Volume 12, Issue 1 (March 2025)
J. Food Qual. Hazards Control 2025, 12(1): 37-45 |
Back to browse issues page
Ethics code: Not applicable
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abbasi S, Rafati A, Hosseini S, Roohinejad S, Hahshemi S, Hashemi H. Effects of Environmental Stress on the Viability of Lactobacillus plantarum Encapsulated in Double Emulsions. J. Food Qual. Hazards Control 2025; 12 (1) :37-45
URL: http://jfqhc.ssu.ac.ir/article-1-1188-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1188-en.html
Department of Food Science and Technology, Sarv. C., Islamic Azad University, Sarvestan, Iran , alireza_rafati@iau.ac.ir
Full-Text [PDF 536 kb]
(529 Downloads)
| Abstract (HTML) (769 Views)
Table 1: Effects of food heat processing on the viability of probiotic Lactobacillus plantarum
encapsulated in a double water/oil/water emulsion system
Table 2: Effects of NaCl concentration on the viability of probiotic Lactobacillus plantarum encapsulated in a double
water/oil/water emulsion system
Table 3: Effect of lysozyme, penicillin, and bile salt on the viability of probiotic Lactobacillus plantarum encapsulated in a double water/oil/water emulsion system
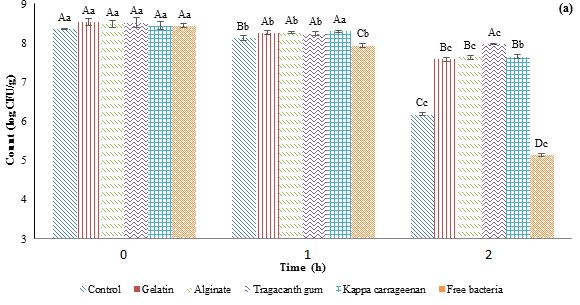
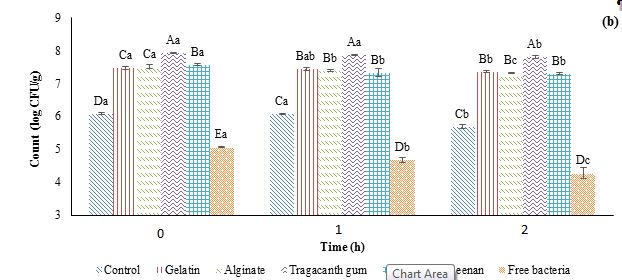
Figure 1: Effects of simulated gastric (a) and intestinal (b) conditions on the viability of probiotic Lactobacillus plantarum encapsulated in a double water/oil/water emulsion system. CFU=Colony Forming Unit.
Different capital letters indicate statistically significant differences (p˂0.05) between samples at the same time and different small letters indicate statistically significant differences (p˂0.05) during the time.
Full-Text: (217 Views)
Effects of Environmental Stress on the Viability of Lactobacillus plantarum Encapsulated in Double Emulsions
S. Abbasi 1, A. Rafati [1]** , S.M.H. Hosseini 2, S. Roohinejad 3, S.-S. Hahshemi 3, H. Hashemi 2
1. Department of Food Science and Technology, Sarv. C., Islamic Azad University, Sarvestan, Iran.
2. Department of Food Science and Technology, School of Agriculture, Shiraz University, Shiraz, Iran.
3. Burn and Wound Healing Research Center, Shiraz University of Medical Science, Shiraz, Iran.
S. Abbasi 1, A. Rafati [1]**
1. Department of Food Science and Technology, Sarv. C., Islamic Azad University, Sarvestan, Iran.
2. Department of Food Science and Technology, School of Agriculture, Shiraz University, Shiraz, Iran.
3. Burn and Wound Healing Research Center, Shiraz University of Medical Science, Shiraz, Iran.
HIGHLIGHTS
- Double Emulsion is a suitable method for protecting probiotics against harsh conditions.
- Internal water phase gelation improves the viability of probiotics.
- Tragacanth gum exhibited higher protective effect in comparison with gelatin, alginate, and carrageenan.
| Article type Original article |
ABSTRACT Background: The primary objective of encapsulating probiotics is to enhance their survival rate during food processing and the challenging conditions of the gastrointestinal tract. Methods: In this specific investigation, Lactobacillus plantarum was introduced into the Inner aqueous phase (W1) of Double Emulsions (DEs) referred to as Water-in-Oil-in-Water (W1/O/W2). This entrapment process involved inducing a transition from solid to gel state of W1 using gelatin, alginate, tragacanth gum, and carrageenan across multiple samples. The study then explored the resistance of L. plantarum to various environmental pressures, including thermal treatments (such as pasteurization at 72 °C for 40 s, microwave heating at 72 °C for 40 s, and sterilization at 145 °C for 40 s), as well as exposure to sodium chloride (NaCl), bile salt, lysozyme, and penicillin. Additionally, the viability of the encapsulated probiotics was investigated in simulated gastrointestinal conditions. Results: It was found that the sensitivity of free bacterial cells to heat processing was significantly higher compared to encapsulated bacteria. Among the different samples, those containing tragacanth gum exhibited the highest cell viability when subjected to various heat treatments (14.67% reduction for microwave, 13.72% reduction for pasteurization). Furthermore, the study demonstrated that DEs effectively improved the survival of probiotics against NaCl, bile salt, lysozyme, and penicillin. Generally, the gastric conditions (0.55 to 3.30 log Colony Forming Unit (CFU)/g reduction) had a more detrimental impact on probiotic viability compared to the intestinal conditions (0.1 to 0.8 log CFU/g reduction). Conclusion: Ultimately, DE samples containing tragacanth gum in the W1 phase displayed the most effective protective effects. This encapsulation technique holds potential for various applications in dairy, meat, and other fermented products. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Environment Lactobacillus plantarum Emulsions Probiotics. |
||
| Article history Received: 20 Aug 2024 Revised: 02 Nov 2024 Accept: 02 Mar 2025 |
||
| Abbreviations CFU=Colony Forming Unit DEs=Double Emulsions MRS=De Man–Rogosa–Sharpe |
To cite: Abbasi S., Rafati A., Hosseini S.M.H., Roohinejad S., Hahshemi S.-S., Hashemi H. (2025). Effects of environmental stress on the viability of Lactobacillus plantarum encapsulated in double emulsions. Journal of Food Quality and Hazards Control. 12: 37-45.
Introduction
Introduction
The health-promoting properties of probiotics are increasingly attracting attention with respect to food products containing these microorganisms. These live microorganisms are the guests which take place in the human intestine and provide health benefits for their host (Hill et al., 2014). It has been reported that a food product is required to contain at least 107 Colony Forming Unit (CFU)/g of these microorganisms to confer proper health benefits (Frakolaki et al., 2021). Dairy foods including cheese (Ortakci et al., 2012), yogurt (El-Dieb et al., 2012), and ice cream (Zanjani et al., 2018) are the most suitable food products to be enriched with probiotics. Also, several attempts are being made to enrich other types of food products with this microorganism such as baked food products (Irani et al., 2021). However, creating functional encapsulation systems that can effectively shield these microorganisms from the harsh conditions encountered during the baking process poses a significant and complex challenge ( Hosseinialhashemi et al., 2021; Zhang et al., 2018).
A microencapsulation system is a matrix or membrane in which the microorganisms such as probiotics are embedded, thereby increasing their viability in food products or the intestinal tract. These methods include extrusion (Sousa et al., 2015), emulsification (Frakolaki et al., 2021), spray drying (Zhang et al., 2015), freeze drying (Dianawati et al., 2013), complex coacervation (De Almeida Paula et al., 2019), ionic gelation (Song et al., 2013), and electrostatic deposition (Anselmo et al., 2016). It is noteworthy that there are still some challenges assigned to these techniques such as low stability during storage (Weinbreck et al., 2010), decreasing liability during the thermal process and in vitro digestion, as well the particle size which is responsible to sensory reception of food products including these systems (Nag, 2011).
The substitution of certain portions of oil with water in Oil-in-Water (O/W) emulsions allows for the incorporation of diverse functional compounds in Water-in-Oil-in-Water (W/O/W) emulsions. Consequently, this makes it an appropriate choice for the development of functional O/W emulsions ( Abbasi et al., 2023; Berendsen et al., 2015; Frank et al., 2012; Giroux et al., 2013; Jiménez-Colmenero, 2013) and fat replacers (Cofrades et al., 2013; Jiménez-Colmenero, 2013). Two-step emulsification is a common method to prepare double emulsions in form of W/O/W emulsion (Balcaen et al., 2016; Koubaa et al., 2018b). The first stage involves utilizing high shear forces to create a Water-in-Oil (W/O) emulsion. Subsequently, lower shear forces are employed to ensure the stability and integrity of the internal water droplets, preventing them from collapsing or dispersing. Consequently, the second step results in coarse droplets and broad distribution of particle size in double emulsions (Koubaa et al., 2018a; Oppermann et al., 2015). Unexpectedly, the droplet size of the resulting double emulsion is somehow responsible for the volume of the water which is retained after the second step, and this is independent of the emulsification device (Schuch et al., 2014).
The emulsions are wide-spreading encapsulation systems that are represented by their scalability, small-scale carriers, and enhancing the viability of the probiotic ( Liu et al., 2019; Manojlović et al., 2010). W1/O/W2 emulsion is a Double Emulsion (DE) that includes oil globules containing water droplets (W1 or interior aqueous phase), and the oil globules are simultaneously dispersed in water as exterior aqueous phase or W2. W1 is a potent environment to embed the probiotics due to their small-scale size (less than one µm) (De Almeida Paula et al., 2019). Several works of literature have reported that double emulsions are proper devices to enhance the viability of these cells during various processes (El Kadri et al., 2018; Hernández-Rodríguez et al., 2014) and digestion (Pimentel-González et al., 2009; Shima et al., 2006, 2009; Wang et al., 2020). It is reported that double emulsions containing Lactobacillus plantarum and L. paracasei resulted in the maintenance of viable cells in high quantities after the production process of Oaxaca cheese (Rodríguez-Huezo et al., 2014), yogurt (El Kadri et al., 2018), as well as under in-vitro digestion condition. Also, when L. rhamnosus and L. acidophilus were encapsulated in a similar encapsulation system, the system showed survival effects during in-vitro digestion conditions as well as resulting in an enhancement of colon adhesion of these microorganisms (Pimentel-González et al., 2009; Shima et al., 2006, 2009; Wang et al., 2020).
To the best of our knowledge, there is presently no existing literature examining the impact of internal water phase gelation on the viability of probiotics. Therefore, the primary aim of this research was to evaluate the survival of L. plantarum subsequent to its encapsulation within the W1 of DEs. Additionally, we examined how the gelation of W1, achieved through the use of gelatin, alginate, tragacanth gum, and carrageenan, influenced the survival of probiotic cells. The viability of these cells was evaluated under a range of environmental stress, including heat treatments (such as pasteurization, microwave heating, and sterilization), exposure to sodium chloride (NaCl), bile salt, lysozyme, and penicillin, as well as simulated gastrointestinal conditions.
Materials and methods
Materials
Polyglycerol Polyricinoleate (PGPR) was obtained from Danisco Co. (Copenhagen, Denmark), Tween 80, gelatin, sodium alginate, carrageenan, De Man–Rogosa–Sharpe (MRS) broth, and agar were sourced from Merck Millipore Co. (Darmstadt, Germany). Tragacanth gum was procured from Dena Emulsion Co. (Shiraz, Iran), and olive oil was provided by Oila Company (Tehran, Iran). Others chemicals were of analytical grade.
