Volume 12, Issue 3 (September 2025)
J. Food Qual. Hazards Control 2025, 12(3): 230-239 |
Back to browse issues page
Ethics code: IR.TBZMED.VCR.REC.1401.022
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yousefi M, Andishmand H, Abedi-Firoozjah R, Khorram S, Ostadrahimi A. Detoxification of Aflatoxin B1 on Dried White Mulberry (Morus alba L.) Using Dielectric Barrier Discharge Plasma. J. Food Qual. Hazards Control 2025; 12 (3) :230-239
URL: http://jfqhc.ssu.ac.ir/article-1-1257-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1257-en.html
Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran , ostadrahimi@tbzmed.ac.ir
Full-Text [PDF 563 kb]
(146 Downloads)
| Abstract (HTML) (279 Views)
Table 1: Central composite experimental design with process variables (uncoded) and observed responses
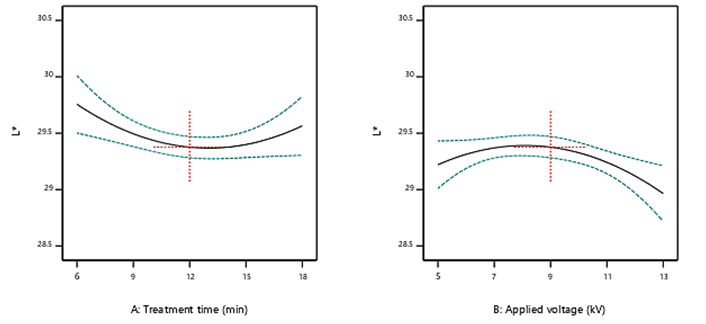
Figure 2: A 3D graph demonstrating effects of cold plasma applied voltage and treatment time on the Total Phenolic Content (TPC) of dried mulberries
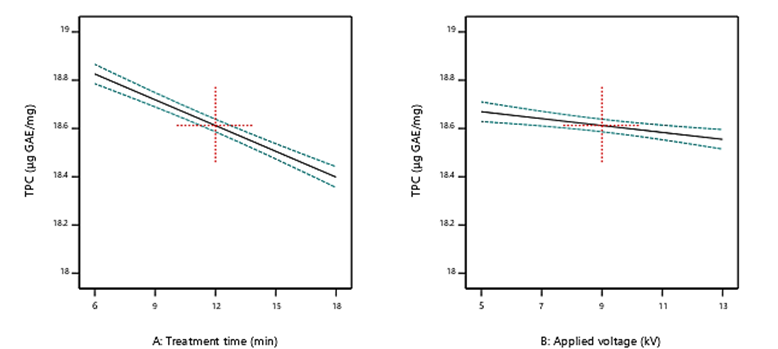
Figure 3: A 3D graph demonstrating effects of cold plasma applied voltage and treatment time on the lightness (L*) of dried mulberries
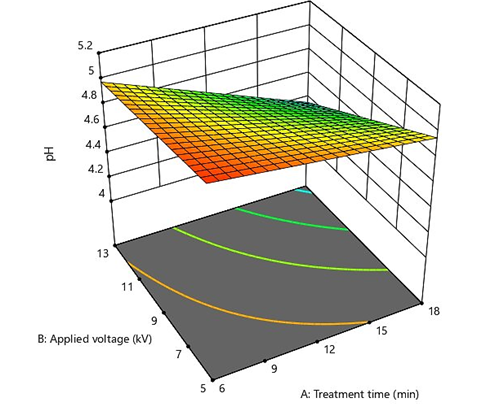
Figure 4: A 3D graph demonstrating effects of cold plasma applied voltage and treatment time on the decrease rate of aflatoxin B1 (AFB1) from the surface of dried mulberries
Full-Text: (4 Views)
Detoxification of Aflatoxin B1 on Dried White Mulberry (Morus alba L.) Using Dielectric Barrier Discharge Plasma
M. Yousefi 1, H. Andishmand 2,3, R. Abedi-Firoozjah 4, S. Khorram 5, A. Ostadrahimi 6**
1. Food and Beverages Safety Research Center, Urmia University of Medical Sciences, Urmia, Iran
2. Department of Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3. Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4. Student Research Committee, Department of Food Science and Technology, School of Nutrition Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran
5. Research Institute for Applied Physics and Astronomy, University of Tabriz, Tabriz, Iran
6. Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
HIGHLIGHTS
M. Yousefi 1, H. Andishmand 2,3, R. Abedi-Firoozjah 4, S. Khorram 5, A. Ostadrahimi 6**
1. Food and Beverages Safety Research Center, Urmia University of Medical Sciences, Urmia, Iran
2. Department of Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3. Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4. Student Research Committee, Department of Food Science and Technology, School of Nutrition Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran
5. Research Institute for Applied Physics and Astronomy, University of Tabriz, Tabriz, Iran
6. Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition & Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
- Cold plasma treatment reduced Aflatoxin B1 in dried mulberries by up to 62.6%.
- Treatment time had a stronger effect on phenolic degradation than applied voltage.
- Phenolic content and color were affected but sensory impact requires further study.
- Cold plasma shows promise as a non-thermal food safety technology for dried fruits.
| Article type Original article |
ABSTRACT Background: Aflatoxin B1 (AFB1) is an extremely toxic mycotoxin usually found in dried fruits, including mulberries, posing significant health risks. This study investigated the potential of cold plasma treatment to decrease AFB1 contamination in dried white mulberries (Morus alba L.) and its effects on product quality. Methods: A total of 5 kg of fresh white mulberries were harvested at full ripeness from Urmia, Iran, during June–July 2023. The fruits were sun-dried using traditional methods and artificially contaminated with AFB1 (2000 µg/kg). Samples (5 g per replicate, total n = 51) were treated using a cold plasma jet system positioned 1 cm above water-containing glass beakers with mulberries immersed in water. Treatments were applied at voltages of 5, 9, and 13 kV for 6, 12, and 18 min according to a Central Composite Design. AFB1 levels were quantified by High-Performance Liquid Chromatography coupled with immunoaffinity column cleanup. Quality parameters including pH, Total Phenolic Content (TPC), and color (L*) were measured. Statistical analysis was performed using Design-Expert software version 13 (Stat-Ease, Inc., Minneapolis, USA), employing analysis of Variance (ANOVA) and regression modeling. Results: Cold plasma treatment decreased AFB1 content by up to 62.6% at 13 kV and 18 min. pH decreased due to the combined effects of treatment time and voltage, whereas TPC decreased significantly with each factor individually, whereas color (L*) showed no significant change (p>0.05). Conclusion: Cold plasma effectively reduced AFB1 contamination in dried mulberries with minimal impact on color but caused decreases in pH (from 5.18 to 4.12) and TPC (by approximately 32%), indicating some quality degradation. Further studies should evaluate sensory attributes, microbial safety, and storage stability to confirm industrial applicability. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Aflatoxins Cold Plasma Mulberry Food Contamination Food Preservation |
||
| Article history Received: 3 Sep 2024 Revised: 16 Jul 2025 Accepted: 23 Sep 2025 |
||
| Abbreviations AFB1=Aflatoxin B1 DBD=Dielectric Barrier Discharge 2FI =Two-Factor Interaction IAC=Immunoaffinity Column TPC=Total Phenolic Content |
To cite: Yousefi M., Andishmand H., Abedi-Firoozjah R., Khorram S., Ostadrahimi A. (2025). Detoxification of aflatoxin b1 on dried white mulberry (Morus alba L.) using dielectric barrier discharge plasma. Journal of Food Quality and Hazards Control. 12: 230-239.
Introduction
Introduction
Dried fruits have high nutritional value for the human diet. However, these environmental products are also very suitable for the growth of various fungi and subsequent contamination by mycotoxins produced by these organisms (Heshmati and Mozaffari Nejad, 2015). To this end, the European :union:, in order to prevent the possible dangers of over-consumption of these products, has set the maximum permissible levels of aflatoxin B1 (AFB1) and total aflatoxin in dried fruits at 2 and 4 ug/kg, respectively (González-Curbelo and Kabak, 2023). AFB1 poses serious health risks, including liver cancer, growth impairment in children, immune suppression, and liver damage. It is categorized as a Group 1 carcinogen by International Agency for Research on Cancer (IARC) and is considered a key food safety concern worldwide. In addition to AFB1, other mycotoxins such as fumonisins and Ochratoxin A (OTA) are also relevant concerns in dried fruits. Ochratoxin A (OTA), primarily produced by Penicillium and Aspergillus species, is nephrotoxic and categorized as a possible human carcinogen (Group 2B) by the International Agency for Research on Cancer (IARC). Fumonisins, produced mainly by Fusarium species, though more common in cereals, have also been sporadically detected in dried fruit products. The presence of multiple mycotoxins highlights the importance of efficient decontamination strategies to ensure food safety in dried fruit consumption. In developing countries, due to the traditional methods of drying, dried fruits are vulnerable to contamination by a variety of fungi, followed by mycotoxins (González-Curbelo and Kabak, 2023). Dried mulberries are no exception. In this regard, a study at Hamadan (Heshmati et al., 2017) showed that more than 45% of dried berries sampled in 2016-2017 from different parts of Iran were contaminated with AFB1 more than the European :union: maximum permissible levels by using the High-Performance Liquid Chromatography (HPLC) method. In another study conducted in Pakistan (Luttfullah and Hussain, 2011), researchers stated that the percentage of contaminated dried berries with aflatoxins was about 26%. This percent was higher than the amount of aflatoxin observed in raisins (20%), dates (10%), dried apricots (20%), pistachios (20%) and pine nuts (20%). Also, in another study, higher levels of AFB1 were reported in berries compared to carob and grapes (Kaymak et al., 2018).
Most berries are eaten dried. Due to the short harvest time and also the high sensitivity of these products to storage, fresh berries are sensitive to microbial spoilage owing to the high level of humidity, even in cold storage conditions. Therefore, for long-term storage as well as domestic consumption and exportation of these products, the methods of freezing, drying, and making mulberry paste and marmalade are used (Samokhvalova et al., 2021). The method of drying mulberries is very important, which is both industrial and traditional. In general, the industrial method is preferable to the traditional method, but traditional drying methods (sunlight) are still used widely due to the ease of work and cheapness. However, a significant reduction in the product quality and quantity is observed in traditional methods due to exposure of fruits to microbial pathogens, aflatoxins, and contaminants caused by insect infestation (Doymaz, 2004). Therefore, developing strategies to deal with dried fruits contamination is necessary.
