Volume 12, Issue 1 (March 2025)
J. Food Qual. Hazards Control 2025, 12(1): 73-80 |
Back to browse issues page
Ethics code: Not applicable
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Parihar H, Singh U, Pathak R, Chaturvedi P, Dayal R, Tirumalai P. Investigating the Individual and Combined Effect of Essential Oils and Probiotics against Staphylococcus aureus. J. Food Qual. Hazards Control 2025; 12 (1) :73-80
URL: http://jfqhc.ssu.ac.ir/article-1-1260-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1260-en.html
Department of Botany (Agriculture Sciences), Dayalbagh Educational Institute, Uttar Pradesh, India, Food and Dairy Testing Laboratory, Department of Botany (Agriculture Sciences), Faculty of Science (Dairy Campus), Dayalbagh Educational Institute, Dayalbagh, Agra – 282005. Uttar Pradesh. India , premtsaran@dei.ac.in
Full-Text [PDF 770 kb]
(440 Downloads)
| Abstract (HTML) (830 Views)
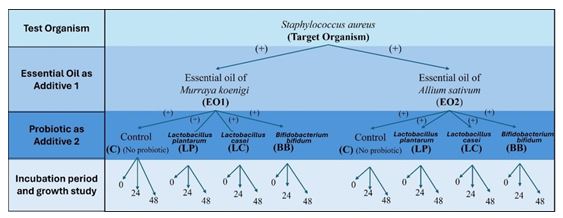
Figure 1: Experimental design for examining the effects of essential oil on Staphylococcus aureus
Table 1: Concentration of Staphylococcus aureus (Colony Forming Units (CFU)/ml) in the presence of probiotic strains combined with essential oils of curry leaf and garlic (EO1 and EO2).
Full-Text: (297 Views)
Investigating the Individual and Combined Effect of Essential Oils and Probiotics against Staphylococcus aureus
H. Parihar 1, U. Singh 2, R. Pathak 3, P. Chaturvedi 1, R. Dayal 1, P.S. Tirumalai 1,4,[*]*
1. Department of Botany (Agriculture Sciences), Dayalbagh Educational Institute, Uttar Pradesh, India
2. Department of Electrical Engineering, Indian Institute of Technology Bombay, Mumbai. India
3. Department of Home Science, Dayalbagh Educational Institute, Uttar Pradesh, India
4. Food and Dairy Testing Laboratory, Department of Botany (Agriculture Sciences), Faculty of Science (Dairy Campus), Dayalbagh Educational Institute, Dayalbagh, Agra – 282005. Uttar Pradesh. India
H. Parihar 1, U. Singh 2, R. Pathak 3, P. Chaturvedi 1, R. Dayal 1, P.S. Tirumalai 1,4,[*]*
1. Department of Botany (Agriculture Sciences), Dayalbagh Educational Institute, Uttar Pradesh, India
2. Department of Electrical Engineering, Indian Institute of Technology Bombay, Mumbai. India
3. Department of Home Science, Dayalbagh Educational Institute, Uttar Pradesh, India
4. Food and Dairy Testing Laboratory, Department of Botany (Agriculture Sciences), Faculty of Science (Dairy Campus), Dayalbagh Educational Institute, Dayalbagh, Agra – 282005. Uttar Pradesh. India
HIGHLIGHTS
- The inhibitory effect of curry leaf essential oil on Staphylococcus aureus is superior to that of garlic essential oil.
- Lactobacillus casei showed greater efficacy in suppressing S. aureus than Lactobacillus plantarum and Bifidobacterium bifidum.