Microorganism preparation
Initially, a solitary colony from the L. plantarum stock was introduced into MRS broth and left to incubate overnight at 37 °C in a shaker incubator. Following this, 10 ml of the inoculum was combined with 90 ml of fresh MRS broth and incubated at 37 °C for 48 h. The resultant microbial suspension was subjected to centrifugation at 6,000 g for four min at 6 °C (Tantratian and Pradeamchai, 2020). The resulting pellet was subsequently washed three times with sterile saline solution and promptly applied for the encapsulation procedure and production of samples.
Preparation of double emulsions
The W1/O/W2 emulsion was prepared using a two-step technique initially introduced by Bou et al. (2014) with some modifications. At the first W1, O and W2 phases were prepared and then converted to DEs. For the W1 phase, L. plantarum was added into a sterile saline solution at a concentration of 11 log CFU/g. In separate samples, gelatin, alginate, tragacanth gum, and carrageenan were added at a concentration of 2% wt. The alginate sample included calcium chloride (CaCl2) (100 mm), while the carrageenan sample contained potassium chloride (KCl) (100 mm). Following preparation, the W1 phase was stored in tubes to shield it from light. W2 was formed by dissolving Tween 80 (4% w/v) in distilled water. The oil phase (O) comprised PGPR (6% wt) dissolved in olive oil, which was stirred at 65 °C for 20 min. The W1/O primary emulsion was generated by the addition of 20 g of W1 to 80 g of the oil phase (O) and stirring at 500 rpm (35 °C) for 20 min. Subsequently, the emulsion was homogenized using a T25 Ultra-Turrax (IKA, Germany) at 13,000 rpm for three min, followed by an additional three min at 15,000 rpm. To produce the W1/O/W2 emulsions, 40 g of the W1/O primary emulsion was homogenized with 60 g of W2 at 13,000 rpm for four min, followed by 10,000 rpm for another 4 min at 35 °C.
Effect of heat processing
The samples were placed in glass tubes and exposed to different treatments (Soltani Lak et al., 2021). Pasteurization involved subjecting the samples to a temperature of 72 °C for 40 s using a water bath. Microwave heating was performed at 72 °C for 40 s. Sterilization was achieved by exposing the samples to a temperature of 145 °C for 40 s using an oil bath. After each treatment, the samples were rapidly cooled to 4 °C using a cold-water bath. The survivability of L. plantarum was then assessed using the following method. The probiotics count in the samples was measured by a serial dilution method and then culturing on an MRS agar medium at 37 °C for two overnight.
Effect of NaCl concentration
The assessment of the survival of both free L. plantarum and encapsulated L. plantarum in the presence of NaCl was conducted following the methodology described by Soltani Lak et al. (2021) by adding 0, 1, and 3% NaCl to the MRS agar culture. The count of sustention was evaluated after 0, 1, and 2 h by MRS agar method and incubation for three days at 37 °C.
Effects of lysozyme, penicillin, and bile salt
The effectiveness of free and encapsulated L. plantarum against lysozyme, penicillin G, and bile salt was individually assessed following the protocol described by Soltani Lak et al. (2021) . This involved supplementing the MRS agar medium with 1 μg/ml of penicillin G, 5 mg/ml lysozyme, and either 0.5 or 1% w/v of bile salt. Enumeration of bacterial colonies was conducted at 24 h post-exposure.
Effect of simulated gastrointestinal conditions
The impact of simulated gastric and intestinal conditions on the viability of all samples was assessed following the methodology outlined by Moayyedi et al. (2018) with some modifications. To prepare the simulated gastric fluid, a mixture of 0.9% w/v NaCl and 0.3% w/v pepsin was dispersed in deionized water and adjusted to pH 1.5 using one M HCl. Similarly, for the simulated intestinal fluid, a combination of 0.6% w/v bile salt, 0.6% NaCl, and 0.2% w/v pancreatin was added to deionized water and adjusted to pH 6. All solutions underwent sterilization through a 0.22 μm syringe filter. For digestion analysis, 10 ml of sample and 10 ml of the prepared solutions were mixed and shaken at 50 rpm and 37 °C. Enumeration was conducted after 0, 1, and 2 h of digestion on MRS agar plates, followed by incubation for three days at 37 °C.
Statistical analysis
All trials were replicated three times unless stated otherwise. Statistical analysis was carried out using one-way analysis of variance (ANOVA) with a significance level of 0.05. Duncan's multiple range tests were then employed to identify significant differences between the mean values. The analysis was conducted using SAS® software, version 9.1, developed by SAS Institute Inc., NC, USA.
Results and discussion
Viability against food heat processing
Heat processing is a critical aspect of food processing that can influence the survivability of probiotics. The effects of different heat treatments on the free L. plantarum and encapsulated L. plantarum bacteria (by application of gelatin, alginate, tragacanth gum, and carrageenan) are shown in Table 1. These treatments included pasteurization (72 °C for 40 s), microwave heating (72 °C for 40 s), and sterilization (145 °C for 40 s). The survival outcomes against these heat treatments are represented in Table 1. As expected, the various heat treatments exerted distinct effects on the survivability of L. plantarum. For free L. plantarum, the initial count decreased from 8.37 log to 4.68, 4.13, and 0 log following microwave heating, pasteurization, and sterilization, respectively. Notably, sterilization resulted in a complete reduction of bacterial count across all samples. Additionally, the sample containing tragacanth gum demonstrated superior protective effects compared to other samples. In this particular sample, the microbial count decreased from 8.38 to 7.15 log CFU/ml after microwave treatment, whereas pasteurization and sterilization reduced it further to 7.23 and 0 log CFU/ml, respectively. This trend aligns with findings from Kim et al. (2001), who noted L. acidophilus survived at 53 °C but exhibited reduced viability at 60 °C. Similarly, Mandal et al. (2006) observed that thermal condition at 55, 60, and 65 °C were fatal for free bacteria, whereas encapsulated forms demonstrated survival.
A microencapsulation system is a matrix or membrane in which the microorganisms such as probiotics are embedded, thereby increasing their viability in food products or the intestinal tract. These methods include extrusion (Sousa et al., 2015), emulsification (Frakolaki et al., 2021), spray drying (Zhang et al., 2015), freeze drying (Dianawati et al., 2013), complex coacervation (De Almeida Paula et al., 2019), ionic gelation (Song et al., 2013), and electrostatic deposition (Anselmo et al., 2016). It is noteworthy that there are still some challenges assigned to these techniques such as low stability during storage (Weinbreck et al., 2010), decreasing liability during the thermal process and in vitro digestion, as well the particle size which is responsible to sensory reception of food products including these systems (Nag, 2011).
The substitution of certain portions of oil with water in Oil-in-Water (O/W) emulsions allows for the incorporation of diverse functional compounds in Water-in-Oil-in-Water (W/O/W) emulsions. Consequently, this makes it an appropriate choice for the development of functional O/W emulsions ( Abbasi et al., 2023; Berendsen et al., 2015; Frank et al., 2012; Giroux et al., 2013; Jiménez-Colmenero, 2013) and fat replacers (Cofrades et al., 2013; Jiménez-Colmenero, 2013). Two-step emulsification is a common method to prepare double emulsions in form of W/O/W emulsion (Balcaen et al., 2016; Koubaa et al., 2018b). The first stage involves utilizing high shear forces to create a Water-in-Oil (W/O) emulsion. Subsequently, lower shear forces are employed to ensure the stability and integrity of the internal water droplets, preventing them from collapsing or dispersing. Consequently, the second step results in coarse droplets and broad distribution of particle size in double emulsions (Koubaa et al., 2018a; Oppermann et al., 2015). Unexpectedly, the droplet size of the resulting double emulsion is somehow responsible for the volume of the water which is retained after the second step, and this is independent of the emulsification device (Schuch et al., 2014).
The emulsions are wide-spreading encapsulation systems that are represented by their scalability, small-scale carriers, and enhancing the viability of the probiotic ( Liu et al., 2019; Manojlović et al., 2010). W1/O/W2 emulsion is a Double Emulsion (DE) that includes oil globules containing water droplets (W1 or interior aqueous phase), and the oil globules are simultaneously dispersed in water as exterior aqueous phase or W2. W1 is a potent environment to embed the probiotics due to their small-scale size (less than one µm) (De Almeida Paula et al., 2019). Several works of literature have reported that double emulsions are proper devices to enhance the viability of these cells during various processes (El Kadri et al., 2018; Hernández-Rodríguez et al., 2014) and digestion (Pimentel-González et al., 2009; Shima et al., 2006, 2009; Wang et al., 2020). It is reported that double emulsions containing Lactobacillus plantarum and L. paracasei resulted in the maintenance of viable cells in high quantities after the production process of Oaxaca cheese (Rodríguez-Huezo et al., 2014), yogurt (El Kadri et al., 2018), as well as under in-vitro digestion condition. Also, when L. rhamnosus and L. acidophilus were encapsulated in a similar encapsulation system, the system showed survival effects during in-vitro digestion conditions as well as resulting in an enhancement of colon adhesion of these microorganisms (Pimentel-González et al., 2009; Shima et al., 2006, 2009; Wang et al., 2020).
To the best of our knowledge, there is presently no existing literature examining the impact of internal water phase gelation on the viability of probiotics. Therefore, the primary aim of this research was to evaluate the survival of L. plantarum subsequent to its encapsulation within the W1 of DEs. Additionally, we examined how the gelation of W1, achieved through the use of gelatin, alginate, tragacanth gum, and carrageenan, influenced the survival of probiotic cells. The viability of these cells was evaluated under a range of environmental stress, including heat treatments (such as pasteurization, microwave heating, and sterilization), exposure to sodium chloride (NaCl), bile salt, lysozyme, and penicillin, as well as simulated gastrointestinal conditions.
Materials and methods
Materials
Polyglycerol Polyricinoleate (PGPR) was obtained from Danisco Co. (Copenhagen, Denmark), Tween 80, gelatin, sodium alginate, carrageenan, De Man–Rogosa–Sharpe (MRS) broth, and agar were sourced from Merck Millipore Co. (Darmstadt, Germany). Tragacanth gum was procured from Dena Emulsion Co. (Shiraz, Iran), and olive oil was provided by Oila Company (Tehran, Iran). Others chemicals were of analytical grade.
Microorganism preparation
Initially, a solitary colony from the L. plantarum stock was introduced into MRS broth and left to incubate overnight at 37 °C in a shaker incubator. Following this, 10 ml of the inoculum was combined with 90 ml of fresh MRS broth and incubated at 37 °C for 48 h. The resultant microbial suspension was subjected to centrifugation at 6,000 g for four min at 6 °C (Tantratian and Pradeamchai, 2020). The resulting pellet was subsequently washed three times with sterile saline solution and promptly applied for the encapsulation procedure and production of samples.
Preparation of double emulsions
The W1/O/W2 emulsion was prepared using a two-step technique initially introduced by Bou et al. (2014) with some modifications. At the first W1, O and W2 phases were prepared and then converted to DEs. For the W1 phase, L. plantarum was added into a sterile saline solution at a concentration of 11 log CFU/g. In separate samples, gelatin, alginate, tragacanth gum, and carrageenan were added at a concentration of 2% wt. The alginate sample included calcium chloride (CaCl2) (100 mm), while the carrageenan sample contained potassium chloride (KCl) (100 mm). Following preparation, the W1 phase was stored in tubes to shield it from light. W2 was formed by dissolving Tween 80 (4% w/v) in distilled water. The oil phase (O) comprised PGPR (6% wt) dissolved in olive oil, which was stirred at 65 °C for 20 min. The W1/O primary emulsion was generated by the addition of 20 g of W1 to 80 g of the oil phase (O) and stirring at 500 rpm (35 °C) for 20 min. Subsequently, the emulsion was homogenized using a T25 Ultra-Turrax (IKA, Germany) at 13,000 rpm for three min, followed by an additional three min at 15,000 rpm. To produce the W1/O/W2 emulsions, 40 g of the W1/O primary emulsion was homogenized with 60 g of W2 at 13,000 rpm for four min, followed by 10,000 rpm for another 4 min at 35 °C.
