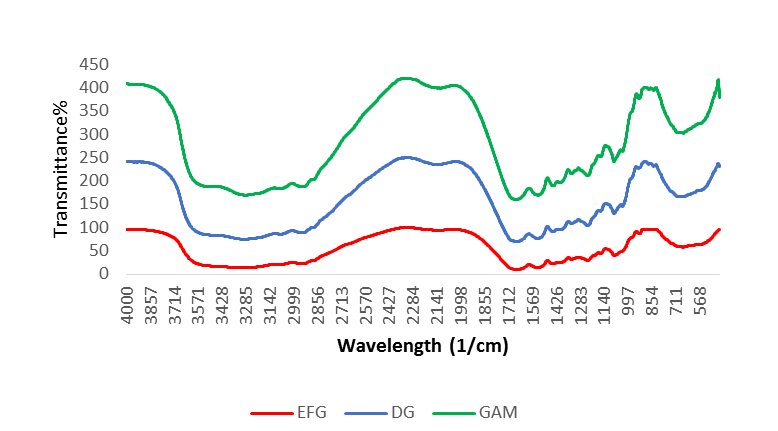
Figure 1: Fourier Transform Infrared Spectroscopy (FTIR) spectra of extracted fish gelatin (Extracted Fish Gelatin (EFG), not modified, Deaminated Gelatin (DG), and Gelatin/Agar Mix (GAM))
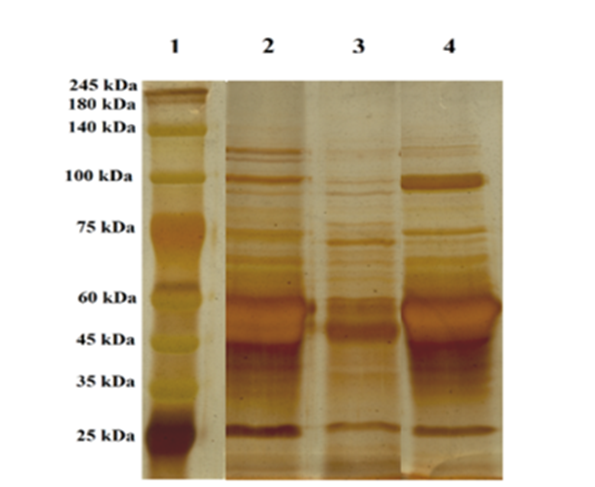
Figure 2: Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) patterns for samples (1=Marker; 2=Gelatin/agar mixture; 3=Control; 4=Deaminated gelatin), Gel (7.5 %), resolving, colored by silver nitrate method
F. Shakeri 1, Z. Bahmani 2**
1. Department of Food Science and Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2. National Fish Processing Research Center, Iranian Fisheries Science Research Institute, Agricultural Research, Education and Extension Organization, Bandar Anzali, Iran
- Gelatin was extracted from fish skin (Thunnus tonggol) by acid treatment method with 19.39±0.25% yield.
- Gelatin physicochemical structure was modified by two methods of deamination and adding agar (2% w/v).
- The results of FTIR and SDS page tests showed that the gel strength, melting, and isoelectric point of extracted gelatin were improved.
| Article type Original article |
ABSTRACT Background: In the past decade, gelatin extraction from fish skin has been intensively investigated. In comparison to mammalian gelatin, fish gelatin has weaker gelatinous and rheological properties, which limits its widespread application. Deamidation and addition of agar hydrocolloid performed to improve the physical and functional properties of fish gelatin. Methods: Gelatin extraction from fish skin was done using acid pretreatment and applying heat. Agar was also extracted from Gracilaria persica algae by alkaline method and after adding it to gelatin, the values of melting point, gel strength, isoelectric point, and type of constituent peptides and amide bands of gelatin were determined. Results: Gel strength and melting point in unmodified treatment (control) were 92.65g, and 18.5 oC, respectively. These values were increased to (170.85 g: 24.7 oC), and (108.78 g: 21.8 oC), respectively, through deamination process with 1N NaOH solution for 12 h and Agar addition (2% w/v agar extracted from Gracilaria persica). The isoelectric point of the deaminated sample decreased from 8.94 to 5.90, but no noticeable change in the isoelectric point was seen in the modified sample with agar (8.25). Fourier Transform Infrared Spectroscopy spectra showed that the deamination and agar addition to gelatin caused changes in the amide bonds, covalent bonding sites, and crosslinking of gelatin powder; consequently, increasing gel strength and melting point. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis results showed that the two methods of deamination and addition of agar led to an increase in the molecular weight of gelatin. Conclusion: The findings indicated that gelatin and agar interacted successfully. The gelatin/agar mixture exhibited the highest gel strength and melting point and in the deaminated sample, the increase in gel strength and melting point was attributed to the reduction in the isoelectric point of the modified gelatin, which caused the gelatin strands to come closer together and form stronger hydrogen bonds. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Gelatin Agar Deamination Electrophoresis Tuna |
||
| Article history Received: 01 Sep 2024 Revised: 10 Feb 2025 Accepted: 20 Jul 2025 |
||
| Abbreviations FTIR=Fourier Transform Infrared Spectroscopy IEP=Isoelectric Point SDS-PAGE=Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
To cite: Shakeri F., Bahmani Z., Mirmoghtadaie L. (2025). Effect of deamination and agar addition on physical properties of gelatin extracted from Thunnus tonggol skin. Journal of Food Quality and Hazards Control. 12: 183-191.
Introduction
Introduction
Gelatin, as a byproduct of partial collagen hydrolysis, has a wide range of applications in the food industry, photography, and pharmaceutical sectors (Lv et al., 2019). Since there is growing concern about infectious diseases and ethical issues with gelatin made from cows or pigs, fish gelatin has received more attention in recent years (Badii and Howell, 2006). By-products of the fish canning industry have great potential to produce high-quality gelatin with different melting temperatures and gel settings (Boran and Rigenstein, 2010). However, the lower content of hydroxyproline, proline, and other amino acid in fish gelatin compared to mammalian gelatin, resulted in weaker rheological characteristics, melting point, and gel strength (Tu et al., 2015). The limitation of using fish gelatin might be attributed to its deficiency in certain amino acids (Binsi et al., 2009). In addition, the quality and physical properties of fish gelatin are strongly influenced by the variety or texture of the fish, as well as the variables of the extraction process, such as pH, temperature, and the duration of the pretreatment of the extraction process (Ahmed, 2017). Acidic gelatin has a higher isoelectric point due to limited hydrolysis of asparagine and glutamine chains. Removal of amide groups by deamidation method reduces pH and is suitable for home use (Abedi and Pourmohammadi, 2021). However, the structural change of dietary protein can improve its functional qualities (Foegeding and Davis, 2011). Therefore, some nontoxic chemical modification of fish gelatin, such as deamination, could potentially improve its functional properties. The primary conversion of amide groups at glutamine (Gln) and asparagine (Asn) residues to carboxyl groups, resulting in glutamic and aspartic acid, is known as deamination (Abedi and Pourmohammadi, 2021). In this method, protein solubility could potentially enhance by converting glutamine and asparagine amino acids to their deaminated counterparts (Hamada and Swanson, 1994; Mirmoghtadaie et al., 2009). Another way to improve the structural properties of gelatin is addition of hydrocolloids such as agar (Somboon et al., 2014). Agar is an ionic biopolymer that is a complex combination of anionic agaropectin and nonionic agarose that contains acidic side groups such as sulfate and pyruvate. Since gelatin and agar have opposing charges, they can be combined electrostatically at neutral pH (Toyama et al., 2011). On the other hand, agar is an affordable hydrocolloid with high gelling property and is widely used in the medical and pharmaceutical sectors, as well as in laboratory investigations (Romero et al., 2008; Sinthusamran et al., 2016). The interactions between gelatin and agar are critical in the creation of novel food systems. Intermolecular interactions can cause the creation of noncovalent polysaccharide-gelatin complexes, incompatibility, or covalent complexes (Derkach et al., 2022). Until now, there has been no study on the chemical modification of fish gelatin by deamination method. The main purpose of this study is to modify and improve structural properties of fish gelatin by deamination and addition of agar extracted from Gracilaria persica.
Materials and methods
Materials
Fish skins (1,000±100 g) were obtained from the Tuna fish market in Bandar Abbas and stored at -20 °C until use time, and 10 Kg of G. persica red algae is collected from Qeshm Island beaches during low tide and other laboratory materials used, such as H2SO4, NaOH, C6H8O7, Citric acid anhydrous, and activated carbon were purchased from Merck Chemicals Co. (Darmstadt, Germany).
Gelatin extraction
First, the cut samples were washed with distilled water and acetone was added to them to remove fat. In the next step, the skin was washed, cleaned and treated with NaOH solution (0.2% w/v) for 40 min to separate minerals. Next, after neutralizing the pH: 7, the skin was immersed in 0.8% (w/v) citric acid at room temperature for 60 min, then distilled water was used to neutralize the pH. Following this, gelatin was extracted from the swollen skin (collagen) using distilled water (60 °C) for 17 h in a water bath shaker. For the chemical purification of the gelatin solution, one ml of egg albumin was added to the gelatin solution during boiling to absorb impurities such as copper and other heavy metals, and then to separate the impurities from the solution, it was filtered through Whatman No. 4 filter paper (Whatman International, Ltd. Maid stone, England), Buchner funnel was also used. The dissolved gelatin was separated from the remaining pieces of skin using a two-layer filter cloth. Finally, the gelatin solution was dried in the oven at 70 °C, then powdered and packed in waterproof bags (Koli et al., 2013).
Agar extraction from G. persica
Alga G. persica was collected from the shores of the Persian Gulf in Qeshm Island. After washing, the algae masses were placed under the sunlight for 24 h and then dried in oven at 60 °C for 8 h. After being ground with an industrial grinder and passed through a sieve with 60 meshes, the agar powders were stored in polyethylene bags until use. The agar was extracted from the produced algae using the procedure of Sinthusamran et al. (2016). First, the powdered algae were soaked in 5% NaOH for 24 h at room temperature, with ratio of 1:50 (w/v). The mixture was stirred at 90 °C for 3 h. Following alkaline treatment, the algae were rinsed in distilled water until the pH reached to seven. Finally, the treated algae were mixed with distilled water in a 1:50 (w/v) ratio at 95 °C for 2 h, and were passed through Buchner funnel and Whatman No. 4 filter paper under pressure. The extract was cooled at room temperature (25 to 26 °C) to form a gel, then it was frozen for 24 h and then thawed for about 4 h at room temperature. This thawed solution was dried and frozen for 24 h using a freeze dryer (Sinthusamran et al., 2016; Yarnpakdee et al., 2015).
Modifying the functional characteristics of fish gelatin
-Preparation of gelatin/agar gels
Two g of agar powder was mixed by 100 ml of water and autoclaved for 15 min at 1.2 atm and 121 °C. Twenty g of gelatin powder were mixed with 70 or 80 ml of water to create 20% (w/v) gelatin solution. The gelatin was dissolved in the solution by heating it to 65 °C. After that, the gelatin-agar solution was mixed with 0.5 ml of glutaraldehyde reagent (0.5 ml of glutaraldehyde, 0.5 ml of ethanol, and 0.1 ml of 0.1 N HCl), and homogenized for 10 s. The gelatin-agar was allowed to cross-link for 30 min at 25 °C (Wakhet et al., 2015)
-Deamination of fish gelatin using alkali treatment
The extracted fish gelatin was deaminated using the procedure of Paraman et al. (2007), with a partial modification. The fish gelatin was mixed with deionized water (8% w/v) and adjusted to pH: 11 using 1 N NaOH and then agitated for 12 h at 25 °C. Then the solution was shaked for 30 min at 70 °C. After 30 min of treatment, the solution was quickly cooled to 30 °C by adding ice cubes and the pH of the solution was adjusted to 7.0. The partially deaminated gelatin solution was dried in an oven and kept at 5 °C in an airtight container (Cabra et al., 2007; Paraman et al., 2007)
-Yield of gelatin extraction
The yield of the gelatin extraction was calculated using the following equation (Wangtueai and Noomhorm, 2009).