- Essential oils exhibit potential as prebiotics for probiotics, thereby influencing microbial dynamics.
| Article type Original article |
ABSTRACT Background: The pathogenic bacteria present in food contribute to its spoilage and can lead to the development of diseases. Chemical preservatives exhibit toxicity and resistance problems, prompting the need for safer alternatives. Natural phytochemicals and probiotics are effective options, as essential oils and probiotics possess robust antibacterial characteristics. The objective of this study is to investigate the combined effects of probiotics (Lactobacillus plantarum, Lactobacillus casei, and Bifidobacterium bifidum) and essential oils derived from Murraya koenigii (curry patha) and Allium sativum (garlic) in inhibiting the growth of Staphylococcus aureus, a major foodborne pathogen. Methods: The study assessed the antibacterial effects of M. koenigii and A. sativum essential oils on S. aureus, both alone and in combination with probiotics (L. plantarum, L. casei, and B. bifidum). Antibacterial activity was measured at zero, 24, and 48 h using a culture plate method with serial dilution and pour plate technique. The Bliss Independent model was used to analyze interactions between control and treatments. Synergy factor and relative inhibition were determined using Python software to evaluate the combined effects of essential oils and probiotics. All treatments were performed in duplicate. Results: M. koenigii and A. sativum essential oils exhibit antibacterial activity against S. aureus, with M. koenigii demonstrating greater potency. Notably, their effectiveness in inhibiting bacterial cells is enhanced when combined with probiotics. In the control group, the colony forming unit/ml of S. aureus was 8.09±0.51, whereas in the presence of M. koenigii essential oil, it significantly reduced to 2±0.2. Conclusion: While both essential oils and probiotics have antibacterial effects on their own, using them together may require careful attention to ensure effectiveness. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Lactobacillus plantarum Lacticaseibacillus casei Bifidobacterium bifidum Garlic. |
||
| Article history Received: 07 Sep 2024 Revised: 30 Nov 2024 Accept: 12 Mar 2025 |
||
| Abbreviations CFU=Colony Forming Unit LAB=Lactic Acid Bacteria RI=Relative Inhibition SCDM=Soybean Casein Digestive Medium SF=Synergy Factor |
To cite: Parihar H., Singh U., Pathak R., Chaturvedi P., Dayal R., Tirumalai P.S. (2025). Investigating the individual and combined effect of essential oils and probiotics against Staphylococcus aureus. Journal of Food Quality and Hazards Control. 12: 73-80.
Introduction
Introduction
Microbial contamination of food involves a wide range of microorganisms, including pathogenic species, leading to food spoilage, reduced food quality, and foodborne illnesses (Abebe et al., 2020). Staphylococcus aureus is a commensal organism and opportunistic pathogen capable of causing various infections in humans and animals. It commonly colonizes the skin, mucous membranes, and gut of both humans and animals. Due to its presence on the skin and in the nasopharynx, S. aureus is easily shed, making it a major contributor to foodborne illnesses globally. The incidence of foodborne illnesses has been rising in recent years, mainly due to the consumption of S. aureus contaminated food (Todd, 2020). Historically, chemical preservatives have played a key role in preventing foodborne diseases and maintaining food safety. However, concerns over chemical residues and antimicrobial resistance have driven interest in natural alternatives (Davies et al., 2021; Narayana et al., 2024). Among natural alternatives, curry leaf (Murraya koenigii) and garlic (Allium sativum) essential oils have gained significant attention for their strong antibacterial properties. Curry leaf essential oil, rich in bioactive compounds such as α-pinene, β-caryophyllene, and linalool, has shown potent inhibitory effects against S. aureus by disrupting bacterial cell membranes and interfering with metabolic processes (Sharma et al., 2022, Li et al 2021). Similarly, garlic essential oil, which contains sulfur-rich compounds like allicin and diallyl disulfide, exhibits strong antimicrobial activity by targeting bacterial cell walls and enzyme systems, leading to the inhibition of S. aureus growth (Kumar et al., 2022). The combined use of these essential oils offers a promising natural approach to controlling foodborne pathogens, reducing reliance on chemical preservatives, and enhancing food safety (Amouei et al., 2021).