Effect of heat processing
The samples were placed in glass tubes and exposed to different treatments (Soltani Lak et al., 2021). Pasteurization involved subjecting the samples to a temperature of 72 °C for 40 s using a water bath. Microwave heating was performed at 72 °C for 40 s. Sterilization was achieved by exposing the samples to a temperature of 145 °C for 40 s using an oil bath. After each treatment, the samples were rapidly cooled to 4 °C using a cold-water bath. The survivability of L. plantarum was then assessed using the following method. The probiotics count in the samples was measured by a serial dilution method and then culturing on an MRS agar medium at 37 °C for two overnight.
Effect of NaCl concentration
The assessment of the survival of both free L. plantarum and encapsulated L. plantarum in the presence of NaCl was conducted following the methodology described by Soltani Lak et al. (2021) by adding 0, 1, and 3% NaCl to the MRS agar culture. The count of sustention was evaluated after 0, 1, and 2 h by MRS agar method and incubation for three days at 37 °C.
Effects of lysozyme, penicillin, and bile salt
The effectiveness of free and encapsulated L. plantarum against lysozyme, penicillin G, and bile salt was individually assessed following the protocol described by Soltani Lak et al. (2021) . This involved supplementing the MRS agar medium with 1 μg/ml of penicillin G, 5 mg/ml lysozyme, and either 0.5 or 1% w/v of bile salt. Enumeration of bacterial colonies was conducted at 24 h post-exposure.
Effect of simulated gastrointestinal conditions
The impact of simulated gastric and intestinal conditions on the viability of all samples was assessed following the methodology outlined by Moayyedi et al. (2018) with some modifications. To prepare the simulated gastric fluid, a mixture of 0.9% w/v NaCl and 0.3% w/v pepsin was dispersed in deionized water and adjusted to pH 1.5 using one M HCl. Similarly, for the simulated intestinal fluid, a combination of 0.6% w/v bile salt, 0.6% NaCl, and 0.2% w/v pancreatin was added to deionized water and adjusted to pH 6. All solutions underwent sterilization through a 0.22 μm syringe filter. For digestion analysis, 10 ml of sample and 10 ml of the prepared solutions were mixed and shaken at 50 rpm and 37 °C. Enumeration was conducted after 0, 1, and 2 h of digestion on MRS agar plates, followed by incubation for three days at 37 °C.
Statistical analysis
All trials were replicated three times unless stated otherwise. Statistical analysis was carried out using one-way analysis of variance (ANOVA) with a significance level of 0.05. Duncan's multiple range tests were then employed to identify significant differences between the mean values. The analysis was conducted using SAS® software, version 9.1, developed by SAS Institute Inc., NC, USA.
Results and discussion
Viability against food heat processing
Heat processing is a critical aspect of food processing that can influence the survivability of probiotics. The effects of different heat treatments on the free L. plantarum and encapsulated L. plantarum bacteria (by application of gelatin, alginate, tragacanth gum, and carrageenan) are shown in Table 1. These treatments included pasteurization (72 °C for 40 s), microwave heating (72 °C for 40 s), and sterilization (145 °C for 40 s). The survival outcomes against these heat treatments are represented in Table 1. As expected, the various heat treatments exerted distinct effects on the survivability of L. plantarum. For free L. plantarum, the initial count decreased from 8.37 log to 4.68, 4.13, and 0 log following microwave heating, pasteurization, and sterilization, respectively. Notably, sterilization resulted in a complete reduction of bacterial count across all samples. Additionally, the sample containing tragacanth gum demonstrated superior protective effects compared to other samples. In this particular sample, the microbial count decreased from 8.38 to 7.15 log CFU/ml after microwave treatment, whereas pasteurization and sterilization reduced it further to 7.23 and 0 log CFU/ml, respectively. This trend aligns with findings from Kim et al. (2001), who noted L. acidophilus survived at 53 °C but exhibited reduced viability at 60 °C. Similarly, Mandal et al. (2006) observed that thermal condition at 55, 60, and 65 °C were fatal for free bacteria, whereas encapsulated forms demonstrated survival.
Table 1: Effects of food heat processing on the viability of probiotic Lactobacillus plantarum
encapsulated in a double water/oil/water emulsion system
| 0 | Microwave (72 °C for 40 s) |
Pasteurization (72 °C for 40 s) |
Sterilization (145 °C for 40 s) |
|
| Control | 8.36±0.03 A | 6.10±0.03 C | 6.20±0.02 C | **ND |
| Gelatin | 8.37±0.04 A | 7.05±0.04 B | 7.10±0.02 B | ND |
| Alginate | 8.35±0.01 A | 7.08±0.03 B | 7.12±0.03 B | ND |
| Tragacanth gum | 8.38±0.03 A | 7.15±0.02 A | 7.23±0.02 A | ND |
| Kappa carrageenan | 8.34±0.04 A | 7.07±0.02 B | 7.14±0.02 B | ND |
| Free bacteria | 8.37±0.04 A | 4.68±0.07 D | 4.13±0.03 D | ND |
*Data represent Mean±Standard Deviation (SD) of three independent repeats.
Values with different superscripted letters in each column indicate significant differences (p<0.05)
**ND=Not Detectable
Values with different superscripted letters in each column indicate significant differences (p<0.05)
**ND=Not Detectable
Viability against NaCl concentration
Table 2 illustrates the impact of varying NaCl concentrations (0, 1, and 3%) on the viability of both free and encapsulated L. plantarum. Notably, the reduction in free cell counts became significantly greater with increasing salt concentrations, with the most substantial reduction observed at 3% NaCl. Specifically, free L. plantarum exhibited the highest reduction, reaching 2.08 log CFU/ml at 3% NaCl. Encapsulated forms utilizing alginate, tragacanth gum, and carrageenan gelling agents, however, showed no reduction in viability, highlighting the protective role of these agents against salt-induced stress. At a 1% NaCl concentration, there was no significant impact on the viability of encapsulated bacteria, whereas the count of free bacteria was notably reduced, further indicating the susceptibility of free L. plantarum to NaCl. The protective effects of probiotic encapsulation against different stress conditions were reported previously by Mohammadi-Gouraji et al. (2017) and Peighambardoust et al. (2011). Soltani Lak et al. (2021) studied the stability of encapsulated L. reuteri against varying concentrations of NaCl. They reported that the effect of NaCl at 4% concentration was more pronounced than at 2%, though the type of wall materials significantly influenced outcomes. Encapsulated samples had maintained higher cell viability compared to free cells.
Table 2 illustrates the impact of varying NaCl concentrations (0, 1, and 3%) on the viability of both free and encapsulated L. plantarum. Notably, the reduction in free cell counts became significantly greater with increasing salt concentrations, with the most substantial reduction observed at 3% NaCl. Specifically, free L. plantarum exhibited the highest reduction, reaching 2.08 log CFU/ml at 3% NaCl. Encapsulated forms utilizing alginate, tragacanth gum, and carrageenan gelling agents, however, showed no reduction in viability, highlighting the protective role of these agents against salt-induced stress. At a 1% NaCl concentration, there was no significant impact on the viability of encapsulated bacteria, whereas the count of free bacteria was notably reduced, further indicating the susceptibility of free L. plantarum to NaCl. The protective effects of probiotic encapsulation against different stress conditions were reported previously by Mohammadi-Gouraji et al. (2017) and Peighambardoust et al. (2011). Soltani Lak et al. (2021) studied the stability of encapsulated L. reuteri against varying concentrations of NaCl. They reported that the effect of NaCl at 4% concentration was more pronounced than at 2%, though the type of wall materials significantly influenced outcomes. Encapsulated samples had maintained higher cell viability compared to free cells.
Table 2: Effects of NaCl concentration on the viability of probiotic Lactobacillus plantarum encapsulated in a double
water/oil/water emulsion system
| NaCl concentration (%) | |||
| 0 | 1 | 3 | |
| Control | 8.34±0.02 B | 8.31±0.01 D | 7.32±0.02 C |
| Gelatin | 8.48±0.04 A | 8.48±0.02 A | 8.48±0.01 A |
| Alginate | 8.40±0.07 AB | 8.39±0.01 B | 8.37±0.02 B |
| Tragacanth gum | 8.46±0.09 AB | 8.51±0.01 A | 8.48±0.01 A |
| Kappa carrageenan | 8.43±0.09 AB | 8.35±0.03 C | 8.35±0.01 B |
| Free bacteria | 8.42±0.06 AB | 8.25±0.02 E | 6.34±0.04 D |
*Data represent Mean±Standard Deviation (SD) of three independent repeats.
Values with different superscripted letters in each column indicate significant differences (p<0.05).
Values with different superscripted letters in each column indicate significant differences (p<0.05).
Viability against lysozyme, penicillin, and bile salt
Table 3 outlines the impact of internal water phase gelation through double emulsion with gelatin, alginate, tragacanth gum, and carrageenan on the survivability of all samples, both control and encapsulated, when exposed to bile salt. Bile salt, a significant component of gastric fluid, can greatly influence bacterial viability. This study evaluated the effects of 0.5 and 1% bile salt concentrations on L. plantarum viability. The findings revealed a notable (p<0.05) decline in the survival of control samples and encapsulated L. plantarum following bile salt addition. Particularly, a significant reduction was observed in free L. plantarum, (from 8.43 to 6.18 log CFU/ml) after exposure to 1% bile salt. These results underscored the protective effects of all gelation agents—gelatin, alginate, tragacanth gum, and carrageenan—against bile salt, with tragacanth gum exhibiting the highest protective efficacy (reducing the count to only 0.07 log CFU/ml after exposure to 1% bile salt). Among the gelling agents, the sample containing carrageenan with 0.33 log CFU/ml reduction had the lowest protective effect. The same findings were observed by Krasaekoopt et al. (2004) and Lee and Heo (2000). Chen et al. (2017) and Hernández-Gómez et al. (2021) showed that encapsulation could reduce the cell reduction in the gastric condition containing bile salt. Various studies have emphasized the significance of probiotic bacteria's viability when exposed to bile salt, highlighting the importance of identifying bile salt-resistant probiotics for food applications (Allain et al., 2018; Singhal et al., 2019). Chandramouli et al. (2004) noted the shielding effects of CaCl2 and alginate on probiotics in the presence of bile salt. They also reported that increasing the concentration of sodium alginate has a significant impact on viability of probiotics.
Table 3 shows the impact of lysozyme addition on the survivability of bacteria. The inclusion of lysozyme led to a notable decline in the survival of L. plantarum. Particularly notable was the highest cell reduction observed in free L. plantarum, with a decrease to 2.34 log CFU/ml. Among the various gelling agents examined in this study, tragacanth gum-containing samples exhibited the highest protective effect, with a reduction of only 0.13 log CFU/ml, while carrageenan-containing samples displayed the lowest protection, with a decline of 0.28 log CFU/ml.
Table 3 presents the effects of gelling agent at the inner phase of double emulsion with gelatin, alginate, tragacanth gum, and carrageenan on the viability of free and encapsulated L. plantarum in presence of penicillin. The impact of penicillin on probiotic viability and cell reduction was previously investigated by Moayyedi et al. (2018). Penicillin demonstrated a significant effect on the survival of all samples, with the highest reduction observed in free L. plantarum at 3.71 log CFU/ml. In our study, tragacanth gum-containing samples exhibited the highest protective effect with a decline of 1.11 log CFU/ml, while gelatin-containing samples displayed the lowest protection with a decline of 1.28 log CFU/ml. These findings corroborate previous research by Moayyedi et al. (2018), which highlighted the application of encapsulation techniques using hydrocolloids to enhance protection against penicillin.