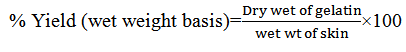
-Determination of Isoelectric Point (IEP)
The transparency of a 2% (w/v) gelatin solution with varied pH values was measured at 660 nm to determine the IEP (spectrophotometer 722, China). The IEP value of gelatin is the pH at which the solution shows the least transparency (Zhang et al., 2011).
-Determination of gel strength
The gel strength was calculated using the Sha et al. (2019) technique. The distilled water was used to dissolve the gelatin (6.67%), which was thoroughly mixed for 30 min at 60 °C in shaking water bath (SS40-D Shaking Bath, Grant Instruments Ltd., Cambridge, England). The flat bottom bottle (with 40.1 mm diameter and 52 mm height), was filled with the gelatin solution and kept at 9-10 °C for 17-18 h. A texture analyzer (TA.XT Plus, Stable Micro Systems Ltd., Surrey, England) was used to the determine gel strength (Sha et al., 2019).
-Determination of melting point
Gelatin solutions with a concentration of 6.67% (w/v) were produced in test tubes. Test tubes were refrigerated for 16 to 18 h before being transferred to a cold water bath (4 °C). The experiment was carried out at a heating rate of 0.2 °C/min. The melting point was determined by the temperature at which the 0.5% methyl-red chloroform began dropping in the gel (Tabarestani et al., 2010).
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR/FT-NIR spectrometer (Perkin Elmer, USA) fitted with an Attenuated Total Reflectance accessory (ATR) equipped with Zn, Se, and Attenuated Total Reflectance (ATR) crystal was used to show the spectra of modified and unmodified gelatin (Aewsiri et al., 2013)
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The SDS-PAGE analysis was carried out in accordance with Norziah et al. (2014) technique, with some modifications. Gelatin solution (4 mg/ml) was mixed with sample buffer containing mercaptoethanol at a ratio of 1:2 (v/v) in a falcon tube. The mixture was warmed at 90 °C for 5 min before getting analyzed with a Mini-PROTEANs Tetra Cell unit (Bio-Rad Laboratories, CA, USA) that was equipped with four stacking gels and 7.5% resolving gel at a continuous current of 25 mA for 90 min. The gel was colored with a Bio-safe Coomassie Brilliant Blue solution and was destained by mixing it with deionized water (Norziah et al., 2014).
Statistical analysis
The experimental data of gel strength, melting point, and functional properties were compared between two groups using t-test procedure, and the data were analyzed using SPSS 22 software. One Way ANOVA was used to compare the means and Least Significant Difference (LSD) was used to compare the treatments at p≤0.05. The SPSS 22 software was used to analyze all data.
Results and discussions
Yield of gelatin extraction
Gelatin was extracted from skin with acidic method then its physicochemical structure was modified. Each step of the gelatin extraction process, such as degreasing and demineralization, and hot water washing and extraction, affected the production yield (Asih et al., 2019). Preparation of gelatin was done under acidic pretreatment conditions by citric acid. The acid extraction method along with the extraction conditions have important roles in determining the extraction efficiency (Shakila et al., 2012). Organic acids (lactic, acetic, etc.) perform better than inorganic acids (sulfuric and hydrochloric acids) due to their higher acid strength, which ultimately lead to the production of gelatin with better quality and more efficiency (Tavakolipour, 2018). The extraction yield of gelatin in this research was 19.39±0.25%. In a research conducted by Zhou and Regenstein (2005), by using 0.05 mol/L citric acids, 16% efficiency was obtained. They demonstrated that acidic treatment promotes increased gelatin yield. In contrast, acidic circumstances result in weak gel strength (Zhou and Regenstein, 2005). Rawdkuen et al. (2010) stated that it was difficult to remove gelatin without expanding the skin matrix with acid. A modest acid treatment is generally required to disrupt these cross-links and allow extraction in the context of acid pretreatments. The yield of skin pretreated with acetic acid was greater (p<0.05) than that of skin pretreated with sulfuric acid+acetic acid. The process of skin swelling was influenced by the acid type utilized, with the ultimate pH and total H+ concentration of the solution playing the most significant roles (Benjakul et al., 2010). Moreover, compared to pretreatment with other organic and inorganic acids, acetic acid pretreatment was shown to produce gelatin with higher yield and quality. Acetic acid destabilizes cross-links in the telopeptide region, amide bonds within the collagen triple helix, and non-covalent intra- and intermolecular cross-links, leading to a compact structure and increased extraction yields. Behnam et al. (2010) produced type A (The control sample) gelatin from the skin and bones of lizardfish (Saurida tumbil). Gelatin was extracted with yields of 10.7 and 5.1%, respectively (Taheri et al., 2010).
IEP
In this study, the IEP of deaminated gelatin was altered to a lower pH value, which might be attributed to glutamine and asparagine deamination (Nagarajan et al., 2012). Acid-processed gelatins have an isoelectric point in the pH range of 6.0-9.5, while alkali-processed gelatins have an isoelectric point between 4.8 and 5.2 (Boran and Rigenstein, 2010). The higher IEP of acid-processed gelatin is due to restricted hydrolysis of the asparagine and glutamine side chains. However, the side chains of these amino acids are readily hydrolyzed to aspartic and glutamic acids in the alkaline process, resulting in gelatin molecules with lower IEPs (Cole, 2000). These findings were consistent with those of Aewsiri et al. (2008), who stated that type A gelatin made by the acid method showed an IEP between pH 6 and 9. Protein deamination, the conversion of amide groups to carboxylic groups, enhances solubility and gelling qualities of protein by adding negative charges (Chen et al., 2021). As the pH of gelatin solution approaches the isoelectric pH, the driving force of identical intermolecular charges between gelatin molecules weakens, allowing the gelatin chains to draw closer together and produce stronger hydrogen bonds (Behnam et al., 2010). In the modified sample with agar, no significant change was observed between the isoelectric pH and pH. The IEP of gelatin, which was measured in both unmodified and modified samples, might be a critical factor to consider when applying it in different products with varying pH levels. Table 1, shows the IEP and pH of the gelatins. The average pH of the control sample was reported to be 6.7±0.77. In the deaminated gelatin, the pH was slightly higher than the control (unmodified) sample (7.5±0.30) and in the sample modified with agar, the pH was higher than the other two samples (7.8±1.20). In extracted gelatin, the isoelectric pH (8.94±1.34) remained high due to acidic pre-treatment and incomplete hydrolysis of amino acids asparagine and glutamine. However, in the deaminated sample, due to the process of deamination and removal of amine groups from the gelatin molecule, the isoelectric pH was decreased. The isoelectric pH in the sample modified with agar did not change significantly compared to the control sample (8.25±0.30). For gelatin type A (The control sample), produced through acidic treatment, the IEP value is often around nine (Enrione et al., 2020).
Materials and methods
Materials
Fish skins (1,000±100 g) were obtained from the Tuna fish market in Bandar Abbas and stored at -20 °C until use time, and 10 Kg of G. persica red algae is collected from Qeshm Island beaches during low tide and other laboratory materials used, such as H2SO4, NaOH, C6H8O7, Citric acid anhydrous, and activated carbon were purchased from Merck Chemicals Co. (Darmstadt, Germany).
Gelatin extraction
First, the cut samples were washed with distilled water and acetone was added to them to remove fat. In the next step, the skin was washed, cleaned and treated with NaOH solution (0.2% w/v) for 40 min to separate minerals. Next, after neutralizing the pH: 7, the skin was immersed in 0.8% (w/v) citric acid at room temperature for 60 min, then distilled water was used to neutralize the pH. Following this, gelatin was extracted from the swollen skin (collagen) using distilled water (60 °C) for 17 h in a water bath shaker. For the chemical purification of the gelatin solution, one ml of egg albumin was added to the gelatin solution during boiling to absorb impurities such as copper and other heavy metals, and then to separate the impurities from the solution, it was filtered through Whatman No. 4 filter paper (Whatman International, Ltd. Maid stone, England), Buchner funnel was also used. The dissolved gelatin was separated from the remaining pieces of skin using a two-layer filter cloth. Finally, the gelatin solution was dried in the oven at 70 °C, then powdered and packed in waterproof bags (Koli et al., 2013).
Agar extraction from G. persica
Alga G. persica was collected from the shores of the Persian Gulf in Qeshm Island. After washing, the algae masses were placed under the sunlight for 24 h and then dried in oven at 60 °C for 8 h. After being ground with an industrial grinder and passed through a sieve with 60 meshes, the agar powders were stored in polyethylene bags until use. The agar was extracted from the produced algae using the procedure of Sinthusamran et al. (2016). First, the powdered algae were soaked in 5% NaOH for 24 h at room temperature, with ratio of 1:50 (w/v). The mixture was stirred at 90 °C for 3 h. Following alkaline treatment, the algae were rinsed in distilled water until the pH reached to seven. Finally, the treated algae were mixed with distilled water in a 1:50 (w/v) ratio at 95 °C for 2 h, and were passed through Buchner funnel and Whatman No. 4 filter paper under pressure. The extract was cooled at room temperature (25 to 26 °C) to form a gel, then it was frozen for 24 h and then thawed for about 4 h at room temperature. This thawed solution was dried and frozen for 24 h using a freeze dryer (Sinthusamran et al., 2016; Yarnpakdee et al., 2015).
Modifying the functional characteristics of fish gelatin
-Preparation of gelatin/agar gels
Two g of agar powder was mixed by 100 ml of water and autoclaved for 15 min at 1.2 atm and 121 °C. Twenty g of gelatin powder were mixed with 70 or 80 ml of water to create 20% (w/v) gelatin solution. The gelatin was dissolved in the solution by heating it to 65 °C. After that, the gelatin-agar solution was mixed with 0.5 ml of glutaraldehyde reagent (0.5 ml of glutaraldehyde, 0.5 ml of ethanol, and 0.1 ml of 0.1 N HCl), and homogenized for 10 s. The gelatin-agar was allowed to cross-link for 30 min at 25 °C (Wakhet et al., 2015)
-Deamination of fish gelatin using alkali treatment
The extracted fish gelatin was deaminated using the procedure of Paraman et al. (2007), with a partial modification. The fish gelatin was mixed with deionized water (8% w/v) and adjusted to pH: 11 using 1 N NaOH and then agitated for 12 h at 25 °C. Then the solution was shaked for 30 min at 70 °C. After 30 min of treatment, the solution was quickly cooled to 30 °C by adding ice cubes and the pH of the solution was adjusted to 7.0. The partially deaminated gelatin solution was dried in an oven and kept at 5 °C in an airtight container (Cabra et al., 2007; Paraman et al., 2007)
-Yield of gelatin extraction
The yield of the gelatin extraction was calculated using the following equation (Wangtueai and Noomhorm, 2009).