In today's world, the use of modern and non-thermal methods owing to the advantages such as faster processing time, low cost, and low side effects, to detoxify foods are very important. Cold Atmospheric Pressure Plasma (CAPP) is one of the newest and most promising technologies to reduce food mycotoxins. Cold Atmospheric Pressure Plasma (CAPP) degrades mycotoxins primarily through the action of Reactive Oxygen Species (ROS) such as hydroxyl radicals (•OH), ozone (O₃), and singlet oxygen (¹O₂), as well as Reactive Nitrogen Species (RNS) like nitric oxide (NO) and nitrogen dioxide (NO₂). These reactive species attack and break down the molecular structure of mycotoxins, especially targeting critical bonds like the lactone ring and furan ring in AFB1, leading to detoxification into less harmful compounds. The advantages of cold plasma include the usage of ambient air as a gas, rapid degradation at ambient temperature and atmospheric pressure, low cost, low substantial change in food quality, and the impact on an extensive range of microorganisms and toxins (Shi et al., 2017b). Recent developments in plasma engineering and the development of cold plasma technology at atmospheric pressure, as well as the increasing number of researches pertained to effects of cold plasma on various compounds in foods, have led many researchers to study the reduction of mycotoxins using cold plasma. Therefore, this study aimed to examine the effect of cold plasma on reducing AFB1 contamination and evaluating quality changes in dried mulberries.
Material and method
The standard of AFB1 (CAS No. 1162-65-8, Sigma-Aldrich, St. Louis, MO, USA) was purchased from Sigma-Aldrich. Gallic acid (G7384, Merck, Germany) and Folin-Ciocalteu’s phenol reagent (F9252, Merck, Germany) were obtained from Merck Chemical Co. (Darmstadt, Germany).
Collection of mulberries
Fresh mulberries were harvested at full ripeness from a mulberry plantation in Urmia city, Iran. The fruits were dried using traditional sun-drying methods without any pre-treatment. The mulberries were spread in a single layer on clean plastic trays and placed under direct sunlight for four days, with typical ambient daytime temperatures ranging from 27°C to 34°C and relative humidity between 35–50%. During nighttime, trays were covered with a clean net to prevent contamination. After drying, the samples were permitted to cool at room temperature, packed in polyethylene bags, then kept at 4°C until further analysis. The original moisture content of the dried mulberries was examined by oven-drying at 105°C to a constant weight, and was found to be 12.8±0.5%.
Contamination of dried mulberries with AFB1
Dried mulberries were first confirmed free from aflatoxin contamination. To artificially contaminate the samples, 1 mg of AFB1 powder was dissolved in 1 ml chloroform to prepare a stock solution. This solution was diluted with chloroform to gain a working concentration of 100 µg/ml. For each treatment, 5 g of dried mulberries were weighed into sterile Petri dishes and uniformly spiked with 100 µl of the 100 µg/ml AFB1 solution, delivering 10 µg of AFB1 per 5 g sample (equivalent to 2000 µg/kg or 2000 ppb initial contamination level). The spiked samples were left in a fume hood overnight to ensure complete chloroform evaporation before the treatment.
Cold plasma treatment
Cold plasma was produced using a custom-built plasma jet system. The device consisted of a single electrode linked to a high-voltage power supply, producing plasma jets directed toward the sample. The plasma jet was positioned approximately 1 cm above the surface of a glass container filled with deionized water. Dried mulberries were immersed in the water during plasma exposure to ensure uniform treatment. The system operated under ambient air without additional gas flow. The electrode configuration was a single-pin electrode, and the plasma was generated by applying a voltage of 5, 9, or 13 kV, as specified in the experimental design. In this phase, treatment times of 0, 2, 4, 6, 8, 10, 12, 14, 16, and 18 min were employed. After treatment with cold plasma, quality experiments of samples were immediately carried out. Therefore, the intended voltage and time of final experiments were determined based on pre-tests. Cold plasma was applied at 6, 12, and 18 min.
Design of the study
A Central Composite Design (CCD) with five center points and 17 runs using the Response Surface Methodology (RSM) was employed to investigate the impacts of the process variables on the quality properties and detoxification of dried mulberries. As mentioned before, cold plasma treatment with applied voltages of 5, 9, and 13 kV and treatment time of 6, 12, and 18 min were employed to explore the efficacy of cold plasma on dependent variables of pH, Total Phenolic Content (TPC), color, and AFB1 degradation. All models suggested for the variables were analyzed by Analysis of Variance (ANOVA) using SPSS version 16. Following Analysis of Variance (ANOVA), Duncan’s multiple range test (p<0.05) was used to determine significant differences among treatment means.
pH measurement
To measure the pH of mulberries, 10 g of a sample was mixed with 100 ml of deionized water for 2 min, then a pH meter (Metrohm AG, Switzerland) at room temperature was used in the room temperature (Viuda-Martos et al., 2015).
Determination of TPC
To extract phenolic compounds, 200 mg of dried mulberries powder (dry sample) were placed in an ultrasonic bath (model XUBA3, Grant Instruments, UK) with 10 ml of mixture of acetone (M011–500G, Merck, Germany) and water (80/20). The samples were centrifuged at 9000 rpm (25 °C) for 10 min and then filtered using Whatman No. 4 filter papers. The TPC of the extract was measured using Folin Ciocalteu reagent (Bey et al., 2013) with slight modifications. A solution (200 µl) of the extract was blended with 400 µl of sodium carbonate and 750 µl of Folin-Ciocalteu reagent. The mixture was stored at room temperature for 90 min. Then, the absorbance was determined at 720 nm by a spectrophotometer (Unico, UV-2100, USA). The TPC value was stated as μg Gallic Acid Equivalents (GAE)/mg of dry sample.
Measurement of color properties
The surface color of dried mulberries was measured using a HunterLab colorimeter (HunterLab MiniScan XE Plus, Reston, VA, USA) in terms of L* at room temperature (Gençdağ et al., 2021). Other color properties such as a* (red-green axis) and b* (yellow-blue axis) were not measured in this study due to limited scope. However, including these parameters in future research would provide a more complete understanding of the color variations induced by cold plasma treatment, especially since a* and b* values can reflect changes in pigment composition and visual quality.
Extraction and analysis of AFB1
The aflatoxin determination in dried mulberries was according to AOAC official method 999.07 with slight modifications (Karaca and Nas, 2006).
In brief, 5 g of dried mulberries were homogenized with 300 ml mixture of methanol-water (8:2) and 5 g NaCl using a blender for 5 min and filtered by a filter paper. Then, 20 ml of the mixture was diluted using 130 ml of deionized water and filtered through a glass microfiber filter. Next, 75 ml of the filtrate was further purified using an Immunoaffinity Column (IAC) specific for AFB1 (Aflatest®, Vicam, MA, USA). The IAC was equilibrated at room temperature and conditioned with 10 ml of Phosphate-Buffered Saline (PBS) at a flow rate of 1–2 ml/min. Subsequently, 75 ml of the diluted extract was passed through the column at a controlled flow rate of 3 ml/min. The IAC was then washed with 20 ml water. AFB1 was eluted using HPLC grade methanol and saved into a vial. Limit of Detection (LOD), and Limit of Quantification (LOQ) values were 0.36±0.04 µg/kg, and 0.9±0.05 µg/kg.
To determine AFB1, separation was carried out by a HPLC equipped with a Wellchrom K-1001 pump, a Rheodyne Model 7125 injector, and a RF10AXL fluorescence detector coupled with a Eurochrom 2000 integrator; all these equipment were from Knauer (Berlin, Germany). The analytical column utilized was a Chromolith Performance column (RP-18e, 100×4.6 mm) from Merck (Darmstadt, Germany). Water and methanol (55:45 v/v) with a flow rate of 1.3 ml/min was used as a mobile phase under isocratic conditions. The fluorescence detector was run at the 365 nm excitation wavelength and the 435 nm emission wavelength. A standard solution of AFB1 was made at 100 ppm, then it was utilized to prepare the working standards of AFB1 for HPLC analysis.
The HPLC system was calibrated using a sequence of AFB1 standard solutions prepared at a determined concentrations to establish a calibration curve with a correlation coefficient (R²) greater than 0.99. Quality control samples and blanks were analyzed alongside the samples to ensure accuracy and precision. Although internal standards were not used, method repeatability was confirmed by analyzing replicate samples, and standard recovery tests were performed to verify the extraction and detection efficiency.
Results and discussion
Despite the growing research on cold plasma for mycotoxin degradation, limited studies have focused specifically on dried mulberries, a popular dried fruit prone to aflatoxin contamination. This study fills this gap by evaluating both toxin detoxification and quality parameters under realistic drying and treatment conditions relevant to this fruit.
Unlike previous studies mainly focused on grains or fresh produce, this research provides novel insights into the efficacy of cold plasma for reducing AFB1 on dried mulberries while simultaneously assessing its effects on phenolic content, pH, and color. This dual focus helps clarify the trade-offs between toxin degradation and preservation of nutritional and sensory qualities in dried fruits.
Response surface methodology
The results of the Central Composite Design (CCD) experiments are presented in Table 1. Additionally, Table 2 summarizes the model fitting and variance analysis for each response variable. Among the tested models, the quadratic model provided the best fit for AFB1 degradation (R² = 0.9924, R²adj = 0.9899), indicating a strong predictive capacity and minimal deviation between predicted and experimental values. This suggests that both individual factors (treatment time and voltage), as well as their interaction and higher-order terms, significantly influenced AFB1 reduction. Similarly, pH followed a Two-Factor Interaction (2FI) model with a high R² (0.8870), showing that the interaction between voltage and treatment time played an important role. In contrast, TPC was adequately explained by a linear model (R² = 0.9414), where both voltage and time exerted independent but additive effects without significant interaction. For color (L), a quadratic model was suggested (R² = 0.7443), but with a lower adjusted R² (0.6591), reflecting the relatively minor changes in lightness and weaker dependence on plasma parameters. Overall, the best-fitted model was the quadratic model for AFB1 degradation, confirming that toxin detoxification was more sensitive to the combined and nonlinear effects of voltage and treatment duration compared to quality attributes.
pH measurement
pH is an important quality property of most food products. Any intense changes in the pH of edible products can cause an unfavorable impact on their shelf life (Shiekh and Benjakul, 2020). In this study, the pH of treated dried mulberries ranged between 4.12 and 5.18 (Table 1). According to the results, the impact of CAP on the pH of samples followed a 2FI model, so that heightening the treatment time and applied voltage led to a decrease in pH of dried mulberries (Figure 1). This phenomenon was clearer in higher applied voltages on the same treatment time.