Table 3 outlines the impact of internal water phase gelation through double emulsion with gelatin, alginate, tragacanth gum, and carrageenan on the survivability of all samples, both control and encapsulated, when exposed to bile salt. Bile salt, a significant component of gastric fluid, can greatly influence bacterial viability. This study evaluated the effects of 0.5 and 1% bile salt concentrations on L. plantarum viability. The findings revealed a notable (p<0.05) decline in the survival of control samples and encapsulated L. plantarum following bile salt addition. Particularly, a significant reduction was observed in free L. plantarum, (from 8.43 to 6.18 log CFU/ml) after exposure to 1% bile salt. These results underscored the protective effects of all gelation agents—gelatin, alginate, tragacanth gum, and carrageenan—against bile salt, with tragacanth gum exhibiting the highest protective efficacy (reducing the count to only 0.07 log CFU/ml after exposure to 1% bile salt). Among the gelling agents, the sample containing carrageenan with 0.33 log CFU/ml reduction had the lowest protective effect. The same findings were observed by Krasaekoopt et al. (2004) and Lee and Heo (2000). Chen et al. (2017) and Hernández-Gómez et al. (2021) showed that encapsulation could reduce the cell reduction in the gastric condition containing bile salt. Various studies have emphasized the significance of probiotic bacteria's viability when exposed to bile salt, highlighting the importance of identifying bile salt-resistant probiotics for food applications (Allain et al., 2018; Singhal et al., 2019). Chandramouli et al. (2004) noted the shielding effects of CaCl2 and alginate on probiotics in the presence of bile salt. They also reported that increasing the concentration of sodium alginate has a significant impact on viability of probiotics.
Table 3 shows the impact of lysozyme addition on the survivability of bacteria. The inclusion of lysozyme led to a notable decline in the survival of L. plantarum. Particularly notable was the highest cell reduction observed in free L. plantarum, with a decrease to 2.34 log CFU/ml. Among the various gelling agents examined in this study, tragacanth gum-containing samples exhibited the highest protective effect, with a reduction of only 0.13 log CFU/ml, while carrageenan-containing samples displayed the lowest protection, with a decline of 0.28 log CFU/ml.
Table 3 presents the effects of gelling agent at the inner phase of double emulsion with gelatin, alginate, tragacanth gum, and carrageenan on the viability of free and encapsulated L. plantarum in presence of penicillin. The impact of penicillin on probiotic viability and cell reduction was previously investigated by Moayyedi et al. (2018). Penicillin demonstrated a significant effect on the survival of all samples, with the highest reduction observed in free L. plantarum at 3.71 log CFU/ml. In our study, tragacanth gum-containing samples exhibited the highest protective effect with a decline of 1.11 log CFU/ml, while gelatin-containing samples displayed the lowest protection with a decline of 1.28 log CFU/ml. These findings corroborate previous research by Moayyedi et al. (2018), which highlighted the application of encapsulation techniques using hydrocolloids to enhance protection against penicillin.
Table 3: Effect of lysozyme, penicillin, and bile salt on the viability of probiotic Lactobacillus plantarum encapsulated in a double water/oil/water emulsion system
| Initial count | Bile salt (%) | Lysozyme | Penicillin | ||
| 0.5 | 1 | ||||
| Control | 8.35±0.02 B | 8.28±0.01 C | 7.19±0.03 D | 7.16±0.03 D | 6.78±0.04 C |
| Gelatin | 8.49±0.04 A | 8.39±0.02 B | 8.21±0.02 B | 8.24±0.03 B | 7.21±0.03 B |
| Alginate | 8.41±0.07 AB | 8.36±0.02 B | 8.19±0.02 B | 8.21±0.02 B | 7.22±0.02 B |
| Tragacanth gum | 8.47±0.09 AB | 8.45±0.01 A | 8.40±0.04 A | 8.34±0.04 A | 7.36±0.02 A |
| Kappa carrageenan | 8.43±0.09 AB | 8.35±0.01 B | 8.10±0.02 C | 8.15±0.02 C | 7.16±0.02 B |
| Free bacteria | 8.43±0.06 AB | 7.69±0.05 D | 6.18±0.02 E | 6.09±0.02 E | 4.72±0.07 D |
*Data represent Mean±Standard Deviation (SD) deviation of three independent repeats.
Values with different superscripted letters in each column indicate significant differences (p<0.05).
Values with different superscripted letters in each column indicate significant differences (p<0.05).
Viability under simulated gastrointestinal conditions
Figure 1 presents the effects of simulated gastric and intestinal conditions on the viability of both control and encapsulated samples within different gelation agents (gelatin, alginate, tragacanth gum, and carrageenan) at the inner phase of the W/O/W emulsion. In this assessment, the counts were recorded at zero, one, and two h after mixing simulated gastric and intestinal fluids with the bacterial samples, reported as log CFU/ml of sample.
The results from the simulated gastric test revealed a significant impact of the gelling agent type on L. plantarum survival. Across all samples, a decrease in L. plantarum count was observed during storage, with free L. plantarum exhibiting the highest reduction. Specifically, the count decreased from an initial 8.43 to 7.92 log CFU/ml after 1 h and 5.13 log CFU/ml after 2 h. Conversely, the sample containing tragacanth gum showed the lowest reduction, with the initial count of 8.51 log CFU/ml decreasing to 8.29 log CFU/ml after 1 h and 7.96 log CFU/ml after 2 h.
The decrease in bacterial count under simulated intestinal conditions was notably lower compared to the simulated gastric conditions (Figure 1b). Free L. plantarum exhibited the highest reduction against the intestinal condition, with reductions of 0.38 and 0.79 log CFU/ml after 1 and 2 h. The sample containing tragacanth gum demonstrated the highest protective effect. Similar findings to our study were reported by Coghetto et al. (2016), who observed substantial decreases in the survival of probiotics encapsulated in sodium alginate or sodium alginate-citric pectin through electro spraying under simulated intestinal conditions. These researchers highlighted the protective influence of the encapsulation material on L. plantarum viability, reporting reductions of 4.02 log CFU/ml for free cells and 2.07 log CFU/ml for encapsulated cells.
Figure 1 presents the effects of simulated gastric and intestinal conditions on the viability of both control and encapsulated samples within different gelation agents (gelatin, alginate, tragacanth gum, and carrageenan) at the inner phase of the W/O/W emulsion. In this assessment, the counts were recorded at zero, one, and two h after mixing simulated gastric and intestinal fluids with the bacterial samples, reported as log CFU/ml of sample.
The results from the simulated gastric test revealed a significant impact of the gelling agent type on L. plantarum survival. Across all samples, a decrease in L. plantarum count was observed during storage, with free L. plantarum exhibiting the highest reduction. Specifically, the count decreased from an initial 8.43 to 7.92 log CFU/ml after 1 h and 5.13 log CFU/ml after 2 h. Conversely, the sample containing tragacanth gum showed the lowest reduction, with the initial count of 8.51 log CFU/ml decreasing to 8.29 log CFU/ml after 1 h and 7.96 log CFU/ml after 2 h.
The decrease in bacterial count under simulated intestinal conditions was notably lower compared to the simulated gastric conditions (Figure 1b). Free L. plantarum exhibited the highest reduction against the intestinal condition, with reductions of 0.38 and 0.79 log CFU/ml after 1 and 2 h. The sample containing tragacanth gum demonstrated the highest protective effect. Similar findings to our study were reported by Coghetto et al. (2016), who observed substantial decreases in the survival of probiotics encapsulated in sodium alginate or sodium alginate-citric pectin through electro spraying under simulated intestinal conditions. These researchers highlighted the protective influence of the encapsulation material on L. plantarum viability, reporting reductions of 4.02 log CFU/ml for free cells and 2.07 log CFU/ml for encapsulated cells.


Figure 1: Effects of simulated gastric (a) and intestinal (b) conditions on the viability of probiotic Lactobacillus plantarum encapsulated in a double water/oil/water emulsion system. CFU=Colony Forming Unit.
Different capital letters indicate statistically significant differences (p˂0.05) between samples at the same time and different small letters indicate statistically significant differences (p˂0.05) during the time.
Conclusion
This research focused on encapsulating L. plantarum within various internal phase gelled double emulsions, including gelatin, alginate, tragacanth gum, and carrageenan. The study examined bacterial survival under different heat treatments (pasteurization, microwave heating, and sterilization), environmental stressors (NaCl, bile salt, lysozyme, penicillin), and simulated gastrointestinal conditions. Notably, under simulated gastric conditions, bacterial reduction was more pronounced compared to simulated intestinal conditions. In the heat processing test, encapsulated L. plantarum demonstrated better survival rates compared to free cells, highlighting the significant role of gelling agents in protecting L. plantarum. Of all gelling agents tested, tragacanth gum exhibited the strongest protective effects. Results showed that the sensitivity of unencapsulated cells to heat processing was significantly higher than encapsulated cells. The sample containing tragacanth gum showed the highest cell viability when subjected to various heat processes. Furthermore, the study showed that DEs effectively increased the probiotics survival against NaCl, bile salt, lysozyme, and penicillin. Finally, the gastric conditions had a more detrimental effect on probiotic viability compared to the intestinal conditions. The study suggests that future research should explore the effects of oil and external water phase gelation on probiotic viability.
Author contributions
A.R., S.M.H.H., S.R., S.-S.H. and H.H. designed the study; S.A. conducted the experimental work and analyzed the data; S.A., A.R., S.M.H.H., S.R., S.-S.H. and H.H. wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors would like to thank the Shiraz University, Shiraz, Iran.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Ethical consideration
Not applicable.