The use of probiotics, though more widely recognized today, has its origins in ancient civilizations that understood the health benefits of fermented foods. Probiotics, predominantly sourced from the Lactobacilli and Bifidobacteria genera, are renowned for their robust antibacterial properties, mediated through the production of bacteriocins, hydrogen peroxide, diacetyl, organic acids, and other inhibitory compounds (Darbandi et al., 2022; Monika et al., 2021; Šalomskienė et al., 2015). Probiotics and essential oils, with their complementary mechanisms, offer a synergistic approach to fighting pathogenic infections a concept that has been utilized in the production of fermented foods throughout history. This synergy not only offers therapeutic potential for gastrointestinal infections but also holds promise for the development of flavored fermented products. With increasing public awareness of natural foods and rising concerns over microbial resistance to conventional preservatives, the integration of essential oils containing probiotics represents a viable strategy for enhancing food safety. Investigating the synergistic potential of probiotics and essential oils presents a promising frontier in combating foodborne pathogens. This study examines the combined effects of probiotics (Lactobacillus plantarum, Lactobacillus casei, and Bifidobacterium bifidum) and essential oils from curry leaf (M. koenigii) and garlic (A. sativum) against S. aureus, a major foodborne pathogen. By analyzing their interaction, this research aims to identify new natural strategies for improving food safety.
Materials and methods
Essential oils and cultures
Commercially available Essential oils of M. koenigii (Curry Leaves) and A. Sativum (Garlic) from Ambrosia Natural Products Pvt. Ltd, New Delhi, India were used. They were produced by steam distillation and stored at 4 ºC until the analysis. L. casei (ATCC 12116) and S. aureus (ATCC 5345) were obtained from the National Collection of Industrial Microorganisms, Pune, India. Additionally, B. bifidum (NRRL/ATCC 29521) and L. plantarum (NRRL/ATCC 8014) were sourced from the Northern Regional Research Laboratory, part of the Agricultural Research Service under the United States Department of Agriculture in the USA.
Culture preparation and maintenance
The following culture media were used in the study: mannitol salt agar (M118–500G, HiMedia, India), Lactobacillus MRS Agar Media (M614–500G, HiMedia, India), Soybean Casein Digestive Medium (SCDM; M011–500G, HiMedia, India), and muller hinton agar (M173–100G, HiMedia, India). Cultures of all strains were stored as stock cultures in soybean casein digestive agar slants at 4 °C (Pan et al., 2023). Prior to their utilization in the experiments, the pathogenic microorganisms and probiotics were revived in SCDM broth for use.
Experimental methodology
The experiment was conducted in duplicate to ensure the reliability and consistency of the results. The experimental design of tests is shown in Figure 1.
The use of probiotics, though more widely recognized today, has its origins in ancient civilizations that understood the health benefits of fermented foods. Probiotics, predominantly sourced from the Lactobacilli and Bifidobacteria genera, are renowned for their robust antibacterial properties, mediated through the production of bacteriocins, hydrogen peroxide, diacetyl, organic acids, and other inhibitory compounds (Darbandi et al., 2022; Monika et al., 2021; Šalomskienė et al., 2015). Probiotics and essential oils, with their complementary mechanisms, offer a synergistic approach to fighting pathogenic infections a concept that has been utilized in the production of fermented foods throughout history. This synergy not only offers therapeutic potential for gastrointestinal infections but also holds promise for the development of flavored fermented products. With increasing public awareness of natural foods and rising concerns over microbial resistance to conventional preservatives, the integration of essential oils containing probiotics represents a viable strategy for enhancing food safety. Investigating the synergistic potential of probiotics and essential oils presents a promising frontier in combating foodborne pathogens. This study examines the combined effects of probiotics (Lactobacillus plantarum, Lactobacillus casei, and Bifidobacterium bifidum) and essential oils from curry leaf (M. koenigii) and garlic (A. sativum) against S. aureus, a major foodborne pathogen. By analyzing their interaction, this research aims to identify new natural strategies for improving food safety.
Materials and methods
Essential oils and cultures
Commercially available Essential oils of M. koenigii (Curry Leaves) and A. Sativum (Garlic) from Ambrosia Natural Products Pvt. Ltd, New Delhi, India were used. They were produced by steam distillation and stored at 4 ºC until the analysis. L. casei (ATCC 12116) and S. aureus (ATCC 5345) were obtained from the National Collection of Industrial Microorganisms, Pune, India. Additionally, B. bifidum (NRRL/ATCC 29521) and L. plantarum (NRRL/ATCC 8014) were sourced from the Northern Regional Research Laboratory, part of the Agricultural Research Service under the United States Department of Agriculture in the USA.