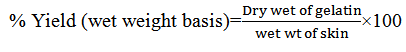
-Determination of Isoelectric Point (IEP)
The transparency of a 2% (w/v) gelatin solution with varied pH values was measured at 660 nm to determine the IEP (spectrophotometer 722, China). The IEP value of gelatin is the pH at which the solution shows the least transparency (Zhang et al., 2011).
-Determination of gel strength
The gel strength was calculated using the Sha et al. (2019) technique. The distilled water was used to dissolve the gelatin (6.67%), which was thoroughly mixed for 30 min at 60 °C in shaking water bath (SS40-D Shaking Bath, Grant Instruments Ltd., Cambridge, England). The flat bottom bottle (with 40.1 mm diameter and 52 mm height), was filled with the gelatin solution and kept at 9-10 °C for 17-18 h. A texture analyzer (TA.XT Plus, Stable Micro Systems Ltd., Surrey, England) was used to the determine gel strength (Sha et al., 2019).
-Determination of melting point
Gelatin solutions with a concentration of 6.67% (w/v) were produced in test tubes. Test tubes were refrigerated for 16 to 18 h before being transferred to a cold water bath (4 °C). The experiment was carried out at a heating rate of 0.2 °C/min. The melting point was determined by the temperature at which the 0.5% methyl-red chloroform began dropping in the gel (Tabarestani et al., 2010).
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR/FT-NIR spectrometer (Perkin Elmer, USA) fitted with an Attenuated Total Reflectance accessory (ATR) equipped with Zn, Se, and Attenuated Total Reflectance (ATR) crystal was used to show the spectra of modified and unmodified gelatin (Aewsiri et al., 2013)
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The SDS-PAGE analysis was carried out in accordance with Norziah et al. (2014) technique, with some modifications. Gelatin solution (4 mg/ml) was mixed with sample buffer containing mercaptoethanol at a ratio of 1:2 (v/v) in a falcon tube. The mixture was warmed at 90 °C for 5 min before getting analyzed with a Mini-PROTEANs Tetra Cell unit (Bio-Rad Laboratories, CA, USA) that was equipped with four stacking gels and 7.5% resolving gel at a continuous current of 25 mA for 90 min. The gel was colored with a Bio-safe Coomassie Brilliant Blue solution and was destained by mixing it with deionized water (Norziah et al., 2014).
Statistical analysis
The experimental data of gel strength, melting point, and functional properties were compared between two groups using t-test procedure, and the data were analyzed using SPSS 22 software. One Way ANOVA was used to compare the means and Least Significant Difference (LSD) was used to compare the treatments at p≤0.05. The SPSS 22 software was used to analyze all data.
Results and discussions
Yield of gelatin extraction
Gelatin was extracted from skin with acidic method then its physicochemical structure was modified. Each step of the gelatin extraction process, such as degreasing and demineralization, and hot water washing and extraction, affected the production yield (Asih et al., 2019). Preparation of gelatin was done under acidic pretreatment conditions by citric acid. The acid extraction method along with the extraction conditions have important roles in determining the extraction efficiency (Shakila et al., 2012). Organic acids (lactic, acetic, etc.) perform better than inorganic acids (sulfuric and hydrochloric acids) due to their higher acid strength, which ultimately lead to the production of gelatin with better quality and more efficiency (Tavakolipour, 2018). The extraction yield of gelatin in this research was 19.39±0.25%. In a research conducted by Zhou and Regenstein (2005), by using 0.05 mol/L citric acids, 16% efficiency was obtained. They demonstrated that acidic treatment promotes increased gelatin yield. In contrast, acidic circumstances result in weak gel strength (Zhou and Regenstein, 2005). Rawdkuen et al. (2010) stated that it was difficult to remove gelatin without expanding the skin matrix with acid. A modest acid treatment is generally required to disrupt these cross-links and allow extraction in the context of acid pretreatments. The yield of skin pretreated with acetic acid was greater (p<0.05) than that of skin pretreated with sulfuric acid+acetic acid. The process of skin swelling was influenced by the acid type utilized, with the ultimate pH and total H+ concentration of the solution playing the most significant roles (Benjakul et al., 2010). Moreover, compared to pretreatment with other organic and inorganic acids, acetic acid pretreatment was shown to produce gelatin with higher yield and quality. Acetic acid destabilizes cross-links in the telopeptide region, amide bonds within the collagen triple helix, and non-covalent intra- and intermolecular cross-links, leading to a compact structure and increased extraction yields. Behnam et al. (2010) produced type A (The control sample) gelatin from the skin and bones of lizardfish (Saurida tumbil). Gelatin was extracted with yields of 10.7 and 5.1%, respectively (Taheri et al., 2010).
IEP
In this study, the IEP of deaminated gelatin was altered to a lower pH value, which might be attributed to glutamine and asparagine deamination (Nagarajan et al., 2012). Acid-processed gelatins have an isoelectric point in the pH range of 6.0-9.5, while alkali-processed gelatins have an isoelectric point between 4.8 and 5.2 (Boran and Rigenstein, 2010). The higher IEP of acid-processed gelatin is due to restricted hydrolysis of the asparagine and glutamine side chains. However, the side chains of these amino acids are readily hydrolyzed to aspartic and glutamic acids in the alkaline process, resulting in gelatin molecules with lower IEPs (Cole, 2000). These findings were consistent with those of Aewsiri et al. (2008), who stated that type A gelatin made by the acid method showed an IEP between pH 6 and 9. Protein deamination, the conversion of amide groups to carboxylic groups, enhances solubility and gelling qualities of protein by adding negative charges (Chen et al., 2021). As the pH of gelatin solution approaches the isoelectric pH, the driving force of identical intermolecular charges between gelatin molecules weakens, allowing the gelatin chains to draw closer together and produce stronger hydrogen bonds (Behnam et al., 2010). In the modified sample with agar, no significant change was observed between the isoelectric pH and pH. The IEP of gelatin, which was measured in both unmodified and modified samples, might be a critical factor to consider when applying it in different products with varying pH levels. Table 1, shows the IEP and pH of the gelatins. The average pH of the control sample was reported to be 6.7±0.77. In the deaminated gelatin, the pH was slightly higher than the control (unmodified) sample (7.5±0.30) and in the sample modified with agar, the pH was higher than the other two samples (7.8±1.20). In extracted gelatin, the isoelectric pH (8.94±1.34) remained high due to acidic pre-treatment and incomplete hydrolysis of amino acids asparagine and glutamine. However, in the deaminated sample, due to the process of deamination and removal of amine groups from the gelatin molecule, the isoelectric pH was decreased. The isoelectric pH in the sample modified with agar did not change significantly compared to the control sample (8.25±0.30). For gelatin type A (The control sample), produced through acidic treatment, the IEP value is often around nine (Enrione et al., 2020).
Table 1: pH and Isoelectric Point (IEP) of control, deaminated gelatin, and gelatin/agar mix gel samples (n=3)
| Parameter | Control (mean±SD) | Deaminated gelatin (mean±SD) | Gelatin/agar mix gel (mean±SD) |
| pH | 6.7±0.77 c | 7.5±.030 b | 7.8±1.20 a |
| IEP | 8.94±1.34 a | 5.90 ±1.50 c | 8.25±0.30 b |
*Different letters in each row indicate statistically significant differences.
Gel strength
Gelatin/agar mix showed the highest strength (170.85 g), followed by deaminated gelatin and unmodified gelatin (control), which showed gel strength of 108.78 and 92.65 g, respectively (Table 2). The strength of the gelatin was decreased with decrease in high molecular weight component (Figure 2). The gel strength of the gelatin protein was also increased (p<0.05) after deamination (Table 2).
Gelatin/agar mix showed the highest strength (170.85 g), followed by deaminated gelatin and unmodified gelatin (control), which showed gel strength of 108.78 and 92.65 g, respectively (Table 2). The strength of the gelatin was decreased with decrease in high molecular weight component (Figure 2). The gel strength of the gelatin protein was also increased (p<0.05) after deamination (Table 2).
Table 2: Gel strength and melting point of fish waste gelatin in different control, deaminated gelatin, and gelatin/agar mix gel samples (n=3)
| Samples | Melting point (ºC) (mean±SD) | Gel strength (Bloom) (g) (mean±SD) |
| Control | 18.5±0.5 c | 92.65±0.82 c |
| Deaminated gelatin | 21.83±0.28 b | 108.78±3.1b |
| Agar/gelatin mix gel | 24.7±0.06 a | 170.85±2.3 a |
* Different letters in each column indicate statistically significant differences.
Gel strength is an important physical characteristic of gelatin and an important factor in determining the final price of the gelatin. This is influenced by molecular weight as well as complicated interactions of amino acids that made α/β-chain ratio in the gelatin (Karim and Bhat, 2009). In this study, the gel strength of the unmodified sample was reported to be 92.65 g. In other studies the gelatin strength of Cod, Alaska Pollock, salmon, and hake was reported in the range of 70 to 110 g (Karim and Bhat, 2009), which is in agreement with results of this study. The gel strength of 92.65 g for unmodified fish gelatin was much lower than the gel strength of pig gelatin (147.4 g) and bovine gelatin (107.9 g) reported by Cho et al. (2014). Furthermore, the gel strength of the deaminated and agar-modified samples was higher than that of the unmodified sample. The addition of 2% (w/v) agar to fish gelatin significantly increased the strength of the gelatin gel (p≤0.05). The interaction between gelatin and agar is an effective factor in determining gel physical properties. The strength of the gel is influenced by the formation of hydrogen bonds between gelatin and agar. Agar is a polysaccharide with large number of OH groups at pH: 7.5. Gelatin also has both amine and carboxyl functional groups on the surface of molecule, so there is a possibility of forming hydrogen bonding between the NH2 groups (gelatin) and OH groups (agar) during agar and gelatin mixing (Saxena et al., 2011). Deamination increased the number of negative charges because of changing glutamine and asparagine to glutamic acid and aspartic acids, which shifted the isoelectric point from alkaline pH to acidic pH (Qiu et al., 2013). Fish gelatin application can be expanded by mixing it with other common hydrocolloids. For instance, fish gelatin and pectin have been combined to create a low-fat spread (Karim and Bhat, 2009). Behnam et al. (2010) reported improvement in the functional properties of lizard fish skin gelatin following the addition of glycerol, 0.5% Ketira, and sucrose, likely due to the formation of hydrogen bonds. Formation of hydrogen bonds could increase the stability of the gelatin structure in the aqueous environment (Behnam et al., 2010).
Melting point
Comparison of the melting point of unmodified fish gelatin (control) with modified gelatin is shown in Table 3. The melting temperature of the deaminated sample (21.83±0.28 °C) was increased compared to the control sample (18.5±0.5 °C). The addition of agar raised the melting point of gelatin. Agar/gelatin mixed gel was melted at 24.7±0.6 °C, which was increased significantly compared to the control sample (p≤0.05). The melting point is influenced by a wide variety of parameters including the average of molecular weight, the molecular weight distribution, the concentration of the gelatin solution, the time needed for gel maturation, the temperature of gel maturation, and the pH of the solution. In addition, it is related to the amount of energy required to break the cross-linked junction zones (Cheow et al., 2007). The melting point of the deaminated sample was increased compared to the control sample, which may be due to the change of amide groups to carboxylic groups and the change of protein charge and resulting in an increase in interaction between water and gelatin molecules in the deaminated sample (Abedi and Pourmohammadi, 2021). Also deamination can cause dissociation of aggregated proteins that can improve the molecule flexibility and facilitate protein-water interaction (Chen et al., 2021). In gelatin/agar mixture, gelatin is polycationic, while agar is polyanionic molecule below its isoelectric pH, that can lead to complex coacervates formation (Singh et al., 2007). In addition, the electrostatic, hydrogen-bonding, or hydrophobic interactions resulted in stabilization of the gel network (Djabourov et al., 1989; Doublier et al., 2000).