References
Abbasi S., Rafati A., Hosseini S.M.H., Roohinejad S., Hashemi S.S., Hashemi Gahruie H., Rashidinejad A. (2023). The internal aqueous phase gelation improves the viability of probiotic cells in a double water/oil/water emulsion system. Food Science and Nutrition. 5978-5988. [DOI: 10.1002/fsn3.3532]
Allain T., Chaouch S., Thomas M., Vallée I., Buret A.G., Langella P., Grellier P., Polack B., Bermudez-Humaran L.G., Florent I. (2018). Bile-salt-hydrolases from the probiotic strain Lactobacillus johnsonii La1 mediate anti-giardial activity in vitro and in vivo. Frontiers in Microbiology. 8: 2707. [DOI: 10.3389/fmicb.2017.02707]
Anselmo A.C., McHugh K.J., Webster J., Langer R., Jaklenec A. (2016). Layer‐by‐layer encapsulation of probiotics for delivery to the microbiome. Advanced Materials. 28: 9486-9490. [DOI: 10.1002/adma.201603270]
Balcaen M., Vermeir L., Declerck A., Van Der Meeren P. (2016). Influence of internal water phase gelation on the shear-and osmotic sensitivity of W/O/W-type double emulsions. Food Hydrocolloids. 58: 356-363. [DOI: 10.1016/j.foodhyd. 2016.03.011]
Berendsen R., Güell C., Ferrando M. (2015). A procyanidin-rich extract encapsulated in water-in-oil-in-water emulsions produced by premix membrane emulsification. Food Hydrocolloids. 43: 636-648. [DOI: 10.1016/j.foodhyd.2014.07.023]
Bou R., Cofrades S., Jiménez-Colmenero F. (2014). Physicochemical properties and riboflavin encapsulation in double emulsions with different lipid sources. LWT-Food Science and Technology. 59: 621-628. [DOI: 10.1016/j.lwt.2014.06.044]
Chandramouli V., Kailasapathy K., Peiris P., Jones M. (2004). An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. Journal of Microbiological Methods. 56: 27-35. [DOI: 10.1016/j.mimet.2003.09.002]
Chen M.-J., Tang H.-Y., Chiang M.-L. (2017). Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiology. 66: 20-27. [DOI: 10.1016/j.fm.2017.03.020]
Cofrades S., Antoniou I., Solas M.T., Herrero A.M., Jiménez-Colmenero F. (2013). Preparation and impact of multiple (water-in-oil-in-water) emulsions in meat systems. Food Chemistry. 141: 338-346. [DOI: 10.1016/j.foodchem.2013.02.097]
Coghetto C.C., Brinques G.B., Siqueira N.M., Pletsch J., Soares R.M.D., Ayub M.A.Z. (2016). Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. Journal of Functional Foods. 24: 316-326. [DOI: 10.1016/j.jff.2016.03.036]
De Almeida Paula D., Martins E.M.F., De Almeida Costa N., De Oliveira P.M., De Oliveira E.B., Ramos A.M. (2019). Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. International Journal of Biological Macromolecules. 133: 722-731. [DOI: 10.1016/j.ijbiomac.2019.04.110]
Dianawati D., Mishra V., Shah N.P. (2013). Effect of drying methods of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris on secondary protein structure and glass transition temperature as studied by fourier transform infrared and differential scanning calorimetry. Journal of Dairy Science. 96: 1419-1430. [DOI: 10.3168/jds.2012-6058]
El-Dieb S.M., Abd Rabo F.H.R., Badran S.M., Abd El-Fattah A.M., Elshaghabee F.M.F. (2012). The growth behaviour and enhancement of probiotic viability in bioyoghurt. International Dairy Journal. 22: 44-47. [DOI: 10.1016/j.idairyj.2011.08.003]
El Kadri H., Lalou S., Mantzouridou F., Gkatzionis K. (2018). Utilisation of water-in-oil-water (W1/O/W2) double emulsion in a set-type yogurt model for the delivery of probiotic Lactobacillus paracasei. Food Research International. 107: 325-336. [DOI: 10.1016/j.foodres.2018.02.049]
Frakolaki G., Giannou V., Kekos D., Tzia C. (2021). A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Critical Reviews in Food Science and Nutrition. 61: 1515-1536. [DOI: 10.1080/ 10408398.2020.1761773]
Frank K., Walz E., Gräf V., Greiner R., Köhler K., Schuchmann H.P. (2012). Stability of anthocyanin-rich W/O/W‐emulsions designed for intestinal release in gastrointestinal environment. Journal of Food Science. 77: N50-N57. [DOI: 10.1111/j.1750-3841.2012.02982.x]
Giroux H.J., Constantineau S., Fustier P., Champagne C.P., St-Gelais D., Lacroix M., Britten M. (2013). Cheese fortification using water-in-oil-in-water double emulsions as carrier for water soluble nutrients. International Dairy Journal. 29: 107-114. [DOI: 10.1016/j.idairyj.2012.10.009]
Hernández-Gómez J.G., López-Bonilla A., Trejo-Tapia G., Ávila-Reyes S.V., Jiménez-Aparicio A.R., Hernández-Sánchez H. (2021). In vitro bile salt hydrolase (BSH) activity screening of different probiotic microorganisms. Foods. 10: 674-684. [DOI: 10.3390/foods10030674]
Hernández-Rodríguez L., Lobato-Calleros C., Pimentel-González D.J., Vernon-Carter E.J. (2014). Lactobacillus plantarum protection by entrapment in whey protein isolate: κ-carrageenan complex coacervates. Food Hydrocolloids. 36: 181-188. [DOI: 10.1016/j.foodhyd.2013.09.018]
Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. (2014). Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology and Hepatology. 11: 506-514. [DOI: 10.1038/nrgastro.2014.66]
Hosseinialhashemi M., Tavakoli J., Rafati A., Ahmadi F. (2021). The aplication of Pistacia khinjuk extract nanoemulsion in a biopolymeric coating to improve the shelf life extension of sunflower oil. Food Science and Nutrition. 9: 920-928. [DOI: 10.1002/fsn3.2057]
Irani M., Rafati A., Hashemi S.S., Barba F.J., Koubaa M., Roohinejad S. (2021). Biomass fractionation using emerging technologies. In: Koubaa M., Barba F.J., Roohinejad S. (Editors). Fermentation processes: emerging and conventional technologies. John Wiley and Sons Ltd., New Jersey, U.S. pp: 145-169. [DOI: 10.1002/9781119505822.ch5]
Jiménez-Colmenero F. (2013). Potential applications of multiple emulsions in the development of healthy and functional foods. Food Research International. 52: 64-74. [DOI: 10.1016/j.foodres.2013.02.040]
Kim W.S., Perl L., Park J.H., Tandianus J.E., Dunn N.W. (2001). Assessment of stress response of the probiotic Lactobacillus acidophilus. Current Microbiology. 43: 346-350. [DOI: 10.1007/s002840010314]
Koubaa M., Nikmaram N., Roohinejad S., Rafati A., Greiner R. (2018a). Multilayered emulsions. In: Roohinejad S., Greiner R., Oey I., Wen J. (Editors). Emulsion-based systems for delivery of food active compounds: formation, application, health and safety. John Wiley and Sons Ltd., New Jersey, U.S. pp: 105-119. [DOI: 10.1002/9781119247159.ch4]
Koubaa M., Roohinejad S., Sharma P., Nikmaram N., Hashemi S.S., Abbaspourrad A., Greiner R. (2018b). Multiple emulsions. In: Roohinejad S., Greiner R., Oey I., Wen J. (Editors). Emulsion‐based systems for delivery of food active compounds: formation, application, health and safety. John Wiley and Sons Ltd., New Jersey, U.S. pp: 69-103. [DOI: 10.1002/9781119247159.ch3]
Krasaekoopt W., Bhandari B., Deeth H. (2004). The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. International Dairy Journal. 14: 737-743. [DOI: 10.1016/j.idairyj.2004.01.004]
Lee K.-Y., Heo T.-R. (2000). Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salt solution. Applied and Environmental Microbiology. 66: 869-873. [DOI: 10.1128/AEM.66.2.869-873.2000]
Liu H., Cui S.W., Chen M., Li Y., Liang R., Xu F., Zhong F. (2019). Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: a review. Critical Reviews in Food Science and Nutrition. 59: 2863-2878. [DOI: 10.1080/10408398.2017.1377684]
Mandal S., Puniya A., Singh K. (2006). Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. International Dairy Journal. 16: 1190-1195. [DOI: 10.1016/j.idairyj.2005.10.005]
Manojlović V., Nedović V.A., Kailasapathy K., Zuidam N.J. (2010). Encapsulation of probiotics for use in food products. In: Zuidam N., Nedovic V. (Editors). Encapsulation technologies for active food ingredients and food processing. Springer, New York, NY. pp: 269-302. [DOI: 10.1007/978-1-4419-1008-0_10]
Moayyedi M., Eskandari M.H., Rad A.H.E., Ziaee E., Khodaparast M.H.H., Golmakani M.-T. (2018). Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. Journal of Functional Foods. 40: 391-399. [DOI: 10.1016/j.jff.2017.11.016]
Mohammadi-Gouraji E., Sheikh-Zeinoddin M., Soleimanian-Zad S. (2017). Effects of Persian gum and gum Arabic on the survival of Lactobacillus plantarum PTCC 1896, Escherichia coli, Xanthomonas axonopodis, and Saccharomyces cerevisiae during freeze drying. British Food Journal. 119: 331-341. [DOI: 10.1108/BFJ-09-2016-0442]
Nag A. (2011). Development of a microencapsulation technique for probiotic bacteria Lactobacillus casei 431 using a protein-polysaccharide complex. The degree of masters of technology in Food Technology thesis. Massey University, Palmerston North, New Zealand.
Oppermann A., Renssen M., Schuch A., Stieger M., Scholten E. (2015). Effect of gelation of inner dispersed phase on stability of (W1/O/W2) multiple emulsions. Food Hydrocolloids. 48: 17-26. [DOI: 10.1016/j.foodhyd.2015.01.027]
Ortakci F., Broadbent J.R., McManus W., McMahon D. (2012). Survival of microencapsulated probiotic Lactobacillus paracasei LBC-1e during manufacture of mozzarella cheese and simulated gastric digestion. Journal of Dairy Science, 95: 6274-6281, [DOI: 10.3168/jds.2012-5476]
Peighambardoust S., Tafti A.G., Hesari J. (2011). Application of spray drying for preservation of lactic acid starter cultures: a review. Trends in Food Science and Technology. 22: 215-224. [DOI: 10.1016/j.tifs.2011.01.009]
Pimentel-González D.J, Campos-Montiel R.G, Lobato-Calleros C., Pedroza-Islas R., Vernon-Carter, E.J. (2009). Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Research International. 42: 292-297. [DOI: 10.1016/j.foodres.2008.12.002]
Rodríguez-Huezo M.E, Estrada-Fernández A.G, García-Almendárez B.E, Ludena-Urquizo F., Campos-Montiel R.G, Pimentel-González D.J. (2014). Viability of Lactobacillus plantarum entrapped in double emulsion during Oaxaca cheese manufacture, melting and simulated intestinal conditions. LWT-Food Science and Technology. 59: 768-773. [DOI: 10.1016/ j.lwt.2014.07.004]
Schuch A., Wrenger J., Schuchmann H.P. (2014). Production of W/O/W double emulsions. part II: influence of emulsification device on release of water by coalescence. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 461: 344-351. [DOI: 10.1016/j.colsurfa.2013.11.044]
Shima M., Matsuo T., Yamashita M., Adachi S. (2009). Protection of Lactobacillus acidophilus from bile salts in a model intestinal juice by incorporation into the inner-water phase of a W/O/W emulsion. Food Hydrocolloids. 23: 281-285. [DOI: 10.1016/j.foodhyd.2008.01.008]
Shima M., Morita Y., Yamashita M., Adachi S. (2006). Protection of Lactobacillus acidophilus from the low pH of a model gastric juice by incorporation in a W/O/W emulsion. Food Hydrocolloids. 20: 1164-1169. [DOI: 10.1016/j.foodhyd. 2006.01.001]
Singhal N., Maurya A.K., Mohanty S., Kumar M., Virdi J.S. (2019). Evaluation of bile salt hydrolases, cholesterol-lowering capabilities, and probiotic potential of Enterococcus faecium isolated from rhizosphere. Frontiers in Microbiology. 10: 1567. [DOI: 10.3389/fmicb.2019.01567]
Soltani Lak A., Marhamatizadeh M.H., Fattahi H. (2021). Stability of encapsulated Lactobacillus reuteri during harsh conditions, storage period, and simulated in vitro conditions. Journal of Food Quality. 2021: 3872190. [DOI: 10.1155/2021/3872190]
Song H., Yu W., Gao M., Liu X., Ma X. (2013). Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydrate Polymers. 96: 181-189. [DOI: 10.1016/j.carbpol.2013.03.068]
Sousa S., Gomes A.M., Pintado M.M., Silva J.P., Costa P., Amaral M.H., Duarte A.C., Rodrigues D., Rocha-Santos T.A.P., Freitas A.C. (2015). Characterization of freezing effect upon stability of, probiotic loaded, calcium-alginate microparticles. Food and Bioproducts Processing. 93: 90-97. [DOI: 10.1016/ j.fbp.2013.11.007]
Tantratian S., Pradeamchai M. (2020). Select a protective agent for encapsulation of Lactobacillus plantarum. LWT. 123: 109075. [DOI: 10.1016/j.lwt.2020.109075]
Wang L., Song M., Zhao Z., Chen X., Cai J., Cao Y., Xiao J. (2020). Lactobacillus acidophilus loaded pickering double emulsion with enhanced viability and colon-adhesion efficiency. LWT. 121: 108928. [DOI: 10.1016/j.lwt.2019.108928]
Weinbreck F., Bodnár I., Marco M.L. (2010). Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products?. International Journal of Food Microbiology. 136: 364-367. [DOI: 10.1016/j.ijfoodmicro.2009.11.004]
Zanjani M.A.K., Ehsani M.R., Ghiassi Tarzi B., Sharifan A. (2018). Promoting Lactobacillus casei and Bifidobacterium adolescentis survival by microencapsulation with different starches and chitosan and poly L‐lysine coatings in ice cream. Journal of Food Processing and Preservation. 42: e13318. [DOI: 10.1111/jfpp.13318]
Zhang L., Taal M.A., Boom R.M., Chen X.D., Schutyser M.A.I. (2018). Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT. 87: 318-325. [DOI: 10.1016/j.lwt.2017.09.005]
Zhang Y., Lin J., Zhong Q. (2015). The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Research International. 71: 9-15. [DOI: 10.1016/j.foodres. 2015.02.017]
This research focused on encapsulating L. plantarum within various internal phase gelled double emulsions, including gelatin, alginate, tragacanth gum, and carrageenan. The study examined bacterial survival under different heat treatments (pasteurization, microwave heating, and sterilization), environmental stressors (NaCl, bile salt, lysozyme, penicillin), and simulated gastrointestinal conditions. Notably, under simulated gastric conditions, bacterial reduction was more pronounced compared to simulated intestinal conditions. In the heat processing test, encapsulated L. plantarum demonstrated better survival rates compared to free cells, highlighting the significant role of gelling agents in protecting L. plantarum. Of all gelling agents tested, tragacanth gum exhibited the strongest protective effects. Results showed that the sensitivity of unencapsulated cells to heat processing was significantly higher than encapsulated cells. The sample containing tragacanth gum showed the highest cell viability when subjected to various heat processes. Furthermore, the study showed that DEs effectively increased the probiotics survival against NaCl, bile salt, lysozyme, and penicillin. Finally, the gastric conditions had a more detrimental effect on probiotic viability compared to the intestinal conditions. The study suggests that future research should explore the effects of oil and external water phase gelation on probiotic viability.