Culture preparation and maintenance
The following culture media were used in the study: mannitol salt agar (M118–500G, HiMedia, India), Lactobacillus MRS Agar Media (M614–500G, HiMedia, India), Soybean Casein Digestive Medium (SCDM; M011–500G, HiMedia, India), and muller hinton agar (M173–100G, HiMedia, India). Cultures of all strains were stored as stock cultures in soybean casein digestive agar slants at 4 °C (Pan et al., 2023). Prior to their utilization in the experiments, the pathogenic microorganisms and probiotics were revived in SCDM broth for use.
Experimental methodology
The experiment was conducted in duplicate to ensure the reliability and consistency of the results. The experimental design of tests is shown in Figure 1.

Figure 1: Experimental design for examining the effects of essential oil on Staphylococcus aureus
Effect of essential oils against S. aureus
The experiment investigated the effects of two essential oils: M. koenigii (Curry leaves), labeled EO1, and A. sativum (Garlic), labeled EO2, on S. aureus. A volume of 300 µl of each essential oil (EO1 and EO2) was added to separate flasks, each containing 100 ml of SCDM culture medium. A thousands µl of overnight culture of S. aureus was introduced into each of the flasks containing the culture medium in suspension with essential oils, EO1 and EO2. The initial cell concentration of S. aureus in the culture medium post inoculation was estimated using the culture plate method, which involved serial dilution and pour plate method, and was designed as the concentration of S. aureus at zero h. The flasks were subsequently incubated at 37 ºC, and the culture concentration of S. aureus was estimated from both flasks (each containing one of the essential oils) at fixed intervals of 24 and 48 h. The effect of essential oils, EO1 and EO2, on the growth of S. aureus at specific time intervals were thus determined as cell concentrations expressed in Colony Forming Units (CFU)/ml.
Effect of essential oils in the presence of probiotic species
A similar experimental setup, as described earlier, was used to study the effect of essential oils (EO1 and EO2) in the presence of three probiotic species: L. plantarum, L. casei, and B. bifidum. In this setup, both the essential oils and the probiotic species were added to assess their combined effect on the growth of S. aureus. For EO1, 300 µl was added to three separate flasks, each containing 100 ml of SCDM. L. casei was inoculated into one set of flasks at a concentration of 1,000 µl (7.4 CFU/ml). Similarly, separate sets of three flasks were inoculated with L. plantarum and B. bifidum at concentrations of 6.9 and 7.6 CFU/ml, respectively. Additionally, 1,000 µl of S. aureus was introduced into each flask.
A similar setup was prepared to test the combined effect of the probiotic species with EO2. The flasks were incubated at 37 ºC, and the culture concentration of S. aureus was measured at fixed time intervals of 24 and 48 h. The combined effect of the essential oils (EO1 and EO2) with each probiotic species on the growth of S. aureus was determined by estimating the cell concentration as CFU/ml.
Data analysis methodology
In this study, we evaluated the interactions between probiotics (L. casei, L. plantarum, and B. bifidum) and essential oils (EO1 and EO2) on the inhibition of S. aureus growth. Using the Bliss Independence Model, we calculated the synergy factors for various combinations of probiotics and essential oil oils at 24 and 48 h. These results highlight the synergistic effects of combining probiotics with essential oils in inhibiting S. aureus growth. The Relative Inhibition (RI) values further quantify the effectiveness of these combinations. Overall, our findings can underscore the potential of synergistic combinations of probiotics and essential oils in enhancing antimicrobial effects, providing valuable insights for developing more effective antimicrobial strategies.
Bliss Independence Model
The Bliss Independence Model [R1, R2, and R3] is a widely recognized approach for assessing the interactions between two treatments, denoted as A and B. This model operates on the premise that the effects of these two treatments are independent and additive when applied together. The individual effects of treatments A (probiotic) and B (essential oil) are denoted as EA and EB, respectively.