FTIR spectra analysis
The presence of numerous main absorption bands corresponding to vibrational transitions in the peptide chain characterizes the infrared spectra of gelatin as a protein (Table 3). The FTIR spectrum of gelatin without and with modification by agar addition and deamination method is depicted in Figure 1. The spectra of fish gelatin without modification exhibited the major bands at 3,299 1/cm (amide A, which represents NH stretching paired with hydrogen bonding), 2,939 1/cm (amide B, stretching vibration of C-H groups), 1,536.03 1/cm (amide II, which represents NH bending in conjunction with CN stretching), and 1,238 1/cm (amide III ,CN stretching, NH bending). The modification of gelatin with agar and deamination caused the changes in FTIR spectra of gelatin. In deaminated sample, the amide A band (N-H stretching) was shifted from 3,299 to 3,288 1/cm and the amide B band decreased from 2,937 to 2,135 1/cm. In the sample modified with agar, amide A was observed at 3,277 1/cm, amide B at 2,935 1/cm, and amide II at 1,535 1/cm, which was slightly reduced compared to the control sample.
Melting point
Comparison of the melting point of unmodified fish gelatin (control) with modified gelatin is shown in Table 3. The melting temperature of the deaminated sample (21.83±0.28 °C) was increased compared to the control sample (18.5±0.5 °C). The addition of agar raised the melting point of gelatin. Agar/gelatin mixed gel was melted at 24.7±0.6 °C, which was increased significantly compared to the control sample (p≤0.05). The melting point is influenced by a wide variety of parameters including the average of molecular weight, the molecular weight distribution, the concentration of the gelatin solution, the time needed for gel maturation, the temperature of gel maturation, and the pH of the solution. In addition, it is related to the amount of energy required to break the cross-linked junction zones (Cheow et al., 2007). The melting point of the deaminated sample was increased compared to the control sample, which may be due to the change of amide groups to carboxylic groups and the change of protein charge and resulting in an increase in interaction between water and gelatin molecules in the deaminated sample (Abedi and Pourmohammadi, 2021). Also deamination can cause dissociation of aggregated proteins that can improve the molecule flexibility and facilitate protein-water interaction (Chen et al., 2021). In gelatin/agar mixture, gelatin is polycationic, while agar is polyanionic molecule below its isoelectric pH, that can lead to complex coacervates formation (Singh et al., 2007). In addition, the electrostatic, hydrogen-bonding, or hydrophobic interactions resulted in stabilization of the gel network (Djabourov et al., 1989; Doublier et al., 2000).
FTIR spectra analysis
The presence of numerous main absorption bands corresponding to vibrational transitions in the peptide chain characterizes the infrared spectra of gelatin as a protein (Table 3). The FTIR spectrum of gelatin without and with modification by agar addition and deamination method is depicted in Figure 1. The spectra of fish gelatin without modification exhibited the major bands at 3,299 1/cm (amide A, which represents NH stretching paired with hydrogen bonding), 2,939 1/cm (amide B, stretching vibration of C-H groups), 1,536.03 1/cm (amide II, which represents NH bending in conjunction with CN stretching), and 1,238 1/cm (amide III ,CN stretching, NH bending). The modification of gelatin with agar and deamination caused the changes in FTIR spectra of gelatin. In deaminated sample, the amide A band (N-H stretching) was shifted from 3,299 to 3,288 1/cm and the amide B band decreased from 2,937 to 2,135 1/cm. In the sample modified with agar, amide A was observed at 3,277 1/cm, amide B at 2,935 1/cm, and amide II at 1,535 1/cm, which was slightly reduced compared to the control sample.
Table 3: Main absorption bands of the functional groups of gelatin
| Groups | Wave Number ν, 1/cm | Type of Vibration |
| Amide A | 3,200–3,300 | N–H stretching vibrations |
| Amide B | 2,900–3,000 | C-H stretching vibrations |
| Amide II | 1,480-1,575 | N–H deformation vibrations—80% and С–N stretching |
| Amide III | 1,230-1,300 | C–N stretching vibrations |

Figure 1: Fourier Transform Infrared Spectroscopy (FTIR) spectra of extracted fish gelatin (Extracted Fish Gelatin (EFG), not modified, Deaminated Gelatin (DG), and Gelatin/Agar Mix (GAM))
Characteristics of FTIR absorption band, namely A, B, ІI, and IІІ peaks could be observed in a typical IR spectrum of gelatin structure. Regarding the deaminated gelatin, decreased hydrogen bonding and increased electrostatic repulsion changed the secondary conformation due to deamination and not due to peptide bond breaking (Derkach et al., 2019; Liao et al., 2010). In comparison to control gelatin, gelatin treated with agar displayed lower amide A, B, and II band wavenumber. These alterations indicated that gelatin was more disordered. The amide I peak is the most effective for characterizing gelatin coil structure (Yakimets et al., 2005). The reduction in amide I band of modified gelatin can be due to effect of agar on gelatin helix coil structure. Agar may cause conformational changes in gelatin, resulting in alterations to its properties (Aewsiri et al., 2013). Studies using gelatin and gelatin films with additional polysaccharides, including k-carrageenan, showed similar effects, as measured by FTIR spectroscopy (Voron’ko et al., 2016). The spectra changes suggested the presence of protein-polysaccharide interactions via hydrogen bond (Mohajer et al., 2017).
SDS-PAGE
Acrylamide gel electrophoresis was used to investigate the formation of stable gelatin-agar complex, deaminated, and unmodified samples. To determine the molecular weight of gelatin components, a protein marker with a molecular weight of 25 to 245 KDa was used. Gelatin protein patterns showed three distinct bands of α1, α2, and β chain (Figure 2). The control sample included α1 and α2 chains and proteins or peptides with a molecular weight of less than 100 KDa. In the agar-modified sample, more bands were observed in the α2 region, 100 KDa, as well as the βregion, 140 KDa. The molecular weight pattern of deaminated gelatin showed a higher position than the control treatment.
SDS-PAGE
Acrylamide gel electrophoresis was used to investigate the formation of stable gelatin-agar complex, deaminated, and unmodified samples. To determine the molecular weight of gelatin components, a protein marker with a molecular weight of 25 to 245 KDa was used. Gelatin protein patterns showed three distinct bands of α1, α2, and β chain (Figure 2). The control sample included α1 and α2 chains and proteins or peptides with a molecular weight of less than 100 KDa. In the agar-modified sample, more bands were observed in the α2 region, 100 KDa, as well as the βregion, 140 KDa. The molecular weight pattern of deaminated gelatin showed a higher position than the control treatment.

Figure 2: Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) patterns for samples (1=Marker; 2=Gelatin/agar mixture; 3=Control; 4=Deaminated gelatin), Gel (7.5 %), resolving, colored by silver nitrate method
Regarding SDS-PAGE analysis, in the unmodified and modified gelatin, when collagen is converted into gelatin, intra- and extra-molecular bonds of collagen chains are broken, resulting in formation of α1, α2, and β chains with lower molecular weight (Muyonga et al., 2004) the presence of bands with low molecular weight in the unmodified sample can indicate a decrease in viscosity and melting temperature of unmodified fish gelatin (Rawdkuen et al., 2010). Kumar et al. (2017) also related low viscosity, melting temperature, and setting point of gelatin gel to high content of low molecular weight peptides (Kumar et al., 2017; Tavernier, 1989). During gelatin extraction and purification, these peptides might have been created by the heating process or by endogenous proteinases. The generation of small peptides was related to the lower bloom and viscosity of fish gelatin (Muyonga et al., 2004). SDS-PAGE patterns of the gelatin/agar mixture revealed high band intensity for α and β components, whereas the main component had the lowest band intensity in the unmodified sample. Bands with higher molecular weight (100-140 KDa) were observed in the sample modified with agar, which could be due to the possible effect of agar on the formation of cross-links between proteins. Diftis et al. (2005) obtained similar results from SDS-PAGE analysis of a gelatin-pectin composite (Diftis et al., 2005). Bands with higher molecular weight were also observed in the deaminated sample, indicating either a more extensive unfolding of the gelatin by deamination or the attachment of a small molecule via cross-linking (Ohtsuka et al., 2001).
Conclusion
This research on the quality attributes of deaminated and agar-modified fish gelatin demonstrated notable improvements in key physicochemical parameters, including gel strength, melting point, molecular weight profile, FTIR spectrum, and isoelectric point. The findings revealed successful interactions between gelatin and agar, with the agar-modified formulation recognized as the most effective treatment. The gelatin/agar mixture showed the highest gel strength and melting point, which can be attributed to the reduced isoelectric point of the modified gelatin, facilitating closer molecular packing and the formation of stronger hydrogen bonds. Consequently, these structural modifications expand the potential for fish gelatin utilization across diverse industrial applications.
Author contributions
F.S. conceptualized and wrote the original draft; F.S. and Z.B. conceived and designed the analysis; Z.B. and L.M. contributed data or analysis tools; L.M. performed the analysis. All authors read and approved the final manuscript.
Acknowledgments
This research work was supported by the Faculty of Nutrition and Food Industry, Shahid Beheshti University of Medical Sciences.
Conflicts of interest
The authors declare that there is no conflict of interests.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-profit sectors.
Ethical consideration
Not applicable.
References
Abedi E., Pourmohammadi K. (2021). Chemical modifications and their effects on gluten protein: an extensive review. Food Chemistry. 343: 128398. [DOI: 10.1016/j.foodchem.2020. 128398]
Aewsiri T., Benjakul S., Visessanguan W., Tanaka M. (2008). Chemical compositions and functional properties of gelatin from pre‐cooked tuna fin. International Journal of Food Science and Technology. 43: 685-693. [DOI: 10.1111/j.1365-2621.2006. 01509.x]
Aewsiri T., Benjakul S., Visessanguan W., Wierenga P.A., Gruppen H. (2013). Emulsifying property and antioxidative activity of cuttlefish skin gelatin modified with oxidized linoleic acid and oxidized tannic acid. Food and Bioprocess Technology. 6: 870-881. [DOI: 10.1007/s11947-011-0636-1]
Ahmed J. (2017). Rheological properties of gelatin and advances in measurement. In: Ahmed J. (Editor). Advances in food rheology and its applications. Woodhead Publishing, Cambridge, UK. pp: 377-404. [DOI:10.1016/B978-0-08-100431-9.00015-2]
Asih I.D., Kemala T., Nurilmala M. (2019). Halal gelatin extraction from Patin fish bone (Pangasius hypophthalmus) by-product with ultrasound-assisted extraction. IOP Conference Series: Earth and Environmental Science. 299: 012061. [DOI: 10.1088/1755-1315/299/1/012061]
Badii F., Howell N.K. (2006). Fish gelatin: structure, gelling properties and interaction with egg albumen proteins. Food Hydrocolloids. 20: 630-640. [DOI: 10.1016/j.foodhyd. 2005.06.006]
Behnam S., Taheri A., Kakoei H. (2010). improvement of lizard fish (saurida tumbil) skin gelatin properties by the coenhancers magnesium sulphate, glycerol, katira, sucrose and ammonium nitrate. Iranian Journal of Food Science and Technology. 7: 21-32.