Author contributions
A.R., S.M.H.H., S.R., S.-S.H. and H.H. designed the study; S.A. conducted the experimental work and analyzed the data; S.A., A.R., S.M.H.H., S.R., S.-S.H. and H.H. wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors would like to thank the Shiraz University, Shiraz, Iran.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Ethical consideration
Not applicable.
References
Abbasi S., Rafati A., Hosseini S.M.H., Roohinejad S., Hashemi S.S., Hashemi Gahruie H., Rashidinejad A. (2023). The internal aqueous phase gelation improves the viability of probiotic cells in a double water/oil/water emulsion system. Food Science and Nutrition. 5978-5988. [DOI: 10.1002/fsn3.3532]
Allain T., Chaouch S., Thomas M., Vallée I., Buret A.G., Langella P., Grellier P., Polack B., Bermudez-Humaran L.G., Florent I. (2018). Bile-salt-hydrolases from the probiotic strain Lactobacillus johnsonii La1 mediate anti-giardial activity in vitro and in vivo. Frontiers in Microbiology. 8: 2707. [DOI: 10.3389/fmicb.2017.02707]
Anselmo A.C., McHugh K.J., Webster J., Langer R., Jaklenec A. (2016). Layer‐by‐layer encapsulation of probiotics for delivery to the microbiome. Advanced Materials. 28: 9486-9490. [DOI: 10.1002/adma.201603270]
Balcaen M., Vermeir L., Declerck A., Van Der Meeren P. (2016). Influence of internal water phase gelation on the shear-and osmotic sensitivity of W/O/W-type double emulsions. Food Hydrocolloids. 58: 356-363. [DOI: 10.1016/j.foodhyd. 2016.03.011]
Berendsen R., Güell C., Ferrando M. (2015). A procyanidin-rich extract encapsulated in water-in-oil-in-water emulsions produced by premix membrane emulsification. Food Hydrocolloids. 43: 636-648. [DOI: 10.1016/j.foodhyd.2014.07.023]
Bou R., Cofrades S., Jiménez-Colmenero F. (2014). Physicochemical properties and riboflavin encapsulation in double emulsions with different lipid sources. LWT-Food Science and Technology. 59: 621-628. [DOI: 10.1016/j.lwt.2014.06.044]
Chandramouli V., Kailasapathy K., Peiris P., Jones M. (2004). An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. Journal of Microbiological Methods. 56: 27-35. [DOI: 10.1016/j.mimet.2003.09.002]
Chen M.-J., Tang H.-Y., Chiang M.-L. (2017). Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiology. 66: 20-27. [DOI: 10.1016/j.fm.2017.03.020]
Cofrades S., Antoniou I., Solas M.T., Herrero A.M., Jiménez-Colmenero F. (2013). Preparation and impact of multiple (water-in-oil-in-water) emulsions in meat systems. Food Chemistry. 141: 338-346. [DOI: 10.1016/j.foodchem.2013.02.097]
Coghetto C.C., Brinques G.B., Siqueira N.M., Pletsch J., Soares R.M.D., Ayub M.A.Z. (2016). Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. Journal of Functional Foods. 24: 316-326. [DOI: 10.1016/j.jff.2016.03.036]
De Almeida Paula D., Martins E.M.F., De Almeida Costa N., De Oliveira P.M., De Oliveira E.B., Ramos A.M. (2019). Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. International Journal of Biological Macromolecules. 133: 722-731. [DOI: 10.1016/j.ijbiomac.2019.04.110]
Dianawati D., Mishra V., Shah N.P. (2013). Effect of drying methods of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris on secondary protein structure and glass transition temperature as studied by fourier transform infrared and differential scanning calorimetry. Journal of Dairy Science. 96: 1419-1430. [DOI: 10.3168/jds.2012-6058]
El-Dieb S.M., Abd Rabo F.H.R., Badran S.M., Abd El-Fattah A.M., Elshaghabee F.M.F. (2012). The growth behaviour and enhancement of probiotic viability in bioyoghurt. International Dairy Journal. 22: 44-47. [DOI: 10.1016/j.idairyj.2011.08.003]
El Kadri H., Lalou S., Mantzouridou F., Gkatzionis K. (2018). Utilisation of water-in-oil-water (W1/O/W2) double emulsion in a set-type yogurt model for the delivery of probiotic Lactobacillus paracasei. Food Research International. 107: 325-336. [DOI: 10.1016/j.foodres.2018.02.049]
Frakolaki G., Giannou V., Kekos D., Tzia C. (2021). A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Critical Reviews in Food Science and Nutrition. 61: 1515-1536. [DOI: 10.1080/ 10408398.2020.1761773]
Frank K., Walz E., Gräf V., Greiner R., Köhler K., Schuchmann H.P. (2012). Stability of anthocyanin-rich W/O/W‐emulsions designed for intestinal release in gastrointestinal environment. Journal of Food Science. 77: N50-N57. [DOI: 10.1111/j.1750-3841.2012.02982.x]
Giroux H.J., Constantineau S., Fustier P., Champagne C.P., St-Gelais D., Lacroix M., Britten M. (2013). Cheese fortification using water-in-oil-in-water double emulsions as carrier for water soluble nutrients. International Dairy Journal. 29: 107-114. [DOI: 10.1016/j.idairyj.2012.10.009]
Hernández-Gómez J.G., López-Bonilla A., Trejo-Tapia G., Ávila-Reyes S.V., Jiménez-Aparicio A.R., Hernández-Sánchez H. (2021). In vitro bile salt hydrolase (BSH) activity screening of different probiotic microorganisms. Foods. 10: 674-684. [DOI: 10.3390/foods10030674]
Hernández-Rodríguez L., Lobato-Calleros C., Pimentel-González D.J., Vernon-Carter E.J. (2014). Lactobacillus plantarum protection by entrapment in whey protein isolate: κ-carrageenan complex coacervates. Food Hydrocolloids. 36: 181-188. [DOI: 10.1016/j.foodhyd.2013.09.018]
Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. (2014). Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology and Hepatology. 11: 506-514. [DOI: 10.1038/nrgastro.2014.66]
Hosseinialhashemi M., Tavakoli J., Rafati A., Ahmadi F. (2021). The aplication of Pistacia khinjuk extract nanoemulsion in a biopolymeric coating to improve the shelf life extension of sunflower oil. Food Science and Nutrition. 9: 920-928. [DOI: 10.1002/fsn3.2057]
Irani M., Rafati A., Hashemi S.S., Barba F.J., Koubaa M., Roohinejad S. (2021). Biomass fractionation using emerging technologies. In: Koubaa M., Barba F.J., Roohinejad S. (Editors). Fermentation processes: emerging and conventional technologies. John Wiley and Sons Ltd., New Jersey, U.S. pp: 145-169. [DOI: 10.1002/9781119505822.ch5]
Jiménez-Colmenero F. (2013). Potential applications of multiple emulsions in the development of healthy and functional foods. Food Research International. 52: 64-74. [DOI: 10.1016/j.foodres.2013.02.040]
Kim W.S., Perl L., Park J.H., Tandianus J.E., Dunn N.W. (2001). Assessment of stress response of the probiotic Lactobacillus acidophilus. Current Microbiology. 43: 346-350. [DOI: 10.1007/s002840010314]
Koubaa M., Nikmaram N., Roohinejad S., Rafati A., Greiner R. (2018a). Multilayered emulsions. In: Roohinejad S., Greiner R., Oey I., Wen J. (Editors). Emulsion-based systems for delivery of food active compounds: formation, application, health and safety. John Wiley and Sons Ltd., New Jersey, U.S. pp: 105-119. [DOI: 10.1002/9781119247159.ch4]
Koubaa M., Roohinejad S., Sharma P., Nikmaram N., Hashemi S.S., Abbaspourrad A., Greiner R. (2018b). Multiple emulsions. In: Roohinejad S., Greiner R., Oey I., Wen J. (Editors). Emulsion‐based systems for delivery of food active compounds: formation, application, health and safety. John Wiley and Sons Ltd., New Jersey, U.S. pp: 69-103. [DOI: 10.1002/9781119247159.ch3]
Krasaekoopt W., Bhandari B., Deeth H. (2004). The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. International Dairy Journal. 14: 737-743. [DOI: 10.1016/j.idairyj.2004.01.004]
Lee K.-Y., Heo T.-R. (2000). Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salt solution. Applied and Environmental Microbiology. 66: 869-873. [DOI: 10.1128/AEM.66.2.869-873.2000]
Liu H., Cui S.W., Chen M., Li Y., Liang R., Xu F., Zhong F. (2019). Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: a review. Critical Reviews in Food Science and Nutrition. 59: 2863-2878. [DOI: 10.1080/10408398.2017.1377684]
Mandal S., Puniya A., Singh K. (2006). Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. International Dairy Journal. 16: 1190-1195. [DOI: 10.1016/j.idairyj.2005.10.005]
Manojlović V., Nedović V.A., Kailasapathy K., Zuidam N.J. (2010). Encapsulation of probiotics for use in food products. In: Zuidam N., Nedovic V. (Editors). Encapsulation technologies for active food ingredients and food processing. Springer, New York, NY. pp: 269-302. [DOI: 10.1007/978-1-4419-1008-0_10]
Moayyedi M., Eskandari M.H., Rad A.H.E., Ziaee E., Khodaparast M.H.H., Golmakani M.-T. (2018). Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. Journal of Functional Foods. 40: 391-399. [DOI: 10.1016/j.jff.2017.11.016]
Mohammadi-Gouraji E., Sheikh-Zeinoddin M., Soleimanian-Zad S. (2017). Effects of Persian gum and gum Arabic on the survival of Lactobacillus plantarum PTCC 1896, Escherichia coli, Xanthomonas axonopodis, and Saccharomyces cerevisiae during freeze drying. British Food Journal. 119: 331-341. [DOI: 10.1108/BFJ-09-2016-0442]
Nag A. (2011). Development of a microencapsulation technique for probiotic bacteria Lactobacillus casei 431 using a protein-polysaccharide complex. The degree of masters of technology in Food Technology thesis. Massey University, Palmerston North, New Zealand.