Most berries are eaten dried. Due to the short harvest time and also the high sensitivity of these products to storage, fresh berries are sensitive to microbial spoilage owing to the high level of humidity, even in cold storage conditions. Therefore, for long-term storage as well as domestic consumption and exportation of these products, the methods of freezing, drying, and making mulberry paste and marmalade are used (Samokhvalova et al., 2021). The method of drying mulberries is very important, which is both industrial and traditional. In general, the industrial method is preferable to the traditional method, but traditional drying methods (sunlight) are still used widely due to the ease of work and cheapness. However, a significant reduction in the product quality and quantity is observed in traditional methods due to exposure of fruits to microbial pathogens, aflatoxins, and contaminants caused by insect infestation (Doymaz, 2004). Therefore, developing strategies to deal with dried fruits contamination is necessary.
In today's world, the use of modern and non-thermal methods owing to the advantages such as faster processing time, low cost, and low side effects, to detoxify foods are very important. Cold Atmospheric Pressure Plasma (CAPP) is one of the newest and most promising technologies to reduce food mycotoxins. Cold Atmospheric Pressure Plasma (CAPP) degrades mycotoxins primarily through the action of Reactive Oxygen Species (ROS) such as hydroxyl radicals (•OH), ozone (O₃), and singlet oxygen (¹O₂), as well as Reactive Nitrogen Species (RNS) like nitric oxide (NO) and nitrogen dioxide (NO₂). These reactive species attack and break down the molecular structure of mycotoxins, especially targeting critical bonds like the lactone ring and furan ring in AFB1, leading to detoxification into less harmful compounds. The advantages of cold plasma include the usage of ambient air as a gas, rapid degradation at ambient temperature and atmospheric pressure, low cost, low substantial change in food quality, and the impact on an extensive range of microorganisms and toxins (Shi et al., 2017b). Recent developments in plasma engineering and the development of cold plasma technology at atmospheric pressure, as well as the increasing number of researches pertained to effects of cold plasma on various compounds in foods, have led many researchers to study the reduction of mycotoxins using cold plasma. Therefore, this study aimed to examine the effect of cold plasma on reducing AFB1 contamination and evaluating quality changes in dried mulberries.
Material and method
The standard of AFB1 (CAS No. 1162-65-8, Sigma-Aldrich, St. Louis, MO, USA) was purchased from Sigma-Aldrich. Gallic acid (G7384, Merck, Germany) and Folin-Ciocalteu’s phenol reagent (F9252, Merck, Germany) were obtained from Merck Chemical Co. (Darmstadt, Germany).
Collection of mulberries
Fresh mulberries were harvested at full ripeness from a mulberry plantation in Urmia city, Iran. The fruits were dried using traditional sun-drying methods without any pre-treatment. The mulberries were spread in a single layer on clean plastic trays and placed under direct sunlight for four days, with typical ambient daytime temperatures ranging from 27°C to 34°C and relative humidity between 35–50%. During nighttime, trays were covered with a clean net to prevent contamination. After drying, the samples were permitted to cool at room temperature, packed in polyethylene bags, then kept at 4°C until further analysis. The original moisture content of the dried mulberries was examined by oven-drying at 105°C to a constant weight, and was found to be 12.8±0.5%.
Contamination of dried mulberries with AFB1
Dried mulberries were first confirmed free from aflatoxin contamination. To artificially contaminate the samples, 1 mg of AFB1 powder was dissolved in 1 ml chloroform to prepare a stock solution. This solution was diluted with chloroform to gain a working concentration of 100 µg/ml. For each treatment, 5 g of dried mulberries were weighed into sterile Petri dishes and uniformly spiked with 100 µl of the 100 µg/ml AFB1 solution, delivering 10 µg of AFB1 per 5 g sample (equivalent to 2000 µg/kg or 2000 ppb initial contamination level). The spiked samples were left in a fume hood overnight to ensure complete chloroform evaporation before the treatment.
Cold plasma treatment
Cold plasma was produced using a custom-built plasma jet system. The device consisted of a single electrode linked to a high-voltage power supply, producing plasma jets directed toward the sample. The plasma jet was positioned approximately 1 cm above the surface of a glass container filled with deionized water. Dried mulberries were immersed in the water during plasma exposure to ensure uniform treatment. The system operated under ambient air without additional gas flow. The electrode configuration was a single-pin electrode, and the plasma was generated by applying a voltage of 5, 9, or 13 kV, as specified in the experimental design. In this phase, treatment times of 0, 2, 4, 6, 8, 10, 12, 14, 16, and 18 min were employed. After treatment with cold plasma, quality experiments of samples were immediately carried out. Therefore, the intended voltage and time of final experiments were determined based on pre-tests. Cold plasma was applied at 6, 12, and 18 min.
Design of the study
A Central Composite Design (CCD) with five center points and 17 runs using the Response Surface Methodology (RSM) was employed to investigate the impacts of the process variables on the quality properties and detoxification of dried mulberries. As mentioned before, cold plasma treatment with applied voltages of 5, 9, and 13 kV and treatment time of 6, 12, and 18 min were employed to explore the efficacy of cold plasma on dependent variables of pH, Total Phenolic Content (TPC), color, and AFB1 degradation. All models suggested for the variables were analyzed by Analysis of Variance (ANOVA) using SPSS version 16. Following Analysis of Variance (ANOVA), Duncan’s multiple range test (p<0.05) was used to determine significant differences among treatment means.
pH measurement
To measure the pH of mulberries, 10 g of a sample was mixed with 100 ml of deionized water for 2 min, then a pH meter (Metrohm AG, Switzerland) at room temperature was used in the room temperature (Viuda-Martos et al., 2015).
Determination of TPC
To extract phenolic compounds, 200 mg of dried mulberries powder (dry sample) were placed in an ultrasonic bath (model XUBA3, Grant Instruments, UK) with 10 ml of mixture of acetone (M011–500G, Merck, Germany) and water (80/20). The samples were centrifuged at 9000 rpm (25 °C) for 10 min and then filtered using Whatman No. 4 filter papers. The TPC of the extract was measured using Folin Ciocalteu reagent (Bey et al., 2013) with slight modifications. A solution (200 µl) of the extract was blended with 400 µl of sodium carbonate and 750 µl of Folin-Ciocalteu reagent. The mixture was stored at room temperature for 90 min. Then, the absorbance was determined at 720 nm by a spectrophotometer (Unico, UV-2100, USA). The TPC value was stated as μg Gallic Acid Equivalents (GAE)/mg of dry sample.
Measurement of color properties
The surface color of dried mulberries was measured using a HunterLab colorimeter (HunterLab MiniScan XE Plus, Reston, VA, USA) in terms of L* at room temperature (Gençdağ et al., 2021). Other color properties such as a* (red-green axis) and b* (yellow-blue axis) were not measured in this study due to limited scope. However, including these parameters in future research would provide a more complete understanding of the color variations induced by cold plasma treatment, especially since a* and b* values can reflect changes in pigment composition and visual quality.
Extraction and analysis of AFB1
The aflatoxin determination in dried mulberries was according to AOAC official method 999.07 with slight modifications (Karaca and Nas, 2006).
In brief, 5 g of dried mulberries were homogenized with 300 ml mixture of methanol-water (8:2) and 5 g NaCl using a blender for 5 min and filtered by a filter paper. Then, 20 ml of the mixture was diluted using 130 ml of deionized water and filtered through a glass microfiber filter. Next, 75 ml of the filtrate was further purified using an Immunoaffinity Column (IAC) specific for AFB1 (Aflatest®, Vicam, MA, USA). The IAC was equilibrated at room temperature and conditioned with 10 ml of Phosphate-Buffered Saline (PBS) at a flow rate of 1–2 ml/min. Subsequently, 75 ml of the diluted extract was passed through the column at a controlled flow rate of 3 ml/min. The IAC was then washed with 20 ml water. AFB1 was eluted using HPLC grade methanol and saved into a vial. Limit of Detection (LOD), and Limit of Quantification (LOQ) values were 0.36±0.04 µg/kg, and 0.9±0.05 µg/kg.
To determine AFB1, separation was carried out by a HPLC equipped with a Wellchrom K-1001 pump, a Rheodyne Model 7125 injector, and a RF10AXL fluorescence detector coupled with a Eurochrom 2000 integrator; all these equipment were from Knauer (Berlin, Germany). The analytical column utilized was a Chromolith Performance column (RP-18e, 100×4.6 mm) from Merck (Darmstadt, Germany). Water and methanol (55:45 v/v) with a flow rate of 1.3 ml/min was used as a mobile phase under isocratic conditions. The fluorescence detector was run at the 365 nm excitation wavelength and the 435 nm emission wavelength. A standard solution of AFB1 was made at 100 ppm, then it was utilized to prepare the working standards of AFB1 for HPLC analysis.
The HPLC system was calibrated using a sequence of AFB1 standard solutions prepared at a determined concentrations to establish a calibration curve with a correlation coefficient (R²) greater than 0.99. Quality control samples and blanks were analyzed alongside the samples to ensure accuracy and precision. Although internal standards were not used, method repeatability was confirmed by analyzing replicate samples, and standard recovery tests were performed to verify the extraction and detection efficiency.
Results and discussion
Despite the growing research on cold plasma for mycotoxin degradation, limited studies have focused specifically on dried mulberries, a popular dried fruit prone to aflatoxin contamination. This study fills this gap by evaluating both toxin detoxification and quality parameters under realistic drying and treatment conditions relevant to this fruit.
Unlike previous studies mainly focused on grains or fresh produce, this research provides novel insights into the efficacy of cold plasma for reducing AFB1 on dried mulberries while simultaneously assessing its effects on phenolic content, pH, and color. This dual focus helps clarify the trade-offs between toxin degradation and preservation of nutritional and sensory qualities in dried fruits.