Binsi P.K., Shamasundar B.A., Dileep A.O., Badii F., Howell N.K. (2009). Rheological and functional properties of gelatin from the skin of Bigeye snapper (Priacanthus hamrur) fish: Influence of gelatin on the gel-forming ability of fish mince. Food Hydrocolloids. 23: 132-145. [DOI: 10.1016/j. foodhyd.2007.12.004]
Boran G., Regenstein J.M. (2010). Fish gelatin. Advances in Food and Nutrition Research. 60: 119-143. [DOI: 10.1016/S1043-4526(10)60005-8]
Cabra V., Arreguin R., Vazquez-Duhalt R., Farres A. (2007). Effect of alkaline deamidation on the structure, surface hydrophobicity, and emulsifying properties of the Z19 α-zein. Journal of Agricultural and Food Chemistry. 55: 439-445. [DOI: 10.1021/ jf061002r]
Chen X., Fu W., Luo Y., Cui C., Suppavorasatit I., Liang L. (2021). Protein deamidation to produce processable ingredients and engineered colloids for emerging food applications. Comprehensive Reviews in Food Science and Food Safety. 20: 3788-3817. [DOI: 10.1111/1541-4337.12759]
Cheow C.S., Norizah M.S., Kyaw Z.Y., Howell N.K. (2007). Preparation and characterisation of gelatins from the skins of sin croaker (Johnius dussumieri) and shortfin scad (Decapterus macrosoma). Food Chemistry. 101: 386-391. [DOI: 10.1016/J.Foodchem.2006.01.046]
Cho S., Ahn J-R., Koo J-S, Kim S-B. (2014). Physicochemical properties of gelatin from jellyfish Rhopilema hispidum. Fisheries and Aquatic Sciences. 17: 299-304. [DOI:10.5657/FAS.2014.0299]
Cole C.G.B. (2000). Gelatin. Encyclopedia of food science and technology. 2nd Edition. John Wiley and Sons, New York, NY. pp: 1183-1188.
Derkach S.R., Kuchina Y.A., Baryshnikov A.V., Kolotova D.S., Voron’ko N.G. (2019). Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers. 11: 1724. [DOI: 10.3390/polym11101724]
Derkach S.R., Voron’ko N.G., Kuchina Y.A. (2022). Intermolecular interactions in the formation of polysaccharide-gelatin complexes: a spectroscopic study. Polymers. 14: 2777. [DOI: 10.3390/polym14142777]
Diftis N.G., Pirzas T.A., Kiosseoglou V.D. (2005). Emulsifying properties of gelatin conjugated to pectin under alkaline conditions. Journal of the Science of Food and Agriculture. 85: 804-808. [DOI: 10.1002/jsfa.2029]
Djabourov M., Clark A.H., Rowlands D.W., Ross-Murphy S.B. (1989). Small-angle x-ray scattering characterization of agarose sols and gels. Macromolecules. 22: 180-188. [DOI: 10.1021/ma00191a035]
Doublier J.-L., Garnier C., Renard D., Sanchez C. (2000). Protein–polysaccharide interactions. Current Opinion in Colloid and Interface Science. 5: 202-214. [DOI: 10.1016/S1359-0294(00)00054-6]
Enrione J., Char C., Pepczynska M., Padilla C., González-Muñoz A., Olguín Y., Quinzio C., Iturriaga L., Díaz-Calderón P. (2020). Rheological and structural study of salmon gelatin with controlled molecular weight. Polymers. 12: 1587. [DOI: 10.3390/polym12071587]
Foegeding E.A., Davis J.P. (2011). Food protein functionality: a comprehensive approach. Food Hydrocolloids. 25: 1853-1864. [DOI: 10.1016/j.foodhyd.2011.05.008]
Hamada J.S., Swanson B. (1994). Deamidation of food proteins to improve functionality. Critical Reviews in Food Science and Nutrition. 34: 283-292. [DOI: 10.1080/10408399409527664]
Karim A.A., Bhat R. (2009). Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids. 23: 563-576. [DOI: 10.1016/j.foodhyd. 2008.07.002]
Koli J.M., Basu S., Venkteshwarlu G., Choukasy M.K., Nayak B.B., (2013). Optimization of fish gelatin extraction from skins and bones: a comparative study. Ecology Environment and Conservation. 19: 47-56.
Kumar D.P., Chandra M.V., Elavarasan K., Shamasundar B. (2017). Structural properties of gelatin extracted from croaker fish (Johnius sp) skin waste. International Journal of Food Properties. 20: S2612-S2625. [DOI: 10.1080/10942912. 2017.1381702]
Liao L., Liu T.-X., Zhao M.-M., Cui C., Yuan B.-E., Tang S.,Yang F. (2010). Functional, nutritional and conformational changes from deamidation of wheat gluten with succinic acid and citric acid. Food Chemistry. 123: 123-130. [DOI: 10.1016/j.foodchem. 2010.04.017]
Lv L.-C., Huang Q.-Y., Ding W., Xiao X.-H., Zhang H.-Y., Xiong L.-X. ( 2019). Fish gelatin: the novel potential applications. Journal of Functional Foods. 63: 103581. [DOI: 10.1016/ j.jff.2019.103581]
Mirmoghtadaie L., Kadivar M., Shahedi M. (2009). Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chemistry. 114: 127-131. [DOI: 10.1016/j.foodchem.2008.09.025]
Mohajer S., Rezaei M., Hosseini S.F. (2017). Physico-chemical and microstructural properties of fish gelatin/agar bio-based blend films. Carbohydrate Polymers. 157: 784-793. [DOI: 10.1016/ j.carbpol.2016.10.061]
Muyonga J., Cole C.G.B., Duodu K.G. (2004). Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocolloids. 18: 581-592. [DOI: 10.1016/j.foodhyd.2003.08.009]
Nagarajan M., Benjakul S., Prodpran T., Songtipya P., Kishimura H. (2012). Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocolloids. 29: 389-397. [DOI: 10.1016/j.foodhyd.2012.04.001]
Norziah M.H., Kee H.Y., Norita M. (2014). Response surface optimization of bromelain-assisted gelatin extraction from surimi processing wastes. Food Bioscience. 5: 9-18. [DOI: 10.1016/j. fbio.2013.10.001]
Ohtsuka T., Umezawa Y., Nio N., Kubota K. (2001). Comparison of deamidation activity of transglutaminases. Journal of Food Science. 66: 25-29. [DOI: 10.1111/j.1365-2621.2001.tb15576.x]
Paraman I., Hettiarachchy N.S., Schaefer C. (2007). Glycosylation and deamidation of rice endosperm protein for improved solubility and emulsifying properties. Cereal Chemistry. 84: 593-599. [DOI: 10.1094/CCHEM-84-6-0593]
Qiu C., Sun W., Cui C., Zhao M. (2013). Effect of citric acid deamidation on in vitro digestibility and antioxidant properties of wheat gluten. Food Chemistry. 141: 2772-2778. [DOI: 10.1016/j.foodchem.2013.05.072]
Rawdkuen S., Sai-Ut S., Benjakul S. (2010). Properties of gelatin films from giant catfish skin and bovine bone: a comparative study. European Food Research and Technology. 231: 907-916. [DOI: 10.1007/s00217-010-1340-5]
Romero J.B., Villanueva R.D., Montaño M.N.E. (2008). Stability of agar in the seaweed Gracilaria eucheumatoides (Gracilariales, Rhodophyta) during postharvest storage. Bioresource Technology. 99: 8151-8155. [DOI: 10.1016/j.biortech. 2008.03. 017]
Saxena A., Tahir A., Kaloti M., Ali J., Bohidar H.B. (2011). Effect of agar—gelatin compositions on the release of salbutamol tablets. International Journal of Pharmaceutical Investigation. 1: 93-98. [DOI: 10.4103/2230-973X.82407]
Sha X.-M., Hu Z.-Z., Ye Y.-H., Xu H., Tu Z.-C. (2019). Effect of extraction temperature on the gelling properties and identification of porcine gelatin. Food Hydrocolloids. 92: 163-172. [DOI:10.1016/j.foodhyd.2019.01.059]
Shakila R.J., Jeevithan E., Varatharajakumar A., Jeyasekaran G., Sukumar D. (2012). Functional characterization of gelatin extracted from bones of red snapper and grouper in comparison with mammalian gelatin. LWT-Food Science and Technology. 48: 30-36. [DOI: 10.1016/j.lwt.2012.03.007]
Singh S.S., Bohidar H.B., Bandyopadhyay S. (2007). Study of gelatin–agar intermolecular aggregates in the supernatant of its coacervate. Colloids and Surfaces B: Biointerfaces. 57: 29-36. [DOI: 10.1016/j.colsurfb.2006.12.017]
Sinthusamran S., Benjakul S., Hemar Y. (2016). Rheological and sensory properties of fish gelatin gels as influenced by agar from Gracilaria tenuistipitata. International Journal of Food Science and Technology. 51: 1530-1536. [DOI: 10.1111/ijfs.13117]
Somboon N., Karrila T.T., Kaewmanee T., Karrila S.J. (2014). Properties of gels from mixed agar and fish gelatin. International Food Research Journal. 21: 485.
Tabarestani H.S., Maghsoudlou Y., Motamedzadegan A., Sadeghi Mahoonak A.R. (2010). Optimization of physico-chemical properties of gelatin extracted from fish skin of rainbow trout (Onchorhynchus mykiss). Bioresource Technology. 101: 6207-6214. [DOI: 10.1016/j.biortech.2010.02.071]
Tavakolipour H. (2011). Extraction and evaluation of gelatin from silver carp waste. World Journal of Fish and Marine Sciences. 3: 10-15.
Tavernier B.H. (1989). Molecular mass distribution of gelatin and physical properties. Photographic Gelatin Proceedings. 1: 217-228.