Oppermann A., Renssen M., Schuch A., Stieger M., Scholten E. (2015). Effect of gelation of inner dispersed phase on stability of (W1/O/W2) multiple emulsions. Food Hydrocolloids. 48: 17-26. [DOI: 10.1016/j.foodhyd.2015.01.027]
Ortakci F., Broadbent J.R., McManus W., McMahon D. (2012). Survival of microencapsulated probiotic Lactobacillus paracasei LBC-1e during manufacture of mozzarella cheese and simulated gastric digestion. Journal of Dairy Science, 95: 6274-6281, [DOI: 10.3168/jds.2012-5476]
Peighambardoust S., Tafti A.G., Hesari J. (2011). Application of spray drying for preservation of lactic acid starter cultures: a review. Trends in Food Science and Technology. 22: 215-224. [DOI: 10.1016/j.tifs.2011.01.009]
Pimentel-González D.J, Campos-Montiel R.G, Lobato-Calleros C., Pedroza-Islas R., Vernon-Carter, E.J. (2009). Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Research International. 42: 292-297. [DOI: 10.1016/j.foodres.2008.12.002]
Rodríguez-Huezo M.E, Estrada-Fernández A.G, García-Almendárez B.E, Ludena-Urquizo F., Campos-Montiel R.G, Pimentel-González D.J. (2014). Viability of Lactobacillus plantarum entrapped in double emulsion during Oaxaca cheese manufacture, melting and simulated intestinal conditions. LWT-Food Science and Technology. 59: 768-773. [DOI: 10.1016/ j.lwt.2014.07.004]
Schuch A., Wrenger J., Schuchmann H.P. (2014). Production of W/O/W double emulsions. part II: influence of emulsification device on release of water by coalescence. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 461: 344-351. [DOI: 10.1016/j.colsurfa.2013.11.044]
Shima M., Matsuo T., Yamashita M., Adachi S. (2009). Protection of Lactobacillus acidophilus from bile salts in a model intestinal juice by incorporation into the inner-water phase of a W/O/W emulsion. Food Hydrocolloids. 23: 281-285. [DOI: 10.1016/j.foodhyd.2008.01.008]
Shima M., Morita Y., Yamashita M., Adachi S. (2006). Protection of Lactobacillus acidophilus from the low pH of a model gastric juice by incorporation in a W/O/W emulsion. Food Hydrocolloids. 20: 1164-1169. [DOI: 10.1016/j.foodhyd. 2006.01.001]
Singhal N., Maurya A.K., Mohanty S., Kumar M., Virdi J.S. (2019). Evaluation of bile salt hydrolases, cholesterol-lowering capabilities, and probiotic potential of Enterococcus faecium isolated from rhizosphere. Frontiers in Microbiology. 10: 1567. [DOI: 10.3389/fmicb.2019.01567]
Soltani Lak A., Marhamatizadeh M.H., Fattahi H. (2021). Stability of encapsulated Lactobacillus reuteri during harsh conditions, storage period, and simulated in vitro conditions. Journal of Food Quality. 2021: 3872190. [DOI: 10.1155/2021/3872190]
Song H., Yu W., Gao M., Liu X., Ma X. (2013). Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydrate Polymers. 96: 181-189. [DOI: 10.1016/j.carbpol.2013.03.068]
Sousa S., Gomes A.M., Pintado M.M., Silva J.P., Costa P., Amaral M.H., Duarte A.C., Rodrigues D., Rocha-Santos T.A.P., Freitas A.C. (2015). Characterization of freezing effect upon stability of, probiotic loaded, calcium-alginate microparticles. Food and Bioproducts Processing. 93: 90-97. [DOI: 10.1016/ j.fbp.2013.11.007]
Tantratian S., Pradeamchai M. (2020). Select a protective agent for encapsulation of Lactobacillus plantarum. LWT. 123: 109075. [DOI: 10.1016/j.lwt.2020.109075]
Wang L., Song M., Zhao Z., Chen X., Cai J., Cao Y., Xiao J. (2020). Lactobacillus acidophilus loaded pickering double emulsion with enhanced viability and colon-adhesion efficiency. LWT. 121: 108928. [DOI: 10.1016/j.lwt.2019.108928]
Weinbreck F., Bodnár I., Marco M.L. (2010). Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products?. International Journal of Food Microbiology. 136: 364-367. [DOI: 10.1016/j.ijfoodmicro.2009.11.004]
Zanjani M.A.K., Ehsani M.R., Ghiassi Tarzi B., Sharifan A. (2018). Promoting Lactobacillus casei and Bifidobacterium adolescentis survival by microencapsulation with different starches and chitosan and poly L‐lysine coatings in ice cream. Journal of Food Processing and Preservation. 42: e13318. [DOI: 10.1111/jfpp.13318]
Zhang L., Taal M.A., Boom R.M., Chen X.D., Schutyser M.A.I. (2018). Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT. 87: 318-325. [DOI: 10.1016/j.lwt.2017.09.005]
Zhang Y., Lin J., Zhong Q. (2015). The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Research International. 71: 9-15. [DOI: 10.1016/j.foodres. 2015.02.017]
* Corresponding author (A. Rafati)
* E-mail: alireza_rafati@iau.ac.ir
ORCID ID: https://orcid.org/0000-0001-8061-697X
* E-mail: alireza_rafati@iau.ac.ir
ORCID ID: https://orcid.org/0000-0001-8061-697X
Type of Study: Original article |
Subject:
Special
Received: 24/08/20 | Accepted: 25/03/02 | Published: 25/03/30
Received: 24/08/20 | Accepted: 25/03/02 | Published: 25/03/30
References
1. Abbasi S., Rafati A., Hosseini S.M.H., Roohinejad S., Hashemi S.S., Hashemi Gahruie H., Rashidinejad A. (2023). The internal aqueous phase gelation improves the viability of probiotic cells in a double water/oil/water emulsion system. Food Science and Nutrition. 5978-5988. [DOI: 10.1002/fsn3.3532] [DOI:10.1002/fsn3.3532] [PMID] [PMCID]
2. Allain T., Chaouch S., Thomas M., Vallée I., Buret A.G., Langella P., Grellier P., Polack B., Bermudez-Humaran L.G., Florent I. (2018). Bile-salt-hydrolases from the probiotic strain Lactobacillus johnsonii La1 mediate anti-giardial activity in vitro and in vivo. Frontiers in Microbiology. 8: 2707. [DOI: 10.3389/fmicb.2017.02707] [DOI:10.3389/fmicb.2017.02707] [PMID] [PMCID]
3. Anselmo A.C., McHugh K.J., Webster J., Langer R., Jaklenec A. (2016). Layer‐by‐layer encapsulation of probiotics for delivery to the microbiome. Advanced Materials. 28: 9486-9490. [DOI: 10.1002/adma.201603270] [DOI:10.1002/adma.201603270] [PMID] [PMCID]
4. Balcaen M., Vermeir L., Declerck A., Van Der Meeren P. (2016). Influence of internal water phase gelation on the shear-and osmotic sensitivity of W/O/W-type double emulsions. Food Hydrocolloids. 58: 356-363. [DOI: 10.1016/j.foodhyd. 2016.03.011] [DOI:10.1016/j.foodhyd.2016.03.011]
5. Berendsen R., Güell C., Ferrando M. (2015). A procyanidin-rich extract encapsulated in water-in-oil-in-water emulsions produced by premix membrane emulsification. Food Hydrocolloids. 43: 636-648. [DOI: 10.1016/j.foodhyd.2014.07.023] [DOI:10.1016/j.foodhyd.2014.07.023]
6. Bou R., Cofrades S., Jiménez-Colmenero F. (2014). Physicochemical properties and riboflavin encapsulation in double emulsions with different lipid sources. LWT-Food Science and Technology. 59: 621-628. [DOI: 10.1016/j.lwt.2014.06.044] [DOI:10.1016/j.lwt.2014.06.044]
7. Chandramouli V., Kailasapathy K., Peiris P., Jones M. (2004). An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. Journal of Microbiological Methods. 56: 27-35. [DOI: 10.1016/j.mimet.2003.09.002] [DOI:10.1016/j.mimet.2003.09.002] [PMID]
8. Chen M.-J., Tang H.-Y., Chiang M.-L. (2017). Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiology. 66: 20-27. [DOI: 10.1016/j.fm.2017.03.020] [DOI:10.1016/j.fm.2017.03.020] [PMID]
9. Cofrades S., Antoniou I., Solas M.T., Herrero A.M., Jiménez-Colmenero F. (2013). Preparation and impact of multiple (water-in-oil-in-water) emulsions in meat systems. Food Chemistry. 141: 338-346. [DOI: 10.1016/j.foodchem.2013.02.097] [DOI:10.1016/j.foodchem.2013.02.097] [PMID]
10. Coghetto C.C., Brinques G.B., Siqueira N.M., Pletsch J., Soares R.M.D., Ayub M.A.Z. (2016). Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. Journal of Functional Foods. 24: 316-326. [DOI: 10.1016/j.jff.2016.03.036] [DOI:10.1016/j.jff.2016.03.036]
11. De Almeida Paula D., Martins E.M.F., De Almeida Costa N., De Oliveira P.M., De Oliveira E.B., Ramos A.M. (2019). Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. International Journal of Biological Macromolecules. 133: 722-731. [DOI: 10.1016/j.ijbiomac.2019.04.110] [DOI:10.1016/j.ijbiomac.2019.04.110] [PMID]
12. Dianawati D., Mishra V., Shah N.P. (2013). Effect of drying methods of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris on secondary protein structure and glass transition temperature as studied by fourier transform infrared and differential scanning calorimetry. Journal of Dairy Science. 96: 1419-1430. [DOI: 10.3168/jds.2012-6058] [DOI:10.3168/jds.2012-6058] [PMID]
13. El-Dieb S.M., Abd Rabo F.H.R., Badran S.M., Abd El-Fattah A.M., Elshaghabee F.M.F. (2012). The growth behaviour and enhancement of probiotic viability in bioyoghurt. International Dairy Journal. 22: 44-47. [DOI: 10.1016/j.idairyj.2011.08.003] [DOI:10.1016/j.idairyj.2011.08.003]
14. El Kadri H., Lalou S., Mantzouridou F., Gkatzionis K. (2018). Utilisation of water-in-oil-water (W1/O/W2) double emulsion in a set-type yogurt model for the delivery of probiotic Lactobacillus paracasei. Food Research International. 107: 325-336. [DOI: 10.1016/j.foodres.2018.02.049] [DOI:10.1016/j.foodres.2018.02.049] [PMID]
15. Frakolaki G., Giannou V., Kekos D., Tzia C. (2021). A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Critical Reviews in Food Science and Nutrition. 61: 1515-1536. [DOI: 10.1080/ 10408398.2020.1761773] [DOI:10.1080/10408398.2020.1761773] [PMID]
16. Frank K., Walz E., Gräf V., Greiner R., Köhler K., Schuchmann H.P. (2012). Stability of anthocyanin-rich W/O/W‐emulsions designed for intestinal release in gastrointestinal environment. Journal of Food Science. 77: N50-N57. [DOI: 10.1111/j.1750-3841.2012.02982.x] [DOI:10.1111/j.1750-3841.2012.02982.x] [PMID]
17. Giroux H.J., Constantineau S., Fustier P., Champagne C.P., St-Gelais D., Lacroix M., Britten M. (2013). Cheese fortification using water-in-oil-in-water double emulsions as carrier for water soluble nutrients. International Dairy Journal. 29: 107-114. [DOI: 10.1016/j.idairyj.2012.10.009] [DOI:10.1016/j.idairyj.2012.10.009]
18. Hernández-Gómez J.G., López-Bonilla A., Trejo-Tapia G., Ávila-Reyes S.V., Jiménez-Aparicio A.R., Hernández-Sánchez H. (2021). In vitro bile salt hydrolase (BSH) activity screening of different probiotic microorganisms. Foods. 10: 674-684. [DOI: 10.3390/foods10030674] [DOI:10.3390/foods10030674] [PMID] [PMCID]