Response surface methodology
The results of the Central Composite Design (CCD) experiments are presented in Table 1. Additionally, Table 2 summarizes the model fitting and variance analysis for each response variable. Among the tested models, the quadratic model provided the best fit for AFB1 degradation (R² = 0.9924, R²adj = 0.9899), indicating a strong predictive capacity and minimal deviation between predicted and experimental values. This suggests that both individual factors (treatment time and voltage), as well as their interaction and higher-order terms, significantly influenced AFB1 reduction. Similarly, pH followed a Two-Factor Interaction (2FI) model with a high R² (0.8870), showing that the interaction between voltage and treatment time played an important role. In contrast, TPC was adequately explained by a linear model (R² = 0.9414), where both voltage and time exerted independent but additive effects without significant interaction. For color (L), a quadratic model was suggested (R² = 0.7443), but with a lower adjusted R² (0.6591), reflecting the relatively minor changes in lightness and weaker dependence on plasma parameters. Overall, the best-fitted model was the quadratic model for AFB1 degradation, confirming that toxin detoxification was more sensitive to the combined and nonlinear effects of voltage and treatment duration compared to quality attributes.
pH measurement
pH is an important quality property of most food products. Any intense changes in the pH of edible products can cause an unfavorable impact on their shelf life (Shiekh and Benjakul, 2020). In this study, the pH of treated dried mulberries ranged between 4.12 and 5.18 (Table 1). According to the results, the impact of CAP on the pH of samples followed a 2FI model, so that heightening the treatment time and applied voltage led to a decrease in pH of dried mulberries (Figure 1). This phenomenon was clearer in higher applied voltages on the same treatment time.
Table 1: Central composite experimental design with process variables (uncoded) and observed responses
| Run | A: Treatment time | B: Applied voltage | pH | TPC | L* | AFB1 |
| (min) | (kV) | (μgGAE/mg) | Degradation (%) | |||
| 1 | 12 | 9 | 4.94 | 18.63 | 29.23 | 31.5 |
| 2 | 12 | 5 | 5.01 | 18.72 | 29.22 | 23.5 |
| 3 | 6 | 5 | 5.11 | 18.83 | 29.58 | 14.3 |
| 4 | 18 | 13 | 4.12 | 18.2 | 29.13 | 59.5 |
| 5 | 12 | 9 | 4.68 | 18.64 | 29.37 | 35.2 |
| 6 | 6 | 13 | 4.96 | 18.78 | 29.3 | 34.9 |
| 7 | 12 | 9 | 4.89 | 18.6 | 29.45 | 31.9 |
| 8 | 18 | 5 | 4.95 | 18.44 | 29.19 | 29.7 |
| 9 | 6 | 13 | 4.98 | 18.81 | 29.32 | 33.1 |
| 10 | 18 | 13 | 4.46 | 18.37 | 29.19 | 62.6 |
| 11 | 18 | 13 | 4.5 | 18.39 | 29.22 | 60.3 |
| 12 | 6 | 5 | 5.18 | 18.86 | 29.64 | 15.4 |
| 13 | 12 | 9 | 4.91 | 18.65 | 29.42 | 33.2 |
| 14 | 6 | 13 | 4.99 | 18.74 | 29.34 | 35.4 |
| 15 | 12 | 9 | 4.89 | 18.62 | 29.41 | 30.8 |
| 16 | 18 | 5 | 4.97 | 18.47 | 29.55 | 31.5 |
| 17 | 6 | 5 | 5.12 | 18.87 | 29.66 | 12.7 |
AFB1=Aflatoxin B1; GAE=Gallic Acid Equivalent; TPC=Total Phenolic Content
Table 2: Statistical modeling and variance analysis of response variables.
Table 2: Statistical modeling and variance analysis of response variables.
| Variable | Suggested model | Model (coded factors) | R2 | R2adj |
| pH | 2FI |
 |
0.8870 | 0.8610 | ||
| TPC | Linear |  |
0.9414 | 0.9330 |
| L* | Quadratic |  |
0.7443 | 0.6591 |
| AFB1 | Quadratic |  |
0.9924 | 0.9899 |
AFB1=Aflatoxin B1; TPC=Total Phenolic Content
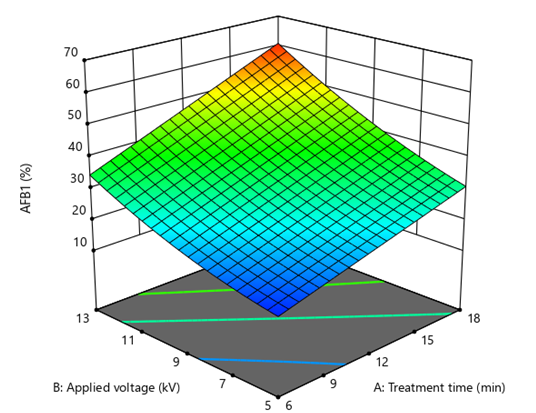
Figure 1: A 3D graph demonstrating effects of cold plasma applied voltage and treatment time on the pH of dried mulberries

Figure 1: A 3D graph demonstrating effects of cold plasma applied voltage and treatment time on the pH of dried mulberries
In general, the interaction of reactive species generated from CAP with the moisture on the surface of food products may form acidic substances and decline the pH value. The reduction in the pH of dried fruits was credited to the reactions of the cold plasma species, such as NOx, O3, and O with the water on the surface of dried food products (Ucar et al., 2021). A similar hypothesis was suggested by Oehmigen et al. (2010), indicating that acidification in CAP treatments may be related to the generation of nitric acid induced by Reactive Nitrogen Species (RNS) such as NO. There are also other studies showing no impact of cold plasma on the pH of food products (Ziuzina et al., 2016).
TPC
Phenolic compounds are natural substances synthesized by various plants as secondary metabolites, which have a substantial role in the coloration, cellular growth, and adjustment of fruit maturation. These compounds offer many health benefits due to their bioactive characteristics such as antioxidant, anti-inflammatory, antimicrobial, anticancer, antidiabetic, and anti-obesity properties (Wang et al., 2020).
Table 1 displays the TPC in dried mulberries after cold plasma exposure. The findings demonstrated no significant interaction effects between applied voltage with treatment time on total phenol content (p=0.23); however, both factors showed significant individual effects on the TPC (p=0.013 for treatment time and p=0.041 for applied voltage). As it is clear from figure 2, the increase in applied voltage and treatment time caused a meaningful decrease in the TPC. The reduction in TPC after cold plasma treatment can be credited to the production of reactive species which contribute in the oxidative degradation and the degradation of the central heterocycle in polyphenolic structures (Sarangapani et al., 2017). Also, the effectiveness of treatment time was considerably greater than that of applied voltage, as indicated by the larger absolute coefficient value for treatment time (-0.21) compared to voltage (-0.05) in the linear model for TPC (Table 2). This quantitatively emphasizes the greater influence of treatment duration over applied voltage in generating reactive species responsible for phenolic degradation.
TPC
Phenolic compounds are natural substances synthesized by various plants as secondary metabolites, which have a substantial role in the coloration, cellular growth, and adjustment of fruit maturation. These compounds offer many health benefits due to their bioactive characteristics such as antioxidant, anti-inflammatory, antimicrobial, anticancer, antidiabetic, and anti-obesity properties (Wang et al., 2020).
Table 1 displays the TPC in dried mulberries after cold plasma exposure. The findings demonstrated no significant interaction effects between applied voltage with treatment time on total phenol content (p=0.23); however, both factors showed significant individual effects on the TPC (p=0.013 for treatment time and p=0.041 for applied voltage). As it is clear from figure 2, the increase in applied voltage and treatment time caused a meaningful decrease in the TPC. The reduction in TPC after cold plasma treatment can be credited to the production of reactive species which contribute in the oxidative degradation and the degradation of the central heterocycle in polyphenolic structures (Sarangapani et al., 2017). Also, the effectiveness of treatment time was considerably greater than that of applied voltage, as indicated by the larger absolute coefficient value for treatment time (-0.21) compared to voltage (-0.05) in the linear model for TPC (Table 2). This quantitatively emphasizes the greater influence of treatment duration over applied voltage in generating reactive species responsible for phenolic degradation.

Figure 2: A 3D graph demonstrating effects of cold plasma applied voltage and treatment time on the Total Phenolic Content (TPC) of dried mulberries
Similar results have been found in the studies of Chutia and Mahanta (2021) and Tappi et al. (2018); however, there are other studies, which show an increased in TPC after treatment with cold plasma, such as the researches of Batista et al. (2021) and Kashfi et al. (2020). They reported that as the processing time of plasma treatment is heightened, the quantity of collisions and covalent bonds increases owing to the elevated electric charge inherently developed, and phenolic compounds are released from vacuole cells owing to the degradation of polysaccharides in cell walls, making them freely available and incrementing their number in the food matrix (Rodríguez et al., 2017). Also, some other studies have found no significant difference in TPC before and after exposure of edible materials to cold plasma such as the study of Amini and Ghoranneviss (2016).
Contradictory impacts of cold plasma treatment on TPC and color changes have been reported in the literature. For instance, while our study observed a significant reduction in TPC with incrementing treatment time and voltage, Batista et al. (2021) and Kashfi et al. (2020) reported an increase in phenolic compounds after plasma exposure. These differences could be credited to some factors including the variation in plasma generation systems, treatment parameters, and food matrices.
Our study employed a plasma jet device with electrode discharges positioned approximately 1 cm above a water layer containing dried mulberries, by means of ambient air as the employed gas. The plasma power, voltage range (5–13 kV), and treatment times (6–18 min) used in our setup differ considerably from those in studies that reported increases in TPC, which typically employed Dielectric Barrier Discharge (DBD) reactors operating at alternative frequencies and with distinct gas compositions, such as pure oxygen or nitrogen.
Moreover, the food matrix composition critically influences plasma effects. The dried mulberries in our study have relatively low moisture content and a complex phenolic profile, which may be more susceptible to oxidative degradation by reactive oxygen and nitrogen species produced in our plasma jet system. In contrast, fresh fruit or vegetable matrices with higher water content and different cell wall structures, such as those used by Batista et al. (2021) for avocado pulp or Kashfi et al. (2020) for peppermint, may experience enhanced extraction or release of bound phenolics due to plasma-induced cell wall disruption.
Reactor design, including electrode configuration and distance to the sample surface, affects plasma species distribution and intensity, influencing the balance between phenolic compound degradation and release. For example, DBD systems generally produce a more diffuse plasma over a larger surface area, whereas plasma jets can deliver localized high-energy species. This could explain why the plasma jets in our study caused phenolic degradation, whereas DBD setups in other studies increased TPC by liberating bound compounds.