Toyama Y., Sahara R., Iino Y., Kubota K. (2011). pH dependence of rheological properties of gelatin gel mixed with agar or agarose. Transactions of the Materials Research Society of Japan. 36: 383-386. [DOI: 10.14723/tmrsj.36.383]
Tu Z.-C., Huang T., Wang H., Sha X.-M., Shi Y., Huang X.-Q., Man Z.-Z., Li D.-J. (2015). Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. Journal of Food Science and Technology. 52: 2166-2174. [DOI: 10.1007/s13197-013-1239-9]
Voron’ko N.G., Derkach S.R., Kuchina Y.A., Sokolan N.I. (2016). The chitosan–gelatin (bio) polyelectrolyte complexes formation in an acidic medium. Carbohydrate Polymers. 138: 265-272. [DOI: 10.1016/j.carbpol.2015.11.059]
Wakhet S., Singh V.K., Sahoo S., Sagiri S.S., Kulanthaivel S., Bhattacharya M.K., Kumar N., Banerjee I., Pal K. (2015). Characterization of gelatin–agar based phase separated hydrogel, emulgel and bigel: a comparative study. Journal of Materials Science: Materials in Medicine. 26: 118. [DOI: 10.1007/s10856-015-5434-2]
Wangtueai S., Noomhorm A. (2009). Processing optimization and characterization of gelatin from lizardfish (Saurida spp.) scales. LWT-Food Science and Technology. 42: 825-834. [DOI: 10.1016/j.lwt.2008.11.014]
Yakimets I., Wellner N., Smith A.C., Wilson R.H., Farhat I., Mitchell J. (2005). Mechanical properties with respect to water content of gelatin films in glassy state. Polymer. 46: 12577-12585. [DOI: 10.1016/j.polymer.2005.10.090]
Yarnpakdee S., Benjakul, S., Kingwascharapong P. (2015). Physico-chemical and gel properties of agar from Gracilaria tenuistipitata from the lake of Songkhla, Thailand. Food Hydrocolloids. 51: 217-226. [DOI: 10.1016/j.foodhyd.2015.05.004]
Zhang F., Xu S., Wang Z. (2011). Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food and Bioproducts Processing. 89: 185-193. [DOI: 10.1016/j. fbp.2010.05.003]
Zhou P., Regenstein J.M. (2005). Effects of alkaline and acid pretreatments on Alaska pollock skin gelatin extraction. Journal of Food Science. 70: c392-c396. [DOI: 10.1111/j.1365-2621.2005.tb11435.x]
Conclusion
This research on the quality attributes of deaminated and agar-modified fish gelatin demonstrated notable improvements in key physicochemical parameters, including gel strength, melting point, molecular weight profile, FTIR spectrum, and isoelectric point. The findings revealed successful interactions between gelatin and agar, with the agar-modified formulation recognized as the most effective treatment. The gelatin/agar mixture showed the highest gel strength and melting point, which can be attributed to the reduced isoelectric point of the modified gelatin, facilitating closer molecular packing and the formation of stronger hydrogen bonds. Consequently, these structural modifications expand the potential for fish gelatin utilization across diverse industrial applications.
Author contributions
F.S. conceptualized and wrote the original draft; F.S. and Z.B. conceived and designed the analysis; Z.B. and L.M. contributed data or analysis tools; L.M. performed the analysis. All authors read and approved the final manuscript.
Acknowledgments
This research work was supported by the Faculty of Nutrition and Food Industry, Shahid Beheshti University of Medical Sciences.
Conflicts of interest
The authors declare that there is no conflict of interests.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-profit sectors.
Ethical consideration
Not applicable.
References
Abedi E., Pourmohammadi K. (2021). Chemical modifications and their effects on gluten protein: an extensive review. Food Chemistry. 343: 128398. [DOI: 10.1016/j.foodchem.2020. 128398]
Aewsiri T., Benjakul S., Visessanguan W., Tanaka M. (2008). Chemical compositions and functional properties of gelatin from pre‐cooked tuna fin. International Journal of Food Science and Technology. 43: 685-693. [DOI: 10.1111/j.1365-2621.2006. 01509.x]
Aewsiri T., Benjakul S., Visessanguan W., Wierenga P.A., Gruppen H. (2013). Emulsifying property and antioxidative activity of cuttlefish skin gelatin modified with oxidized linoleic acid and oxidized tannic acid. Food and Bioprocess Technology. 6: 870-881. [DOI: 10.1007/s11947-011-0636-1]
Ahmed J. (2017). Rheological properties of gelatin and advances in measurement. In: Ahmed J. (Editor). Advances in food rheology and its applications. Woodhead Publishing, Cambridge, UK. pp: 377-404. [DOI:10.1016/B978-0-08-100431-9.00015-2]
Asih I.D., Kemala T., Nurilmala M. (2019). Halal gelatin extraction from Patin fish bone (Pangasius hypophthalmus) by-product with ultrasound-assisted extraction. IOP Conference Series: Earth and Environmental Science. 299: 012061. [DOI: 10.1088/1755-1315/299/1/012061]
Badii F., Howell N.K. (2006). Fish gelatin: structure, gelling properties and interaction with egg albumen proteins. Food Hydrocolloids. 20: 630-640. [DOI: 10.1016/j.foodhyd. 2005.06.006]
Behnam S., Taheri A., Kakoei H. (2010). improvement of lizard fish (saurida tumbil) skin gelatin properties by the coenhancers magnesium sulphate, glycerol, katira, sucrose and ammonium nitrate. Iranian Journal of Food Science and Technology. 7: 21-32.
Binsi P.K., Shamasundar B.A., Dileep A.O., Badii F., Howell N.K. (2009). Rheological and functional properties of gelatin from the skin of Bigeye snapper (Priacanthus hamrur) fish: Influence of gelatin on the gel-forming ability of fish mince. Food Hydrocolloids. 23: 132-145. [DOI: 10.1016/j. foodhyd.2007.12.004]
Boran G., Regenstein J.M. (2010). Fish gelatin. Advances in Food and Nutrition Research. 60: 119-143. [DOI: 10.1016/S1043-4526(10)60005-8]
Cabra V., Arreguin R., Vazquez-Duhalt R., Farres A. (2007). Effect of alkaline deamidation on the structure, surface hydrophobicity, and emulsifying properties of the Z19 α-zein. Journal of Agricultural and Food Chemistry. 55: 439-445. [DOI: 10.1021/ jf061002r]
Chen X., Fu W., Luo Y., Cui C., Suppavorasatit I., Liang L. (2021). Protein deamidation to produce processable ingredients and engineered colloids for emerging food applications. Comprehensive Reviews in Food Science and Food Safety. 20: 3788-3817. [DOI: 10.1111/1541-4337.12759]
Cheow C.S., Norizah M.S., Kyaw Z.Y., Howell N.K. (2007). Preparation and characterisation of gelatins from the skins of sin croaker (Johnius dussumieri) and shortfin scad (Decapterus macrosoma). Food Chemistry. 101: 386-391. [DOI: 10.1016/J.Foodchem.2006.01.046]
Cho S., Ahn J-R., Koo J-S, Kim S-B. (2014). Physicochemical properties of gelatin from jellyfish Rhopilema hispidum. Fisheries and Aquatic Sciences. 17: 299-304. [DOI:10.5657/FAS.2014.0299]
Cole C.G.B. (2000). Gelatin. Encyclopedia of food science and technology. 2nd Edition. John Wiley and Sons, New York, NY. pp: 1183-1188.
Derkach S.R., Kuchina Y.A., Baryshnikov A.V., Kolotova D.S., Voron’ko N.G. (2019). Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers. 11: 1724. [DOI: 10.3390/polym11101724]
Derkach S.R., Voron’ko N.G., Kuchina Y.A. (2022). Intermolecular interactions in the formation of polysaccharide-gelatin complexes: a spectroscopic study. Polymers. 14: 2777. [DOI: 10.3390/polym14142777]
Diftis N.G., Pirzas T.A., Kiosseoglou V.D. (2005). Emulsifying properties of gelatin conjugated to pectin under alkaline conditions. Journal of the Science of Food and Agriculture. 85: 804-808. [DOI: 10.1002/jsfa.2029]
Djabourov M., Clark A.H., Rowlands D.W., Ross-Murphy S.B. (1989). Small-angle x-ray scattering characterization of agarose sols and gels. Macromolecules. 22: 180-188. [DOI: 10.1021/ma00191a035]
Doublier J.-L., Garnier C., Renard D., Sanchez C. (2000). Protein–polysaccharide interactions. Current Opinion in Colloid and Interface Science. 5: 202-214. [DOI: 10.1016/S1359-0294(00)00054-6]
Enrione J., Char C., Pepczynska M., Padilla C., González-Muñoz A., Olguín Y., Quinzio C., Iturriaga L., Díaz-Calderón P. (2020). Rheological and structural study of salmon gelatin with controlled molecular weight. Polymers. 12: 1587. [DOI: 10.3390/polym12071587]
Foegeding E.A., Davis J.P. (2011). Food protein functionality: a comprehensive approach. Food Hydrocolloids. 25: 1853-1864. [DOI: 10.1016/j.foodhyd.2011.05.008]
Hamada J.S., Swanson B. (1994). Deamidation of food proteins to improve functionality. Critical Reviews in Food Science and Nutrition. 34: 283-292. [DOI: 10.1080/10408399409527664]
Karim A.A., Bhat R. (2009). Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids. 23: 563-576. [DOI: 10.1016/j.foodhyd. 2008.07.002]
Koli J.M., Basu S., Venkteshwarlu G., Choukasy M.K., Nayak B.B., (2013). Optimization of fish gelatin extraction from skins and bones: a comparative study. Ecology Environment and Conservation. 19: 47-56.
Kumar D.P., Chandra M.V., Elavarasan K., Shamasundar B. (2017). Structural properties of gelatin extracted from croaker fish (Johnius sp) skin waste. International Journal of Food Properties. 20: S2612-S2625. [DOI: 10.1080/10942912. 2017.1381702]
Liao L., Liu T.-X., Zhao M.-M., Cui C., Yuan B.-E., Tang S.,Yang F. (2010). Functional, nutritional and conformational changes from deamidation of wheat gluten with succinic acid and citric acid. Food Chemistry. 123: 123-130. [DOI: 10.1016/j.foodchem. 2010.04.017]
Lv L.-C., Huang Q.-Y., Ding W., Xiao X.-H., Zhang H.-Y., Xiong L.-X. ( 2019). Fish gelatin: the novel potential applications. Journal of Functional Foods. 63: 103581. [DOI: 10.1016/ j.jff.2019.103581]
Mirmoghtadaie L., Kadivar M., Shahedi M. (2009). Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chemistry. 114: 127-131. [DOI: 10.1016/j.foodchem.2008.09.025]
Mohajer S., Rezaei M., Hosseini S.F. (2017). Physico-chemical and microstructural properties of fish gelatin/agar bio-based blend films. Carbohydrate Polymers. 157: 784-793. [DOI: 10.1016/ j.carbpol.2016.10.061]
Muyonga J., Cole C.G.B., Duodu K.G. (2004). Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocolloids. 18: 581-592. [DOI: 10.1016/j.foodhyd.2003.08.009]
Nagarajan M., Benjakul S., Prodpran T., Songtipya P., Kishimura H. (2012). Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocolloids. 29: 389-397. [DOI: 10.1016/j.foodhyd.2012.04.001]
Norziah M.H., Kee H.Y., Norita M. (2014). Response surface optimization of bromelain-assisted gelatin extraction from surimi processing wastes. Food Bioscience. 5: 9-18. [DOI: 10.1016/j. fbio.2013.10.001]
Ohtsuka T., Umezawa Y., Nio N., Kubota K. (2001). Comparison of deamidation activity of transglutaminases. Journal of Food Science. 66: 25-29. [DOI: 10.1111/j.1365-2621.2001.tb15576.x]
Paraman I., Hettiarachchy N.S., Schaefer C. (2007). Glycosylation and deamidation of rice endosperm protein for improved solubility and emulsifying properties. Cereal Chemistry. 84: 593-599. [DOI: 10.1094/CCHEM-84-6-0593]
Qiu C., Sun W., Cui C., Zhao M. (2013). Effect of citric acid deamidation on in vitro digestibility and antioxidant properties of wheat gluten. Food Chemistry. 141: 2772-2778. [DOI: 10.1016/j.foodchem.2013.05.072]
Rawdkuen S., Sai-Ut S., Benjakul S. (2010). Properties of gelatin films from giant catfish skin and bovine bone: a comparative study. European Food Research and Technology. 231: 907-916. [DOI: 10.1007/s00217-010-1340-5]
Romero J.B., Villanueva R.D., Montaño M.N.E. (2008). Stability of agar in the seaweed Gracilaria eucheumatoides (Gracilariales, Rhodophyta) during postharvest storage. Bioresource Technology. 99: 8151-8155. [DOI: 10.1016/j.biortech. 2008.03. 017]
Saxena A., Tahir A., Kaloti M., Ali J., Bohidar H.B. (2011). Effect of agar—gelatin compositions on the release of salbutamol tablets. International Journal of Pharmaceutical Investigation. 1: 93-98. [DOI: 10.4103/2230-973X.82407]
Sha X.-M., Hu Z.-Z., Ye Y.-H., Xu H., Tu Z.-C. (2019). Effect of extraction temperature on the gelling properties and identification of porcine gelatin. Food Hydrocolloids. 92: 163-172. [DOI:10.1016/j.foodhyd.2019.01.059]
Shakila R.J., Jeevithan E., Varatharajakumar A., Jeyasekaran G., Sukumar D. (2012). Functional characterization of gelatin extracted from bones of red snapper and grouper in comparison with mammalian gelatin. LWT-Food Science and Technology. 48: 30-36. [DOI: 10.1016/j.lwt.2012.03.007]
Singh S.S., Bohidar H.B., Bandyopadhyay S. (2007). Study of gelatin–agar intermolecular aggregates in the supernatant of its coacervate. Colloids and Surfaces B: Biointerfaces. 57: 29-36. [DOI: 10.1016/j.colsurfb.2006.12.017]
Sinthusamran S., Benjakul S., Hemar Y. (2016). Rheological and sensory properties of fish gelatin gels as influenced by agar from Gracilaria tenuistipitata. International Journal of Food Science and Technology. 51: 1530-1536. [DOI: 10.1111/ijfs.13117]
Somboon N., Karrila T.T., Kaewmanee T., Karrila S.J. (2014). Properties of gels from mixed agar and fish gelatin. International Food Research Journal. 21: 485.