The expected combined effect,E AB ( exp )
The experiment investigated the effects of two essential oils: M. koenigii (Curry leaves), labeled EO1, and A. sativum (Garlic), labeled EO2, on S. aureus. A volume of 300 µl of each essential oil (EO1 and EO2) was added to separate flasks, each containing 100 ml of SCDM culture medium. A thousands µl of overnight culture of S. aureus was introduced into each of the flasks containing the culture medium in suspension with essential oils, EO1 and EO2. The initial cell concentration of S. aureus in the culture medium post inoculation was estimated using the culture plate method, which involved serial dilution and pour plate method, and was designed as the concentration of S. aureus at zero h. The flasks were subsequently incubated at 37 ºC, and the culture concentration of S. aureus was estimated from both flasks (each containing one of the essential oils) at fixed intervals of 24 and 48 h. The effect of essential oils, EO1 and EO2, on the growth of S. aureus at specific time intervals were thus determined as cell concentrations expressed in Colony Forming Units (CFU)/ml.
Effect of essential oils in the presence of probiotic species
A similar experimental setup, as described earlier, was used to study the effect of essential oils (EO1 and EO2) in the presence of three probiotic species: L. plantarum, L. casei, and B. bifidum. In this setup, both the essential oils and the probiotic species were added to assess their combined effect on the growth of S. aureus. For EO1, 300 µl was added to three separate flasks, each containing 100 ml of SCDM. L. casei was inoculated into one set of flasks at a concentration of 1,000 µl (7.4 CFU/ml). Similarly, separate sets of three flasks were inoculated with L. plantarum and B. bifidum at concentrations of 6.9 and 7.6 CFU/ml, respectively. Additionally, 1,000 µl of S. aureus was introduced into each flask.
A similar setup was prepared to test the combined effect of the probiotic species with EO2. The flasks were incubated at 37 ºC, and the culture concentration of S. aureus was measured at fixed time intervals of 24 and 48 h. The combined effect of the essential oils (EO1 and EO2) with each probiotic species on the growth of S. aureus was determined by estimating the cell concentration as CFU/ml.
Data analysis methodology
In this study, we evaluated the interactions between probiotics (L. casei, L. plantarum, and B. bifidum) and essential oils (EO1 and EO2) on the inhibition of S. aureus growth. Using the Bliss Independence Model, we calculated the synergy factors for various combinations of probiotics and essential oil oils at 24 and 48 h. These results highlight the synergistic effects of combining probiotics with essential oils in inhibiting S. aureus growth. The Relative Inhibition (RI) values further quantify the effectiveness of these combinations. Overall, our findings can underscore the potential of synergistic combinations of probiotics and essential oils in enhancing antimicrobial effects, providing valuable insights for developing more effective antimicrobial strategies.
Bliss Independence Model
The Bliss Independence Model [R1, R2, and R3] is a widely recognized approach for assessing the interactions between two treatments, denoted as A and B. This model operates on the premise that the effects of these two treatments are independent and additive when applied together. The individual effects of treatments A (probiotic) and B (essential oil) are denoted as EA and EB, respectively.
The expected combined effect,
 |
(Liu et al., 2018) |
The Synergy Factor (SF) is then defined as the ratio of the observed combined effect ( ) to the expected combined effect:
) to the expected combined effect:
 ) to the expected combined effect:
) to the expected combined effect: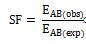 |
(Alengebawy et al., 2021) |
Based on the value of the synergy factor, the interaction between the two treatments can be categorized into three categories. Firstly, a SF greater than one indicates a synergistic interaction, where the combined effect of both treatments exceeds the expected additive effect. This means their combined impact is greater than the sum of their individual effects. Secondly, An SF value equal to one signifies an additive interaction, where the observed combined effect is exactly what would be expected if the effects were simply additive, implying no synergy or antagonism. Conversely, an SF less than one indicates an antagonistic interaction, where the observed combined effect is less than the expected additive effect, suggesting that the combined impact is less than the sum of the individual effects. Thus, the SF provides a straightforward metric to assess whether the combined effects of treatments are synergistic, additive, or antagonistic.