Therefore, plasma system parameters, food matrix characteristics, and treatment conditions collectively contribute to the observed variability in cold plasma’s effect on phenolic content and color. Future studies should standardize the reporting of plasma parameters and include detailed characterization of food matrices to better understand and predict these effects.
Color properties of dried mulberries
The color of dried fruits is an important factor, which affects the optical appearance that has a substantial role in the consumer acceptability. Lightness (L*) is the most important parameter of dried Morus alba. Thereby, in this research, only the lightness index (L) was measured, as it is the most relevant parameter for dried mulberries, which varies in brightness from white to brown. Other indices (a* and b*) were not measured due to scope and equipment limitations but will be included in future studies. The impact of cold plasma on the lightness (0=black, 100=white) of dried mulberries is given in Table 1 and Figure 3. Response Surface Methodology (RSM) suggested a quadratic model for the impact of cold plasma on the lightness of dried mulberries. According to the results, all data were found in a range of 20-30 for the L* in all conditions of treatment. Also, the efficacy of the interaction factor was not significant (p>0.05). Since there was a tight range for the results obtained for color, it can be concluded that the treatment of dried mulberries with cold plasma in the conditions working in this study, did not demonstrate an unfavorable impact on the color of samples. The outcomes were in accordance with the study of Jangi et al. (2021), however, there are studies reporting contradictory results compared to our findings such as Kashfi et al. (2020) and Chen et al. (2020). The varieties in the applied treatment conditions, beside the type of food, are probably the most important factors affecting the function of CAP on the color of edible products.
Contradictory impacts of cold plasma treatment on TPC and color changes have been reported in the literature. For instance, while our study observed a significant reduction in TPC with incrementing treatment time and voltage, Batista et al. (2021) and Kashfi et al. (2020) reported an increase in phenolic compounds after plasma exposure. These differences could be credited to some factors including the variation in plasma generation systems, treatment parameters, and food matrices.
Our study employed a plasma jet device with electrode discharges positioned approximately 1 cm above a water layer containing dried mulberries, by means of ambient air as the employed gas. The plasma power, voltage range (5–13 kV), and treatment times (6–18 min) used in our setup differ considerably from those in studies that reported increases in TPC, which typically employed Dielectric Barrier Discharge (DBD) reactors operating at alternative frequencies and with distinct gas compositions, such as pure oxygen or nitrogen.
Moreover, the food matrix composition critically influences plasma effects. The dried mulberries in our study have relatively low moisture content and a complex phenolic profile, which may be more susceptible to oxidative degradation by reactive oxygen and nitrogen species produced in our plasma jet system. In contrast, fresh fruit or vegetable matrices with higher water content and different cell wall structures, such as those used by Batista et al. (2021) for avocado pulp or Kashfi et al. (2020) for peppermint, may experience enhanced extraction or release of bound phenolics due to plasma-induced cell wall disruption.
Reactor design, including electrode configuration and distance to the sample surface, affects plasma species distribution and intensity, influencing the balance between phenolic compound degradation and release. For example, DBD systems generally produce a more diffuse plasma over a larger surface area, whereas plasma jets can deliver localized high-energy species. This could explain why the plasma jets in our study caused phenolic degradation, whereas DBD setups in other studies increased TPC by liberating bound compounds.
Therefore, plasma system parameters, food matrix characteristics, and treatment conditions collectively contribute to the observed variability in cold plasma’s effect on phenolic content and color. Future studies should standardize the reporting of plasma parameters and include detailed characterization of food matrices to better understand and predict these effects.
Color properties of dried mulberries
The color of dried fruits is an important factor, which affects the optical appearance that has a substantial role in the consumer acceptability. Lightness (L*) is the most important parameter of dried Morus alba. Thereby, in this research, only the lightness index (L) was measured, as it is the most relevant parameter for dried mulberries, which varies in brightness from white to brown. Other indices (a* and b*) were not measured due to scope and equipment limitations but will be included in future studies. The impact of cold plasma on the lightness (0=black, 100=white) of dried mulberries is given in Table 1 and Figure 3. Response Surface Methodology (RSM) suggested a quadratic model for the impact of cold plasma on the lightness of dried mulberries. According to the results, all data were found in a range of 20-30 for the L* in all conditions of treatment. Also, the efficacy of the interaction factor was not significant (p>0.05). Since there was a tight range for the results obtained for color, it can be concluded that the treatment of dried mulberries with cold plasma in the conditions working in this study, did not demonstrate an unfavorable impact on the color of samples. The outcomes were in accordance with the study of Jangi et al. (2021), however, there are studies reporting contradictory results compared to our findings such as Kashfi et al. (2020) and Chen et al. (2020). The varieties in the applied treatment conditions, beside the type of food, are probably the most important factors affecting the function of CAP on the color of edible products.

Figure 3: A 3D graph demonstrating effects of cold plasma applied voltage and treatment time on the lightness (L*) of dried mulberries
AFB1 degradation
The result of treatment with CAP on AFB1 degradation on the surface of dried mulberries is exhibited in table 1. Based on the statistics, the impact of cold plasma on AFB1 reduction followed a 2FI model, in which a significant interaction effect of applied voltage and treatment time was observed, so that increasing the value of both independent variables led to the significant increase (p≤0.05) in degradation of AFB1 (Figure 4). The lowest degradation percentage of AFB1 (12.7%) after CAP treatment was at 6 min and 5 kV voltage, while the highest degradation percent (62.6%) was seen at 18 min and 13 kV applied voltage. The recovery was measured at 80.11±0.02%.
The result of treatment with CAP on AFB1 degradation on the surface of dried mulberries is exhibited in table 1. Based on the statistics, the impact of cold plasma on AFB1 reduction followed a 2FI model, in which a significant interaction effect of applied voltage and treatment time was observed, so that increasing the value of both independent variables led to the significant increase (p≤0.05) in degradation of AFB1 (Figure 4). The lowest degradation percentage of AFB1 (12.7%) after CAP treatment was at 6 min and 5 kV voltage, while the highest degradation percent (62.6%) was seen at 18 min and 13 kV applied voltage. The recovery was measured at 80.11±0.02%.

Figure 4: A 3D graph demonstrating effects of cold plasma applied voltage and treatment time on the decrease rate of aflatoxin B1 (AFB1) from the surface of dried mulberries
Regarding the degradation mechanism of CAP against AFB1, Shi et al. (2017a) have suggested two main pathways. The first path depends on the presence of water in the system, thus forming neutral, radical, and the ionized substances such as hydrogen atom (H) and the hydroxyl radical (OH•) after cold plasma treatment. Such species involved in the hydrogenation, hydration, and cleavage of methoxy group (OCH3), lead to the degradation of AFB1 and the production of one intermediate compound (C16H13O6) and two main products (C16H17O6 and C17H15O7). In our study, this pathway seems more dominant. The second branch includes the incorporation of H2 and aldehyde group (OCH). The reactions of first pathway mainly happen on the double bond (C8-C9) in the furan ring and lactone ring of AFB1. Lactone ring and C8-C9 double bond of furan ring are linked with carcinogenicity, teratogenicity, and mutagenicity of AFB1 (Puligundla et al., 2020). Besides the reactive species, UV photons of CAP can be effective in degradation of AFB1, however, more studies are needed to prove it. UV photons generated during cold plasma treatment contribute to AFB1 degradation by directly breaking chemical bonds within the toxin molecule, leading to its structural breakdown. Additionally, UV radiation helps produce Reactive Oxygen Species (ROS) that further oxidize and degrade AFB1. The joint action of UV photons and plasma-generated reactive species enhances the overall efficiency of AFB1 removal.
In a study conducted by Nishimwe et al. (2021), it was shown that 20 min of CAP treatment with 85 kV voltage applied, significantly decreased AFB1 cytotoxicity and demonstrated potential as a safe AFB1 degradation technology. In a comparable study, Nguyen et al. (2022) demonstrated that applying cold plasma treatment for 20 min at voltage of 80 kV to milk resulted in a significant 65% reduction in aflatoxin levels. Importantly, this detoxification occurred without any noticeable alteration in the milk’s color, indicating that the sensory and visual quality of the product was maintained. This finding reinforces the potential of CAP as an effective non-thermal food processing technology capable of degrading harmful mycotoxins like aflatoxins while preserving key quality attributes. It suggests that cold plasma can be utilized safely in various food matrices to improve food safety without compromising consumer acceptance (Nguyen et al., 2022).
Conclusion
The design of this study could be divided into two sections: (a) the effect of CAP on quality attributes of dried mulberries and (b) its effect on AFB1 degradation from the surface of the samples. Concerning the quality properties of samples, although cold plasma treatment showed promising results for the color of dried mulberries (with no significant change in color), it caused a decrease in the samples’ TPC and pH.
Regarding the AFB1, cold plasma demonstrated an acceptable reduction in the percentage of toxin after treatment. Approximately 62.6 % decrease in AFB1 was observed after 18 min of treatment with cold plasma at a constant applied voltage of 13 kV. Overall, it should be noted that changes in the variables of time and voltage during CAP treatment had a meaningful effect on the majority of experiments.
Cold plasma technology displayed great promise as a useful, non-thermal method for reducing aflatoxin contamination and altering phenolic content in dried mulberries, with potential applications in enhancing food safety and quality. Its ability to generate reactive species without heat makes it appropriate for treating heat-sensitive foodstuffs. Future research should focus on scaling up the technology, evaluating its impacts on microbial safety and sensory properties, and optimizing treatment conditions for various food matrices. This will help establish CAP as a practical tool for the food industry to ensure safer, higher-quality products.
This study had some limitations. First, microbiological safety, sensory attributes, and long-term storage stability of dried mulberries after cold plasma treatment were not assessed, which are important for industrial application. Second, the plasma setup and treatment conditions used here may not directly translate to an industrial scale. Future research should address these aspects to better establish the practical applicability of cold plasma technology in dried fruit processing.
Author contributions
M.Y. performed formal analysis, investigation, methodology, software, and wrote the original draft and wrote–reviewed and edited the manuscript; R.A.-F. handled data curation, formal analysis, software, investigation, and methodology; H.A. contributed to investigation, project administration, data curation, methodology, resources, software, and manuscript writing–review and editing; S.K. was responsible for data curation, project administration, and validation; A.O. provided conceptualization, funding acquisition, project administration, data curation, resources, supervision, and validation. All authors read and approved the final manuscript.