Tabarestani H.S., Maghsoudlou Y., Motamedzadegan A., Sadeghi Mahoonak A.R. (2010). Optimization of physico-chemical properties of gelatin extracted from fish skin of rainbow trout (Onchorhynchus mykiss). Bioresource Technology. 101: 6207-6214. [DOI: 10.1016/j.biortech.2010.02.071]
Tavakolipour H. (2011). Extraction and evaluation of gelatin from silver carp waste. World Journal of Fish and Marine Sciences. 3: 10-15.
Tavernier B.H. (1989). Molecular mass distribution of gelatin and physical properties. Photographic Gelatin Proceedings. 1: 217-228.
Toyama Y., Sahara R., Iino Y., Kubota K. (2011). pH dependence of rheological properties of gelatin gel mixed with agar or agarose. Transactions of the Materials Research Society of Japan. 36: 383-386. [DOI: 10.14723/tmrsj.36.383]
Tu Z.-C., Huang T., Wang H., Sha X.-M., Shi Y., Huang X.-Q., Man Z.-Z., Li D.-J. (2015). Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. Journal of Food Science and Technology. 52: 2166-2174. [DOI: 10.1007/s13197-013-1239-9]
Voron’ko N.G., Derkach S.R., Kuchina Y.A., Sokolan N.I. (2016). The chitosan–gelatin (bio) polyelectrolyte complexes formation in an acidic medium. Carbohydrate Polymers. 138: 265-272. [DOI: 10.1016/j.carbpol.2015.11.059]
Wakhet S., Singh V.K., Sahoo S., Sagiri S.S., Kulanthaivel S., Bhattacharya M.K., Kumar N., Banerjee I., Pal K. (2015). Characterization of gelatin–agar based phase separated hydrogel, emulgel and bigel: a comparative study. Journal of Materials Science: Materials in Medicine. 26: 118. [DOI: 10.1007/s10856-015-5434-2]
Wangtueai S., Noomhorm A. (2009). Processing optimization and characterization of gelatin from lizardfish (Saurida spp.) scales. LWT-Food Science and Technology. 42: 825-834. [DOI: 10.1016/j.lwt.2008.11.014]
Yakimets I., Wellner N., Smith A.C., Wilson R.H., Farhat I., Mitchell J. (2005). Mechanical properties with respect to water content of gelatin films in glassy state. Polymer. 46: 12577-12585. [DOI: 10.1016/j.polymer.2005.10.090]
Yarnpakdee S., Benjakul, S., Kingwascharapong P. (2015). Physico-chemical and gel properties of agar from Gracilaria tenuistipitata from the lake of Songkhla, Thailand. Food Hydrocolloids. 51: 217-226. [DOI: 10.1016/j.foodhyd.2015.05.004]
Zhang F., Xu S., Wang Z. (2011). Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food and Bioproducts Processing. 89: 185-193. [DOI: 10.1016/j. fbp.2010.05.003]
Zhou P., Regenstein J.M. (2005). Effects of alkaline and acid pretreatments on Alaska pollock skin gelatin extraction. Journal of Food Science. 70: c392-c396. [DOI: 10.1111/j.1365-2621.2005.tb11435.x]
*Corresponding author (Z. Bahmani)
* E-mail: zabihbahmani@gmail.com
ORCID ID: https://orcid.org/0000-0001-6569-5577
* E-mail: zabihbahmani@gmail.com
ORCID ID: https://orcid.org/0000-0001-6569-5577
Type of Study: Original article |
Subject:
Special
Received: 24/09/01 | Accepted: 25/07/20 | Published: 25/09/30
Received: 24/09/01 | Accepted: 25/07/20 | Published: 25/09/30
References
1. Abedi E., Pourmohammadi K. (2021). Chemical modifications and their effects on gluten protein: an extensive review. Food Chemistry. 343: 128398. [DOI: 10.1016/j.foodchem.2020. 128398] [DOI:10.1016/j.foodchem.2020.128398] [PMID]
2. Aewsiri T., Benjakul S., Visessanguan W., Tanaka M. (2008). Chemical compositions and functional properties of gelatin from pre‐cooked tuna fin. International Journal of Food Science and Technology. 43: 685-693. [DOI: 10.1111/j.1365-2621.2006. 01509.x] [DOI:10.1111/j.1365-2621.2006.01509.x]
3. Aewsiri T., Benjakul S., Visessanguan W., Wierenga P.A., Gruppen H. (2013). Emulsifying property and antioxidative activity of cuttlefish skin gelatin modified with oxidized linoleic acid and oxidized tannic acid. Food and Bioprocess Technology. 6: 870-881. [DOI: 10.1007/s11947-011-0636-1] [DOI:10.1007/s11947-011-0636-1]
4. Ahmed J. (2017). Rheological properties of gelatin and advances in measurement. In: Ahmed J. (Editor). Advances in food rheology and its applications. Woodhead Publishing, Cambridge, UK. pp: 377-404. [DOI:10.1016/B978-0-08-100431-9.00015-2] [DOI:10.1016/B978-0-08-100431-9.00015-2]
5. Asih I.D., Kemala T., Nurilmala M. (2019). Halal gelatin extraction from Patin fish bone (Pangasius hypophthalmus) by-product with ultrasound-assisted extraction. IOP Conference Series: Earth and Environmental Science. 299: 012061. [DOI: 10.1088/1755-1315/299/1/012061] [DOI:10.1088/1755-1315/299/1/012061]
6. Badii F., Howell N.K. (2006). Fish gelatin: structure, gelling properties and interaction with egg albumen proteins. Food Hydrocolloids. 20: 630-640. [DOI: 10.1016/j.foodhyd. 2005.06.006] [DOI:10.1016/j.foodhyd.2005.06.006]
7. Behnam S., Taheri A., Kakoei H. (2010). improvement of lizard fish (saurida tumbil) skin gelatin properties by the coenhancers magnesium sulphate, glycerol, katira, sucrose and ammonium nitrate. Iranian Journal of Food Science and Technology. 7: 21-32.
8. Binsi P.K., Shamasundar B.A., Dileep A.O., Badii F., Howell N.K. (2009). Rheological and functional properties of gelatin from the skin of Bigeye snapper (Priacanthus hamrur) fish: Influence of gelatin on the gel-forming ability of fish mince. Food Hydrocolloids. 23: 132-145. [DOI: 10.1016/j. foodhyd.2007.12.004] [DOI:10.1016/j.foodhyd.2007.12.004]
9. Boran G., Regenstein J.M. (2010). Fish gelatin. Advances in Food and Nutrition Research. 60: 119-143. [DOI: 10.1016/S1043-4526(10)60005-8] [DOI:10.1016/S1043-4526(10)60005-8] [PMID]
10. Cabra V., Arreguin R., Vazquez-Duhalt R., Farres A. (2007). Effect of alkaline deamidation on the structure, surface hydrophobicity, and emulsifying properties of the Z19 α-zein. Journal of Agricultural and Food Chemistry. 55: 439-445. [DOI: 10.1021/ jf061002r] [DOI:10.1021/jf061002r] [PMID]
11. Chen X., Fu W., Luo Y., Cui C., Suppavorasatit I., Liang L. (2021). Protein deamidation to produce processable ingredients and engineered colloids for emerging food applications. Comprehensive Reviews in Food Science and Food Safety. 20: 3788-3817. [DOI: 10.1111/1541-4337.12759] [DOI:10.1111/1541-4337.12759] [PMID]
12. Cheow C.S., Norizah M.S., Kyaw Z.Y., Howell N.K. (2007). Preparation and characterisation of gelatins from the skins of sin croaker (Johnius dussumieri) and shortfin scad (Decapterus macrosoma). Food Chemistry. 101: 386-391. [DOI: 10.1016/J.Foodchem.2006.01.046] [DOI:10.1016/j.foodchem.2006.01.046]
13. Cho S., Ahn J-R., Koo J-S, Kim S-B. (2014). Physicochemical properties of gelatin from jellyfish Rhopilema hispidum. Fisheries and Aquatic Sciences. 17: 299-304. [DOI:10.5657/FAS.2014.0299] [DOI:10.5657/FAS.2014.0299]
14. Cole C.G.B. (2000). Gelatin. Encyclopedia of food science and technology. 2nd Edition. John Wiley and Sons, New York, NY. pp: 1183-1188.