19. Hernández-Rodríguez L., Lobato-Calleros C., Pimentel-González D.J., Vernon-Carter E.J. (2014). Lactobacillus plantarum protection by entrapment in whey protein isolate: κ-carrageenan complex coacervates. Food Hydrocolloids. 36: 181-188. [DOI: 10.1016/j.foodhyd.2013.09.018] [DOI:10.1016/j.foodhyd.2013.09.018]
20. Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. (2014). Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology and Hepatology. 11: 506-514. [DOI: 10.1038/nrgastro.2014.66] [DOI:10.1038/nrgastro.2014.66] [PMID]
21. Hosseinialhashemi M., Tavakoli J., Rafati A., Ahmadi F. (2021). The aplication of Pistacia khinjuk extract nanoemulsion in a biopolymeric coating to improve the shelf life extension of sunflower oil. Food Science and Nutrition. 9: 920-928. [DOI: 10.1002/fsn3.2057] [DOI:10.1002/fsn3.2057] [PMID] [PMCID]
22. Irani M., Rafati A., Hashemi S.S., Barba F.J., Koubaa M., Roohinejad S. (2021). Biomass fractionation using emerging technologies. In: Koubaa M., Barba F.J., Roohinejad S. (Editors). Fermentation processes: emerging and conventional technologies. John Wiley and Sons Ltd., New Jersey, U.S. pp: 145-169. [DOI: 10.1002/9781119505822.ch5] [DOI:10.1002/9781119505822.ch5]
23. Jiménez-Colmenero F. (2013). Potential applications of multiple emulsions in the development of healthy and functional foods. Food Research International. 52: 64-74. [DOI: 10.1016/j.foodres.2013.02.040] [DOI:10.1016/j.foodres.2013.02.040]
24. Kim W.S., Perl L., Park J.H., Tandianus J.E., Dunn N.W. (2001). Assessment of stress response of the probiotic Lactobacillus acidophilus. Current Microbiology. 43: 346-350. [DOI: 10.1007/s002840010314] [DOI:10.1007/s002840010314] [PMID]
25. Koubaa M., Nikmaram N., Roohinejad S., Rafati A., Greiner R. (2018a). Multilayered emulsions. In: Roohinejad S., Greiner R., Oey I., Wen J. (Editors). Emulsion-based systems for delivery of food active compounds: formation, application, health and safety. John Wiley and Sons Ltd., New Jersey, U.S. pp: 105-119. [DOI: 10.1002/9781119247159.ch4] [DOI:10.1002/9781119247159.ch4]
26. Koubaa M., Roohinejad S., Sharma P., Nikmaram N., Hashemi S.S., Abbaspourrad A., Greiner R. (2018b). Multiple emulsions. In: Roohinejad S., Greiner R., Oey I., Wen J. (Editors). Emulsion‐based systems for delivery of food active compounds: formation, application, health and safety. John Wiley and Sons Ltd., New Jersey, U.S. pp: 69-103. [DOI: 10.1002/9781119247159.ch3] [DOI:10.1002/9781119247159.ch3]
27. Krasaekoopt W., Bhandari B., Deeth H. (2004). The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. International Dairy Journal. 14: 737-743. [DOI: 10.1016/j.idairyj.2004.01.004] [DOI:10.1016/j.idairyj.2004.01.004]
28. Lee K.-Y., Heo T.-R. (2000). Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salt solution. Applied and Environmental Microbiology. 66: 869-873. [DOI: 10.1128/AEM.66.2.869-873.2000] [DOI:10.1128/AEM.66.2.869-873.2000] [PMID] [PMCID]
29. Liu H., Cui S.W., Chen M., Li Y., Liang R., Xu F., Zhong F. (2019). Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: a review. Critical Reviews in Food Science and Nutrition. 59: 2863-2878. [DOI: 10.1080/10408398.2017.1377684] [DOI:10.1080/10408398.2017.1377684] [PMID]
30. Mandal S., Puniya A., Singh K. (2006). Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. International Dairy Journal. 16: 1190-1195. [DOI: 10.1016/j.idairyj.2005.10.005] [DOI:10.1016/j.idairyj.2005.10.005]
31. Manojlović V., Nedović V.A., Kailasapathy K., Zuidam N.J. (2010). Encapsulation of probiotics for use in food products. In: Zuidam N., Nedovic V. (Editors). Encapsulation technologies for active food ingredients and food processing. Springer, New York, NY. pp: 269-302. [DOI: 10.1007/978-1-4419-1008-0_10] [DOI:10.1007/978-1-4419-1008-0_10]
32. Moayyedi M., Eskandari M.H., Rad A.H.E., Ziaee E., Khodaparast M.H.H., Golmakani M.-T. (2018). Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. Journal of Functional Foods. 40: 391-399. [DOI: 10.1016/j.jff.2017.11.016] [DOI:10.1016/j.jff.2017.11.016]
33. Mohammadi-Gouraji E., Sheikh-Zeinoddin M., Soleimanian-Zad S. (2017). Effects of Persian gum and gum Arabic on the survival of Lactobacillus plantarum PTCC 1896, Escherichia coli, Xanthomonas axonopodis, and Saccharomyces cerevisiae during freeze drying. British Food Journal. 119: 331-341. [DOI: 10.1108/BFJ-09-2016-0442] [DOI:10.1108/BFJ-09-2016-0442]
34. Nag A. (2011). Development of a microencapsulation technique for probiotic bacteria Lactobacillus casei 431 using a protein-polysaccharide complex. The degree of masters of technology in Food Technology thesis. Massey University, Palmerston North, New Zealand.
35. Oppermann A., Renssen M., Schuch A., Stieger M., Scholten E. (2015). Effect of gelation of inner dispersed phase on stability of (W1/O/W2) multiple emulsions. Food Hydrocolloids. 48: 17-26. [DOI: 10.1016/j.foodhyd.2015.01.027] [DOI:10.1016/j.foodhyd.2015.01.027]
36. Ortakci F., Broadbent J.R., McManus W., McMahon D. (2012). Survival of microencapsulated probiotic Lactobacillus paracasei LBC-1e during manufacture of mozzarella cheese and simulated gastric digestion. Journal of Dairy Science, 95: 6274-6281, [DOI: 10.3168/jds.2012-5476] [DOI:10.3168/jds.2012-5476] [PMID]
37. Peighambardoust S., Tafti A.G., Hesari J. (2011). Application of spray drying for preservation of lactic acid starter cultures: a review. Trends in Food Science and Technology. 22: 215-224. [DOI: 10.1016/j.tifs.2011.01.009] [DOI:10.1016/j.tifs.2011.01.009]
38. Pimentel-González D.J, Campos-Montiel R.G, Lobato-Calleros C., Pedroza-Islas R., Vernon-Carter, E.J. (2009). Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Research International. 42: 292-297. [DOI: 10.1016/j.foodres.2008.12.002] [DOI:10.1016/j.foodres.2008.12.002]
39. Rodríguez-Huezo M.E, Estrada-Fernández A.G, García-Almendárez B.E, Ludena-Urquizo F., Campos-Montiel R.G, Pimentel-González D.J. (2014). Viability of Lactobacillus plantarum entrapped in double emulsion during Oaxaca cheese manufacture, melting and simulated intestinal conditions. LWT-Food Science and Technology. 59: 768-773. [DOI: 10.1016/ j.lwt.2014.07.004] [DOI:10.1016/j.lwt.2014.07.004]
40. Schuch A., Wrenger J., Schuchmann H.P. (2014). Production of W/O/W double emulsions. part II: influence of emulsification device on release of water by coalescence. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 461: 344-351. [DOI: 10.1016/j.colsurfa.2013.11.044] [DOI:10.1016/j.colsurfa.2013.11.044]
41. Shima M., Matsuo T., Yamashita M., Adachi S. (2009). Protection of Lactobacillus acidophilus from bile salts in a model intestinal juice by incorporation into the inner-water phase of a W/O/W emulsion. Food Hydrocolloids. 23: 281-285. [DOI: 10.1016/j.foodhyd.2008.01.008] [DOI:10.1016/j.foodhyd.2008.01.008]
42. Shima M., Morita Y., Yamashita M., Adachi S. (2006). Protection of Lactobacillus acidophilus from the low pH of a model gastric juice by incorporation in a W/O/W emulsion. Food Hydrocolloids. 20: 1164-1169. [DOI: 10.1016/j.foodhyd. 2006.01.001] [DOI:10.1016/j.foodhyd.2006.01.001]
43. Singhal N., Maurya A.K., Mohanty S., Kumar M., Virdi J.S. (2019). Evaluation of bile salt hydrolases, cholesterol-lowering capabilities, and probiotic potential of Enterococcus faecium isolated from rhizosphere. Frontiers in Microbiology. 10: 1567. [DOI: 10.3389/fmicb.2019.01567] [DOI:10.3389/fmicb.2019.01567] [PMID] [PMCID]
44. Soltani Lak A., Marhamatizadeh M.H., Fattahi H. (2021). Stability of encapsulated Lactobacillus reuteri during harsh conditions, storage period, and simulated in vitro conditions. Journal of Food Quality. 2021: 3872190. [DOI: 10.1155/2021/3872190] [DOI:10.1155/2021/3872190]
45. Song H., Yu W., Gao M., Liu X., Ma X. (2013). Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydrate Polymers. 96: 181-189. [DOI: 10.1016/j.carbpol.2013.03.068] [DOI:10.1016/j.carbpol.2013.03.068] [PMID]
46. Sousa S., Gomes A.M., Pintado M.M., Silva J.P., Costa P., Amaral M.H., Duarte A.C., Rodrigues D., Rocha-Santos T.A.P., Freitas A.C. (2015). Characterization of freezing effect upon stability of, probiotic loaded, calcium-alginate microparticles. Food and Bioproducts Processing. 93: 90-97. [DOI: 10.1016/ j.fbp.2013.11.007] [DOI:10.1016/j.fbp.2013.11.007]
47. Tantratian S., Pradeamchai M. (2020). Select a protective agent for encapsulation of Lactobacillus plantarum. LWT. 123: 109075. [DOI: 10.1016/j.lwt.2020.109075] [DOI:10.1016/j.lwt.2020.109075]
48. Wang L., Song M., Zhao Z., Chen X., Cai J., Cao Y., Xiao J. (2020). Lactobacillus acidophilus loaded pickering double emulsion with enhanced viability and colon-adhesion efficiency. LWT. 121: 108928. [DOI: 10.1016/j.lwt.2019.108928] [DOI:10.1016/j.lwt.2019.108928]
49. Weinbreck F., Bodnár I., Marco M.L. (2010). Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products?. International Journal of Food Microbiology. 136: 364-367. [DOI: 10.1016/j.ijfoodmicro.2009.11.004] [DOI:10.1016/j.ijfoodmicro.2009.11.004] [PMID]
50. Zanjani M.A.K., Ehsani M.R., Ghiassi Tarzi B., Sharifan A. (2018). Promoting Lactobacillus casei and Bifidobacterium adolescentis survival by microencapsulation with different starches and chitosan and poly L‐lysine coatings in ice cream. Journal of Food Processing and Preservation. 42: e13318. [DOI: 10.1111/jfpp.13318] [DOI:10.1111/jfpp.13318]
51. Zhang L., Taal M.A., Boom R.M., Chen X.D., Schutyser M.A.I. (2018). Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT. 87: 318-325. [DOI: 10.1016/j.lwt.2017.09.005] [DOI:10.1016/j.lwt.2017.09.005]
52. Zhang Y., Lin J., Zhong Q. (2015). The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Research International. 71: 9-15. [DOI: 10.1016/j.foodres. 2015.02.017] [DOI:10.1016/j.foodres.2015.02.017]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