Acknowledgments
This study was supported by nutrition research center, Tabriz University of Medical Sciences, Tabriz, Iran.
Conflicts of interest
All authors declare no conflict of interest.
Funding
This research received a grant from nutrition research center, Tabriz University of Medical Sciences, Tabriz, Iran.
Ethical consideration
All study protocols were reviewed and ethically approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (Approval ID: IR.TBZMED.VCR.REC.1401.022).
References
Amini M., Ghoranneviss M. (2016). Effects of cold plasma treatment on antioxidants activity, phenolic contents and shelf life of fresh and dried walnut (Juglans regia L.) cultivars during storage. LWT. 73: 178-184. [DOI: 10.1016/j.lwt.2016.06.014]
Batista J.D.F., Dantas A.M., Dos Santos Fonseca J.V., Madruga M.S., Fernandes F.A.N., Rodrigues S., Da Silva Campelo Borges G. (2021). Effects of cold plasma on avocado pulp (Persea americana Mill.): chemical characteristics and bioactive compounds. Journal of Food Processing and Preservation. 45: e15179. [DOI: 10.1111/jfpp.15179]
Bey M.B., Louaileche H., Zemouri S. (2013). Optimization of phenolic compound recovery and antioxidant activity of light and dark dried fig (Ficus carica L.) varieties. Food Science and Biotechnology. 22: 1613-1619. [DOI: 10.1007/s10068-013-0258-7]
Chen Y., Zhang Y., Jiang L., Chen G., Yu J., Li S., Chen Y. (2020). Moisture molecule migration and quality changes of fresh wet noodles dehydrated by cold plasma treatment. Food Chemistry. 328: 127053. [DOI: 10.1016/j.foodchem.2020.127053]
Chutia H., Mahanta C.L. (2021). Influence of cold plasma voltage and time on quality attributes of tender coconut water (Cocos nucifera L.) and degradation kinetics of its blended beverage. Journal of Food Processing and Preservation. 45: e15372. [DOI: 10.1111/jfpp.15372]
Doymaz İ. (2004). Pretreatment effect on sun drying of mulberry fruits (Morus alba L.). Journal of Food Engineering. 65: 205-209. [DOI: 10.1016/j.jfoodeng.2004.01.016]
Gençdağ E., Görgüç A., Okuroğlu F., Yılmaz F.M. (2021). The effects of power ‐ ultrasound, peroxyacetic acid and sodium chloride washing treatments on the physical and chemical quality characteristics of dried figs. Journal of Food Processing and Preservation. 45: e15009. [DOI: 10.1111/jfpp.15009]
González-Curbelo M.Á., Kabak B. (2023). Occurrence of mycotoxins in dried fruits worldwide, with a focus on aflatoxins and ochratoxin A: a review. Toxins. 15: 576. [DOI: 10.3390/toxins15090576]
Heshmati A., Mozaffari Nejad A.S. (2015). Ochratoxin A in dried grapes in Hamadan province, Iran. Food Additives and Contaminants: Part B. 8: 255-259. [DOI: 10.1080/ 19393210.2015.1074945]
Heshmati A., Zohrevand T., Mousavi Khaneghah A., Mozaffari Nejad A.S., Sant’Ana A.S. (2017). Co-occurrence of aflatoxins and ochratoxin A in dried fruits in Iran: dietary exposure risk assessment. Food and Chemical Toxicology. 106: 202-208. [DOI: 10.1016/j.fct.2017.05.046]
Jangi F., Ebadi M.-T., Ayyari M. (2021). Qualitative changes in hyssop (Hyssopus officinalis L.) as affected by cold plasma, packaging method and storage duration. Journal of Applied Research on Medicinal and Aromatic Plants. 22: 100289. [DOI: 10.1016/j.jarmap.2020.100289]
Karaca H., Nas S. (2006). Aflatoxins, patulin and ergosterol contents of dried figs in Turkey. Food Additives and Contaminants. 23: 502-508. [DOI: 10.1080/02652030600550739]
Kashfi A.S., Ramezan Y., Khani M.R. (2020). Simultaneous study of the antioxidant activity, microbial decontamination and color of dried peppermint (Mentha piperita L.) using low pressure cold plasma. LWT. 123: 109121. [DOI: 10.1016/j.lwt.2020.109121]
Kaymak T., Türker L., Tulay H., Stroka J. (2018). Determination of aflatoxins and ochratoxin A in traditional Turkish concentrated fruit juice products by multi-immunoaffinity column cleanup and LC fluorescence detection: single-laboratory validation. Journal of AOAC International. 101: 1839-1849. [DOI: 10.5740/ jaoacint.17-0463]
Luttfullah G., Hussain A. (2011). Studies on contamination level of aflatoxins in some dried fruits and nuts of Pakistan. Food Control. 22: 426-429. [DOI: 10.1016/j.foodcont.2010.09.015]
Nguyen T., Palmer J., Phan N., Shi H., Keener K., Flint S. (2022). Control of aflatoxin M1 in skim milk by high voltage atmospheric cold plasma. Food Chemistry: 386: 132814. [DOI: 10.1016/j.foodchem.2022.132814]
Nishimwe K., Agbemafle I., Reddy M.B., Keener K., Maier D.E. (2021). Cytotoxicity assessment of aflatoxin B1 after high voltage atmospheric cold plasma treatment. Toxicon. 194: 17-22. [DOI: 10.1016/j.toxicon.2021.02.008]
Oehmigen K., Hähnel M., Brandenburg R., Wilke C., Weltmann K.-D., Von Woedtke T. (2010). The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Processes and Polymers. 7: 250-257. [DOI: 10.1002/ppap.200900077]
Puligundla P., Lee T., Mok C. (2020). Effect of corona discharge plasma jet treatment on the degradation of aflatoxin B1 on glass slides and in spiked food commodities. LWT. 124: 108333. [DOI: 10.1016/j.lwt.2019.108333]
Rodríguez Ó., Gomes W.F., Rodrigues S., Fernandes F.A.N. (2017). Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT. 84: 457-463. [DOI: 10.1016/j.lwt.2017.06.010]
Samokhvalova O., Kasabova K., Shmatchenko N., Zagorulko A., Zahorulko A. (2021). Improving the marmalade technology by adding a multicomponent fruit-and-berry paste. Eastern-European Journal of Enterprise Technologies. 6: 6-14. [DOI: 10.15587/1729-4061.2021.245986]
Sarangapani C., O'Toole G., Cullen P.J., Bourke P. (2017). Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innovative Food Science and Emerging Technologies. 44: 235-241. [DOI: 10.1016/j.ifset.2017.02.012]
Shi H., Cooper B., Stroshine R.L., Ileleji K.E., Keener K.M. (2017a). Structures of degradation products and degradation pathways of aflatoxin B1 by high-voltage atmospheric cold plasma (HVACP) treatment. Journal of Agricultural and Food Chemistry. 65: 6222-6230. [DOI: 10.1021/acs.jafc.7b01604]
Shi H., Ileleji K., Stroshine R.L., Keener K., Jensen J.L. (2017b). Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food and Bioprocess Technology. 10: 1042-1052. [DOI: 10.1007/s11947-017-1873-8]
Shiekh K.A., Benjakul S. (2020). Effect of high voltage cold atmospheric plasma processing on the quality and shelf-life of Pacific white shrimp treated with Chamuang leaf extract. Innovative Food Science and Emerging Technologies. 64: 102435. [DOI: 10.1016/j.ifset.2020.102435]
Tappi S., Ramazzina I., Rizzi F., Sacchetti G., Ragni L., Rocculi P. (2018). Effect of plasma exposure time on the polyphenolic profile and antioxidant activity of fresh-cut apples. Applied Sciences. 8: 1939. [DOI: 10.3390/app8101939]
Ucar Y., Ceylan Z., Durmus M., Tomar O., Cetinkaya T. (2021). Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends in Food Science and Technology. 114: 355-371. [DOI: 10.1016/j.tifs.2021.06.004]
Viuda-Martos M., Barber X., Pérez-Álvarez J.A., Fernández-López J. (2015). Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Industrial Crops and Products. 69: 472-479. [DOI: 10.1016/j.indcrop.2015.03.005]
Wang Z., Li S., Ge S., Lin S. (2020). Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. Journal of Agricultural and Food Chemistry. 68: 3330-3343. [DOI: 10.1021/acs.jafc.9b06574]
Ziuzina D., Misra N.N., Cullen P.J., Keener K., Mosnier J.P., Vilaró I., Gaston E., Bourke P. (2016). Demonstrating the potential of industrial scale in-package atmospheric cold plasma for decontamination of cherry tomatoes. Plasma Medicine. 6: 397-412. [DOI: 10.1615/PlasmaMed.2017019498]
In a study conducted by Nishimwe et al. (2021), it was shown that 20 min of CAP treatment with 85 kV voltage applied, significantly decreased AFB1 cytotoxicity and demonstrated potential as a safe AFB1 degradation technology. In a comparable study, Nguyen et al. (2022) demonstrated that applying cold plasma treatment for 20 min at voltage of 80 kV to milk resulted in a significant 65% reduction in aflatoxin levels. Importantly, this detoxification occurred without any noticeable alteration in the milk’s color, indicating that the sensory and visual quality of the product was maintained. This finding reinforces the potential of CAP as an effective non-thermal food processing technology capable of degrading harmful mycotoxins like aflatoxins while preserving key quality attributes. It suggests that cold plasma can be utilized safely in various food matrices to improve food safety without compromising consumer acceptance (Nguyen et al., 2022).
Conclusion
The design of this study could be divided into two sections: (a) the effect of CAP on quality attributes of dried mulberries and (b) its effect on AFB1 degradation from the surface of the samples. Concerning the quality properties of samples, although cold plasma treatment showed promising results for the color of dried mulberries (with no significant change in color), it caused a decrease in the samples’ TPC and pH.
Regarding the AFB1, cold plasma demonstrated an acceptable reduction in the percentage of toxin after treatment. Approximately 62.6 % decrease in AFB1 was observed after 18 min of treatment with cold plasma at a constant applied voltage of 13 kV. Overall, it should be noted that changes in the variables of time and voltage during CAP treatment had a meaningful effect on the majority of experiments.