15. Derkach S.R., Kuchina Y.A., Baryshnikov A.V., Kolotova D.S., Voron'ko N.G. (2019). Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers. 11: 1724. [DOI: 10.3390/polym11101724] [DOI:10.3390/polym11101724] [PMID] [PMCID]
16. Derkach S.R., Voron'ko N.G., Kuchina Y.A. (2022). Intermolecular interactions in the formation of polysaccharide-gelatin complexes: a spectroscopic study. Polymers. 14: 2777. [DOI: 10.3390/polym14142777] [DOI:10.3390/polym14142777] [PMID] [PMCID]
17. Diftis N.G., Pirzas T.A., Kiosseoglou V.D. (2005). Emulsifying properties of gelatin conjugated to pectin under alkaline conditions. Journal of the Science of Food and Agriculture. 85: 804-808. [DOI: 10.1002/jsfa.2029] [DOI:10.1002/jsfa.2029]
18. Djabourov M., Clark A.H., Rowlands D.W., Ross-Murphy S.B. (1989). Small-angle x-ray scattering characterization of agarose sols and gels. Macromolecules. 22: 180-188. [DOI: 10.1021/ma00191a035] [DOI:10.1021/ma00191a035]
19. Doublier J.-L., Garnier C., Renard D., Sanchez C. (2000). Protein-polysaccharide interactions. Current Opinion in Colloid and Interface Science. 5: 202-214. [DOI: 10.1016/S1359-0294(00)00054-6] [DOI:10.1016/S1359-0294(00)00054-6]
20. Enrione J., Char C., Pepczynska M., Padilla C., González-Muñoz A., Olguín Y., Quinzio C., Iturriaga L., Díaz-Calderón P. (2020). Rheological and structural study of salmon gelatin with controlled molecular weight. Polymers. 12: 1587. [DOI: 10.3390/polym12071587] [DOI:10.3390/polym12071587] [PMID] [PMCID]
21. Foegeding E.A., Davis J.P. (2011). Food protein functionality: a comprehensive approach. Food Hydrocolloids. 25: 1853-1864. [DOI: 10.1016/j.foodhyd.2011.05.008] [DOI:10.1016/j.foodhyd.2011.05.008]
22. Hamada J.S., Swanson B. (1994). Deamidation of food proteins to improve functionality. Critical Reviews in Food Science and Nutrition. 34: 283-292. [DOI: 10.1080/10408399409527664] [DOI:10.1080/10408399409527664] [PMID]
23. Karim A.A., Bhat R. (2009). Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids. 23: 563-576. [DOI: 10.1016/j.foodhyd. 2008.07.002] [DOI:10.1016/j.foodhyd.2008.07.002]
24. Koli J.M., Basu S., Venkteshwarlu G., Choukasy M.K., Nayak B.B., (2013). Optimization of fish gelatin extraction from skins and bones: a comparative study. Ecology Environment and Conservation. 19: 47-56.
25. Kumar D.P., Chandra M.V., Elavarasan K., Shamasundar B. (2017). Structural properties of gelatin extracted from croaker fish (Johnius sp) skin waste. International Journal of Food Properties. 20: S2612-S2625. [DOI: 10.1080/10942912. 2017.1381702] [DOI:10.1080/10942912.2017.1381702]
26. Liao L., Liu T.-X., Zhao M.-M., Cui C., Yuan B.-E., Tang S.,Yang F. (2010). Functional, nutritional and conformational changes from deamidation of wheat gluten with succinic acid and citric acid. Food Chemistry. 123: 123-130. [DOI: 10.1016/j.foodchem. 2010.04.017] [DOI:10.1016/j.foodchem.2010.04.017]
27. Lv L.-C., Huang Q.-Y., Ding W., Xiao X.-H., Zhang H.-Y., Xiong L.-X. ( 2019). Fish gelatin: the novel potential applications. Journal of Functional Foods. 63: 103581. [DOI: 10.1016/ j.jff.2019.103581] [DOI:10.1016/j.jff.2019.103581]
28. Mirmoghtadaie L., Kadivar M., Shahedi M. (2009). Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chemistry. 114: 127-131. [DOI: 10.1016/j.foodchem.2008.09.025] [DOI:10.1016/j.foodchem.2008.09.025]
29. Mohajer S., Rezaei M., Hosseini S.F. (2017). Physico-chemical and microstructural properties of fish gelatin/agar bio-based blend films. Carbohydrate Polymers. 157: 784-793. [DOI: 10.1016/ j.carbpol.2016.10.061] [DOI:10.1016/j.carbpol.2016.10.061] [PMID]
30. Muyonga J., Cole C.G.B., Duodu K.G. (2004). Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocolloids. 18: 581-592. [DOI: 10.1016/j.foodhyd.2003.08.009] [DOI:10.1016/j.foodhyd.2003.08.009]
31. Nagarajan M., Benjakul S., Prodpran T., Songtipya P., Kishimura H. (2012). Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocolloids. 29: 389-397. [DOI: 10.1016/j.foodhyd.2012.04.001] [DOI:10.1016/j.foodhyd.2012.04.001]
32. Norziah M.H., Kee H.Y., Norita M. (2014). Response surface optimization of bromelain-assisted gelatin extraction from surimi processing wastes. Food Bioscience. 5: 9-18. [DOI: 10.1016/j. fbio.2013.10.001] [DOI:10.1016/j.fbio.2013.10.001]
33. Ohtsuka T., Umezawa Y., Nio N., Kubota K. (2001). Comparison of deamidation activity of transglutaminases. Journal of Food Science. 66: 25-29. [DOI: 10.1111/j.1365-2621.2001.tb15576.x] [DOI:10.1111/j.1365-2621.2001.tb15576.x]
34. Paraman I., Hettiarachchy N.S., Schaefer C. (2007). Glycosylation and deamidation of rice endosperm protein for improved solubility and emulsifying properties. Cereal Chemistry. 84: 593-599. [DOI: 10.1094/CCHEM-84-6-0593] [DOI:10.1094/CCHEM-84-6-0593]
35. Qiu C., Sun W., Cui C., Zhao M. (2013). Effect of citric acid deamidation on in vitro digestibility and antioxidant properties of wheat gluten. Food Chemistry. 141: 2772-2778. [DOI: 10.1016/j.foodchem.2013.05.072] [DOI:10.1016/j.foodchem.2013.05.072] [PMID]
36. Rawdkuen S., Sai-Ut S., Benjakul S. (2010). Properties of gelatin films from giant catfish skin and bovine bone: a comparative study. European Food Research and Technology. 231: 907-916. [DOI: 10.1007/s00217-010-1340-5] [DOI:10.1007/s00217-010-1340-5]
37. Romero J.B., Villanueva R.D., Montaño M.N.E. (2008). Stability of agar in the seaweed Gracilaria eucheumatoides (Gracilariales, Rhodophyta) during postharvest storage. Bioresource Technology. 99: 8151-8155. [DOI: 10.1016/j.biortech. 2008.03. 017] [DOI:10.1016/j.biortech.2008.03.017] [PMID]
38. Saxena A., Tahir A., Kaloti M., Ali J., Bohidar H.B. (2011). Effect of agar-gelatin compositions on the release of salbutamol tablets. International Journal of Pharmaceutical Investigation. 1: 93-98. [DOI: 10.4103/2230-973X.82407] [DOI:10.4103/2230-973X.82407] [PMID] [PMCID]
39. Sha X.-M., Hu Z.-Z., Ye Y.-H., Xu H., Tu Z.-C. (2019). Effect of extraction temperature on the gelling properties and identification of porcine gelatin. Food Hydrocolloids. 92: 163-172. [DOI:10.1016/j.foodhyd.2019.01.059] [DOI:10.1016/j.foodhyd.2019.01.059]
40. Shakila R.J., Jeevithan E., Varatharajakumar A., Jeyasekaran G., Sukumar D. (2012). Functional characterization of gelatin extracted from bones of red snapper and grouper in comparison with mammalian gelatin. LWT-Food Science and Technology. 48: 30-36. [DOI: 10.1016/j.lwt.2012.03.007] [DOI:10.1016/j.lwt.2012.03.007]
41. Singh S.S., Bohidar H.B., Bandyopadhyay S. (2007). Study of gelatin-agar intermolecular aggregates in the supernatant of its coacervate. Colloids and Surfaces B: Biointerfaces. 57: 29-36. [DOI: 10.1016/j.colsurfb.2006.12.017] [DOI:10.1016/j.colsurfb.2006.12.017] [PMID]
42. Sinthusamran S., Benjakul S., Hemar Y. (2016). Rheological and sensory properties of fish gelatin gels as influenced by agar from Gracilaria tenuistipitata. International Journal of Food Science and Technology. 51: 1530-1536. [DOI: 10.1111/ijfs.13117] [DOI:10.1111/ijfs.13117]
43. Somboon N., Karrila T.T., Kaewmanee T., Karrila S.J. (2014). Properties of gels from mixed agar and fish gelatin. International Food Research Journal. 21: 485.
44. Tabarestani H.S., Maghsoudlou Y., Motamedzadegan A., Sadeghi Mahoonak A.R. (2010). Optimization of physico-chemical properties of gelatin extracted from fish skin of rainbow trout (Onchorhynchus mykiss). Bioresource Technology. 101: 6207-6214. [DOI: 10.1016/j.biortech.2010.02.071] [DOI:10.1016/j.biortech.2010.02.071] [PMID]
45. Tavakolipour H. (2011). Extraction and evaluation of gelatin from silver carp waste. World Journal of Fish and Marine Sciences. 3: 10-15.
46. Tavernier B.H. (1989). Molecular mass distribution of gelatin and physical properties. Photographic Gelatin Proceedings. 1: 217-228.
47. Toyama Y., Sahara R., Iino Y., Kubota K. (2011). pH dependence of rheological properties of gelatin gel mixed with agar or agarose. Transactions of the Materials Research Society of Japan. 36: 383-386. [DOI: 10.14723/tmrsj.36.383] [DOI:10.14723/tmrsj.36.383]
48. Tu Z.-C., Huang T., Wang H., Sha X.-M., Shi Y., Huang X.-Q., Man Z.-Z., Li D.-J. (2015). Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. Journal of Food Science and Technology. 52: 2166-2174. [DOI: 10.1007/s13197-013-1239-9] [DOI:10.1007/s13197-013-1239-9] [PMID] [PMCID]
49. Voron'ko N.G., Derkach S.R., Kuchina Y.A., Sokolan N.I. (2016). The chitosan-gelatin (bio) polyelectrolyte complexes formation in an acidic medium. Carbohydrate Polymers. 138: 265-272. [DOI: 10.1016/j.carbpol.2015.11.059] [DOI:10.1016/j.carbpol.2015.11.059] [PMID]
50. Wakhet S., Singh V.K., Sahoo S., Sagiri S.S., Kulanthaivel S., Bhattacharya M.K., Kumar N., Banerjee I., Pal K. (2015). Characterization of gelatin-agar based phase separated hydrogel, emulgel and bigel: a comparative study. Journal of Materials Science: Materials in Medicine. 26: 118. [DOI: 10.1007/s10856-015-5434-2] [DOI:10.1007/s10856-015-5434-2] [PMID]
51. Wangtueai S., Noomhorm A. (2009). Processing optimization and characterization of gelatin from lizardfish (Saurida spp.) scales. LWT-Food Science and Technology. 42: 825-834. [DOI: 10.1016/j.lwt.2008.11.014] [DOI:10.1016/j.lwt.2008.11.014]
52. Yakimets I., Wellner N., Smith A.C., Wilson R.H., Farhat I., Mitchell J. (2005). Mechanical properties with respect to water content of gelatin films in glassy state. Polymer. 46: 12577-12585. [DOI: 10.1016/j.polymer.2005.10.090] [DOI:10.1016/j.polymer.2005.10.090]
53. Yarnpakdee S., Benjakul, S., Kingwascharapong P. (2015). Physico-chemical and gel properties of agar from Gracilaria tenuistipitata from the lake of Songkhla, Thailand. Food Hydrocolloids. 51: 217-226. [DOI: 10.1016/j.foodhyd.2015.05.004] [DOI:10.1016/j.foodhyd.2015.05.004]
54. Zhang F., Xu S., Wang Z. (2011). Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food and Bioproducts Processing. 89: 185-193. [DOI: 10.1016/j. fbp.2010.05.003] [DOI:10.1016/j.fbp.2010.05.003]
55. Zhou P., Regenstein J.M. (2005). Effects of alkaline and acid pretreatments on Alaska pollock skin gelatin extraction. Journal of Food Science. 70: c392-c396. [DOI: 10.1111/j.1365-2621.2005.tb11435.x] [DOI:10.1111/j.1365-2621.2005.tb11435.x]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