Cold plasma technology displayed great promise as a useful, non-thermal method for reducing aflatoxin contamination and altering phenolic content in dried mulberries, with potential applications in enhancing food safety and quality. Its ability to generate reactive species without heat makes it appropriate for treating heat-sensitive foodstuffs. Future research should focus on scaling up the technology, evaluating its impacts on microbial safety and sensory properties, and optimizing treatment conditions for various food matrices. This will help establish CAP as a practical tool for the food industry to ensure safer, higher-quality products.
This study had some limitations. First, microbiological safety, sensory attributes, and long-term storage stability of dried mulberries after cold plasma treatment were not assessed, which are important for industrial application. Second, the plasma setup and treatment conditions used here may not directly translate to an industrial scale. Future research should address these aspects to better establish the practical applicability of cold plasma technology in dried fruit processing.
Author contributions
M.Y. performed formal analysis, investigation, methodology, software, and wrote the original draft and wrote–reviewed and edited the manuscript; R.A.-F. handled data curation, formal analysis, software, investigation, and methodology; H.A. contributed to investigation, project administration, data curation, methodology, resources, software, and manuscript writing–review and editing; S.K. was responsible for data curation, project administration, and validation; A.O. provided conceptualization, funding acquisition, project administration, data curation, resources, supervision, and validation. All authors read and approved the final manuscript.
Acknowledgments
This study was supported by nutrition research center, Tabriz University of Medical Sciences, Tabriz, Iran.
Conflicts of interest
All authors declare no conflict of interest.
Funding
This research received a grant from nutrition research center, Tabriz University of Medical Sciences, Tabriz, Iran.
Ethical consideration
All study protocols were reviewed and ethically approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (Approval ID: IR.TBZMED.VCR.REC.1401.022).
References
Amini M., Ghoranneviss M. (2016). Effects of cold plasma treatment on antioxidants activity, phenolic contents and shelf life of fresh and dried walnut (Juglans regia L.) cultivars during storage. LWT. 73: 178-184. [DOI: 10.1016/j.lwt.2016.06.014]
Batista J.D.F., Dantas A.M., Dos Santos Fonseca J.V., Madruga M.S., Fernandes F.A.N., Rodrigues S., Da Silva Campelo Borges G. (2021). Effects of cold plasma on avocado pulp (Persea americana Mill.): chemical characteristics and bioactive compounds. Journal of Food Processing and Preservation. 45: e15179. [DOI: 10.1111/jfpp.15179]
Bey M.B., Louaileche H., Zemouri S. (2013). Optimization of phenolic compound recovery and antioxidant activity of light and dark dried fig (Ficus carica L.) varieties. Food Science and Biotechnology. 22: 1613-1619. [DOI: 10.1007/s10068-013-0258-7]
Chen Y., Zhang Y., Jiang L., Chen G., Yu J., Li S., Chen Y. (2020). Moisture molecule migration and quality changes of fresh wet noodles dehydrated by cold plasma treatment. Food Chemistry. 328: 127053. [DOI: 10.1016/j.foodchem.2020.127053]
Chutia H., Mahanta C.L. (2021). Influence of cold plasma voltage and time on quality attributes of tender coconut water (Cocos nucifera L.) and degradation kinetics of its blended beverage. Journal of Food Processing and Preservation. 45: e15372. [DOI: 10.1111/jfpp.15372]
Doymaz İ. (2004). Pretreatment effect on sun drying of mulberry fruits (Morus alba L.). Journal of Food Engineering. 65: 205-209. [DOI: 10.1016/j.jfoodeng.2004.01.016]
Gençdağ E., Görgüç A., Okuroğlu F., Yılmaz F.M. (2021). The effects of power ‐ ultrasound, peroxyacetic acid and sodium chloride washing treatments on the physical and chemical quality characteristics of dried figs. Journal of Food Processing and Preservation. 45: e15009. [DOI: 10.1111/jfpp.15009]
González-Curbelo M.Á., Kabak B. (2023). Occurrence of mycotoxins in dried fruits worldwide, with a focus on aflatoxins and ochratoxin A: a review. Toxins. 15: 576. [DOI: 10.3390/toxins15090576]
Heshmati A., Mozaffari Nejad A.S. (2015). Ochratoxin A in dried grapes in Hamadan province, Iran. Food Additives and Contaminants: Part B. 8: 255-259. [DOI: 10.1080/ 19393210.2015.1074945]
Heshmati A., Zohrevand T., Mousavi Khaneghah A., Mozaffari Nejad A.S., Sant’Ana A.S. (2017). Co-occurrence of aflatoxins and ochratoxin A in dried fruits in Iran: dietary exposure risk assessment. Food and Chemical Toxicology. 106: 202-208. [DOI: 10.1016/j.fct.2017.05.046]
Jangi F., Ebadi M.-T., Ayyari M. (2021). Qualitative changes in hyssop (Hyssopus officinalis L.) as affected by cold plasma, packaging method and storage duration. Journal of Applied Research on Medicinal and Aromatic Plants. 22: 100289. [DOI: 10.1016/j.jarmap.2020.100289]
Karaca H., Nas S. (2006). Aflatoxins, patulin and ergosterol contents of dried figs in Turkey. Food Additives and Contaminants. 23: 502-508. [DOI: 10.1080/02652030600550739]
Kashfi A.S., Ramezan Y., Khani M.R. (2020). Simultaneous study of the antioxidant activity, microbial decontamination and color of dried peppermint (Mentha piperita L.) using low pressure cold plasma. LWT. 123: 109121. [DOI: 10.1016/j.lwt.2020.109121]
Kaymak T., Türker L., Tulay H., Stroka J. (2018). Determination of aflatoxins and ochratoxin A in traditional Turkish concentrated fruit juice products by multi-immunoaffinity column cleanup and LC fluorescence detection: single-laboratory validation. Journal of AOAC International. 101: 1839-1849. [DOI: 10.5740/ jaoacint.17-0463]
Luttfullah G., Hussain A. (2011). Studies on contamination level of aflatoxins in some dried fruits and nuts of Pakistan. Food Control. 22: 426-429. [DOI: 10.1016/j.foodcont.2010.09.015]
Nguyen T., Palmer J., Phan N., Shi H., Keener K., Flint S. (2022). Control of aflatoxin M1 in skim milk by high voltage atmospheric cold plasma. Food Chemistry: 386: 132814. [DOI: 10.1016/j.foodchem.2022.132814]
Nishimwe K., Agbemafle I., Reddy M.B., Keener K., Maier D.E. (2021). Cytotoxicity assessment of aflatoxin B1 after high voltage atmospheric cold plasma treatment. Toxicon. 194: 17-22. [DOI: 10.1016/j.toxicon.2021.02.008]
Oehmigen K., Hähnel M., Brandenburg R., Wilke C., Weltmann K.-D., Von Woedtke T. (2010). The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Processes and Polymers. 7: 250-257. [DOI: 10.1002/ppap.200900077]
Puligundla P., Lee T., Mok C. (2020). Effect of corona discharge plasma jet treatment on the degradation of aflatoxin B1 on glass slides and in spiked food commodities. LWT. 124: 108333. [DOI: 10.1016/j.lwt.2019.108333]
Rodríguez Ó., Gomes W.F., Rodrigues S., Fernandes F.A.N. (2017). Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT. 84: 457-463. [DOI: 10.1016/j.lwt.2017.06.010]
Samokhvalova O., Kasabova K., Shmatchenko N., Zagorulko A., Zahorulko A. (2021). Improving the marmalade technology by adding a multicomponent fruit-and-berry paste. Eastern-European Journal of Enterprise Technologies. 6: 6-14. [DOI: 10.15587/1729-4061.2021.245986]
Sarangapani C., O'Toole G., Cullen P.J., Bourke P. (2017). Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innovative Food Science and Emerging Technologies. 44: 235-241. [DOI: 10.1016/j.ifset.2017.02.012]
Shi H., Cooper B., Stroshine R.L., Ileleji K.E., Keener K.M. (2017a). Structures of degradation products and degradation pathways of aflatoxin B1 by high-voltage atmospheric cold plasma (HVACP) treatment. Journal of Agricultural and Food Chemistry. 65: 6222-6230. [DOI: 10.1021/acs.jafc.7b01604]
Shi H., Ileleji K., Stroshine R.L., Keener K., Jensen J.L. (2017b). Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food and Bioprocess Technology. 10: 1042-1052. [DOI: 10.1007/s11947-017-1873-8]
Shiekh K.A., Benjakul S. (2020). Effect of high voltage cold atmospheric plasma processing on the quality and shelf-life of Pacific white shrimp treated with Chamuang leaf extract. Innovative Food Science and Emerging Technologies. 64: 102435. [DOI: 10.1016/j.ifset.2020.102435]
Tappi S., Ramazzina I., Rizzi F., Sacchetti G., Ragni L., Rocculi P. (2018). Effect of plasma exposure time on the polyphenolic profile and antioxidant activity of fresh-cut apples. Applied Sciences. 8: 1939. [DOI: 10.3390/app8101939]
Ucar Y., Ceylan Z., Durmus M., Tomar O., Cetinkaya T. (2021). Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends in Food Science and Technology. 114: 355-371. [DOI: 10.1016/j.tifs.2021.06.004]
Viuda-Martos M., Barber X., Pérez-Álvarez J.A., Fernández-López J. (2015). Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Industrial Crops and Products. 69: 472-479. [DOI: 10.1016/j.indcrop.2015.03.005]
Wang Z., Li S., Ge S., Lin S. (2020). Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. Journal of Agricultural and Food Chemistry. 68: 3330-3343. [DOI: 10.1021/acs.jafc.9b06574]
Ziuzina D., Misra N.N., Cullen P.J., Keener K., Mosnier J.P., Vilaró I., Gaston E., Bourke P. (2016). Demonstrating the potential of industrial scale in-package atmospheric cold plasma for decontamination of cherry tomatoes. Plasma Medicine. 6: 397-412. [DOI: 10.1615/PlasmaMed.2017019498]
*Corresponding author (A. Ostadrahimi)
* E-mail: ostadrahimi@tbzmed.ac.ir
ORCID ID: https://orcid.org/0000-0002-1058-1481
* E-mail: ostadrahimi@tbzmed.ac.ir
ORCID ID: https://orcid.org/0000-0002-1058-1481
Type of Study: Original article |
Subject:
Special
Received: 24/09/03 | Accepted: 25/09/23 | Published: 25/09/30
Received: 24/09/03 | Accepted: 25/09/23 | Published: 25/09/30
References
1. Amini M., Ghoranneviss M. (2016). Effects of cold plasma treatment on antioxidants activity, phenolic contents and shelf life of fresh and dried walnut (Juglans regia L.) cultivars during storage. LWT. 73: 178-184. [DOI: 10.1016/j.lwt.2016.06.014] [DOI:10.1016/j.lwt.2016.06.014]
2. Batista J.D.F., Dantas A.M., Dos Santos Fonseca J.V., Madruga M.S., Fernandes F.A.N., Rodrigues S., Da Silva Campelo Borges G. (2021). Effects of cold plasma on avocado pulp (Persea americana Mill.): chemical characteristics and bioactive compounds. Journal of Food Processing and Preservation. 45: e15179. [DOI: 10.1111/jfpp.15179] [DOI:10.1111/jfpp.15179]
3. Bey M.B., Louaileche H., Zemouri S. (2013). Optimization of phenolic compound recovery and antioxidant activity of light and dark dried fig (Ficus carica L.) varieties. Food Science and Biotechnology. 22: 1613-1619. [DOI: 10.1007/s10068-013-0258-7] [DOI:10.1007/s10068-013-0258-7]
4. Chen Y., Zhang Y., Jiang L., Chen G., Yu J., Li S., Chen Y. (2020). Moisture molecule migration and quality changes of fresh wet noodles dehydrated by cold plasma treatment. Food Chemistry. 328: 127053. [DOI: 10.1016/j.foodchem.2020.127053] [DOI:10.1016/j.foodchem.2020.127053] [PMID]
5. Chutia H., Mahanta C.L. (2021). Influence of cold plasma voltage and time on quality attributes of tender coconut water (Cocos nucifera L.) and degradation kinetics of its blended beverage. Journal of Food Processing and Preservation. 45: e15372. [DOI: 10.1111/jfpp.15372] [DOI:10.1111/jfpp.15372]
6. Doymaz İ. (2004). Pretreatment effect on sun drying of mulberry fruits (Morus alba L.). Journal of Food Engineering. 65: 205-209. [DOI: 10.1016/j.jfoodeng.2004.01.016] [DOI:10.1016/j.jfoodeng.2004.01.016]
7. Gençdağ E., Görgüç A., Okuroğlu F., Yılmaz F.M. (2021). The effects of power ‐ ultrasound, peroxyacetic acid and sodium chloride washing treatments on the physical and chemical quality characteristics of dried figs. Journal of Food Processing and Preservation. 45: e15009. [DOI: 10.1111/jfpp.15009] [DOI:10.1111/jfpp.15009]
8. González-Curbelo M.Á., Kabak B. (2023). Occurrence of mycotoxins in dried fruits worldwide, with a focus on aflatoxins and ochratoxin A: a review. Toxins. 15: 576. [DOI: 10.3390/toxins15090576] [DOI:10.3390/toxins15090576] [PMID] [PMCID]
9. Heshmati A., Mozaffari Nejad A.S. (2015). Ochratoxin A in dried grapes in Hamadan province, Iran. Food Additives and Contaminants: Part B. 8: 255-259. [DOI: 10.1080/ 19393210.2015.1074945] [DOI:10.1080/19393210.2015.1074945] [PMID]
10. Heshmati A., Zohrevand T., Mousavi Khaneghah A., Mozaffari Nejad A.S., Sant'Ana A.S. (2017). Co-occurrence of aflatoxins and ochratoxin A in dried fruits in Iran: dietary exposure risk assessment. Food and Chemical Toxicology. 106: 202-208. [DOI: 10.1016/j.fct.2017.05.046] [DOI:10.1016/j.fct.2017.05.046] [PMID]
11. Jangi F., Ebadi M.-T., Ayyari M. (2021). Qualitative changes in hyssop (Hyssopus officinalis L.) as affected by cold plasma, packaging method and storage duration. Journal of Applied Research on Medicinal and Aromatic Plants. 22: 100289. [DOI: 10.1016/j.jarmap.2020.100289] [DOI:10.1016/j.jarmap.2020.100289]
12. Karaca H., Nas S. (2006). Aflatoxins, patulin and ergosterol contents of dried figs in Turkey. Food Additives and Contaminants. 23: 502-508. [DOI: 10.1080/02652030600550739] [DOI:10.1080/02652030600550739] [PMID]
13. Kashfi A.S., Ramezan Y., Khani M.R. (2020). Simultaneous study of the antioxidant activity, microbial decontamination and color of dried peppermint (Mentha piperita L.) using low pressure cold plasma. LWT. 123: 109121. [DOI: 10.1016/j.lwt.2020.109121] [DOI:10.1016/j.lwt.2020.109121]
14. Kaymak T., Türker L., Tulay H., Stroka J. (2018). Determination of aflatoxins and ochratoxin A in traditional Turkish concentrated fruit juice products by multi-immunoaffinity column cleanup and LC fluorescence detection: single-laboratory validation. Journal of AOAC International. 101: 1839-1849. [DOI: 10.5740/ jaoacint.17-0463] [DOI:10.5740/jaoacint.17-0463]
15. Luttfullah G., Hussain A. (2011). Studies on contamination level of aflatoxins in some dried fruits and nuts of Pakistan. Food Control. 22: 426-429. [DOI: 10.1016/j.foodcont.2010.09.015] [DOI:10.1016/j.foodcont.2010.09.015]
16. Nguyen T., Palmer J., Phan N., Shi H., Keener K., Flint S. (2022). Control of aflatoxin M1 in skim milk by high voltage atmospheric cold plasma. Food Chemistry: 386: 132814. [DOI: 10.1016/j.foodchem.2022.132814] [DOI:10.1016/j.foodchem.2022.132814] [PMID]
17. Nishimwe K., Agbemafle I., Reddy M.B., Keener K., Maier D.E. (2021). Cytotoxicity assessment of aflatoxin B1 after high voltage atmospheric cold plasma treatment. Toxicon. 194: 17-22. [DOI: 10.1016/j.toxicon.2021.02.008] [DOI:10.1016/j.toxicon.2021.02.008] [PMID]
18. Oehmigen K., Hähnel M., Brandenburg R., Wilke C., Weltmann K.-D., Von Woedtke T. (2010). The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Processes and Polymers. 7: 250-257. [DOI: 10.1002/ppap.200900077] [DOI:10.1002/ppap.200900077]
19. Puligundla P., Lee T., Mok C. (2020). Effect of corona discharge plasma jet treatment on the degradation of aflatoxin B1 on glass slides and in spiked food commodities. LWT. 124: 108333. [DOI: 10.1016/j.lwt.2019.108333] [DOI:10.1016/j.lwt.2019.108333]
20. Rodríguez Ó., Gomes W.F., Rodrigues S., Fernandes F.A.N. (2017). Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT. 84: 457-463. [DOI: 10.1016/j.lwt.2017.06.010] [DOI:10.1016/j.lwt.2017.06.010]
21. Samokhvalova O., Kasabova K., Shmatchenko N., Zagorulko A., Zahorulko A. (2021). Improving the marmalade technology by adding a multicomponent fruit-and-berry paste. Eastern-European Journal of Enterprise Technologies. 6: 6-14. [DOI: 10.15587/1729-4061.2021.245986] [DOI:10.15587/1729-4061.2021.245986]
22. Sarangapani C., O'Toole G., Cullen P.J., Bourke P. (2017). Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innovative Food Science and Emerging Technologies. 44: 235-241. [DOI: 10.1016/j.ifset.2017.02.012] [DOI:10.1016/j.ifset.2017.02.012]
23. Shi H., Cooper B., Stroshine R.L., Ileleji K.E., Keener K.M. (2017a). Structures of degradation products and degradation pathways of aflatoxin B1 by high-voltage atmospheric cold plasma (HVACP) treatment. Journal of Agricultural and Food Chemistry. 65: 6222-6230. [DOI: 10.1021/acs.jafc.7b01604] [DOI:10.1021/acs.jafc.7b01604] [PMID]
24. Shi H., Ileleji K., Stroshine R.L., Keener K., Jensen J.L. (2017b). Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food and Bioprocess Technology. 10: 1042-1052. [DOI: 10.1007/s11947-017-1873-8] [DOI:10.1007/s11947-017-1873-8]
25. Shiekh K.A., Benjakul S. (2020). Effect of high voltage cold atmospheric plasma processing on the quality and shelf-life of Pacific white shrimp treated with Chamuang leaf extract. Innovative Food Science and Emerging Technologies. 64: 102435. [DOI: 10.1016/j.ifset.2020.102435] [DOI:10.1016/j.ifset.2020.102435]
26. Tappi S., Ramazzina I., Rizzi F., Sacchetti G., Ragni L., Rocculi P. (2018). Effect of plasma exposure time on the polyphenolic profile and antioxidant activity of fresh-cut apples. Applied Sciences. 8: 1939. [DOI: 10.3390/app8101939] [DOI:10.3390/app8101939]
27. Ucar Y., Ceylan Z., Durmus M., Tomar O., Cetinkaya T. (2021). Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends in Food Science and Technology. 114: 355-371. [DOI: 10.1016/j.tifs.2021.06.004] [DOI:10.1016/j.tifs.2021.06.004]
28. Viuda-Martos M., Barber X., Pérez-Álvarez J.A., Fernández-López J. (2015). Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Industrial Crops and Products. 69: 472-479. [DOI: 10.1016/j.indcrop.2015.03.005] [DOI:10.1016/j.indcrop.2015.03.005]
29. Wang Z., Li S., Ge S., Lin S. (2020). Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. Journal of Agricultural and Food Chemistry. 68: 3330-3343. [DOI: 10.1021/acs.jafc.9b06574] [DOI:10.1021/acs.jafc.9b06574] [PMID]
30. Ziuzina D., Misra N.N., Cullen P.J., Keener K., Mosnier J.P., Vilaró I., Gaston E., Bourke P. (2016). Demonstrating the potential of industrial scale in-package atmospheric cold plasma for decontamination of cherry tomatoes. Plasma Medicine. 6: 397-412. [DOI: 10.1615/PlasmaMed.2017019498] [DOI:10.1615/PlasmaMed.2017019498]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