Furthermore, we calculated RI, which measures the extent to which a treatment inhibits the growth of S. aureus compared to a control group, providing a quantitative assessment of the treatment's effectiveness in reducing bacterial growth.
(Econtrol): effect observed in the control group (no treatment) and (Etreatment): effect observed in the treatment group.
RI is calculated using this formula:
Furthermore, we calculated RI, which measures the extent to which a treatment inhibits the growth of S. aureus compared to a control group, providing a quantitative assessment of the treatment's effectiveness in reducing bacterial growth.
(Econtrol): effect observed in the control group (no treatment) and (Etreatment): effect observed in the treatment group.
RI is calculated using this formula:
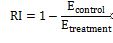 |
(Zhao et al., 2014) |
This formula expresses the relative reduction in bacterial growth due to the treatment compared to the untreated control group. Higher RI indicates greater inhibition of bacterial growth by the treatment.
Statistical analysis
Each analysis was performed in duplicate, and the resulting data were recorded as mean±Standard Deviation (SD). Statistical significance was determined using a by t-test with p<0.05. Basic data analysis was conducted using Microsoft Excel (version 16, 2016), while more advanced statistical computations, including significance testing, were performed using Python.
Results and discussion
Effect of probiotic strains on S. aureus
Table 1 shows the concentration of S. aureus as a control and in the presence of probiotic strains (L. casei, L. plantarum, and B. bifidum) and essential oils. Compared to the control sample, the growth of S. aureus was negatively affected in the presence of L. casei and L. plantarum after both 24 and 48 h of incubation, while it was positively influenced by B. bifidum after 48 h. In a previous experiment, it was observed that the growth of S. aureus is most inhibited by L. casei compared to L. plantarum and B. bifidum, likely due to its higher production of antimicrobial compounds (Parihar et al., 2023). These observations highlight the effect of probiotics on S. aureus.
Statistical analysis
Each analysis was performed in duplicate, and the resulting data were recorded as mean±Standard Deviation (SD). Statistical significance was determined using a by t-test with p<0.05. Basic data analysis was conducted using Microsoft Excel (version 16, 2016), while more advanced statistical computations, including significance testing, were performed using Python.
Results and discussion
Effect of probiotic strains on S. aureus
Table 1 shows the concentration of S. aureus as a control and in the presence of probiotic strains (L. casei, L. plantarum, and B. bifidum) and essential oils. Compared to the control sample, the growth of S. aureus was negatively affected in the presence of L. casei and L. plantarum after both 24 and 48 h of incubation, while it was positively influenced by B. bifidum after 48 h. In a previous experiment, it was observed that the growth of S. aureus is most inhibited by L. casei compared to L. plantarum and B. bifidum, likely due to its higher production of antimicrobial compounds (Parihar et al., 2023). These observations highlight the effect of probiotics on S. aureus.
Table 1: Concentration of Staphylococcus aureus (Colony Forming Units (CFU)/ml) in the presence of probiotic strains combined with essential oils of curry leaf and garlic (EO1 and EO2).
| Staphylococcus aureus in the Presence of Probiotic Strains |
Concentration of S. aureus (CFU/ml) | ||||||
| Treatments without EO | EO1 | EO2 | |||||
| 0 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| S. aureus | 7.6±0.41 |
8.45±0.45 | 8.09±0.51 | 7.2±0.70 b | 2.00±0.2 c | 6.54±0.24 c | 4.00±0.3 c |
| S. aureus+Lactobacillus casei | 7.16±0.71 b | 7.05±0.46 b | 8.18±0.67 a | 6.75±0.35 b | 7.9±04 a | 7.06±0.26 b | |
| S. aureus+Lactobacillus plantarum | 7.18±0.08 b | 6.8±0.17 b | 7.58±0.66 b | 6.98±0.73 b | 7.99±0.29 a | 7.43±0.33 a | |
| S.aureus+Bifidobacterium bifidum | 8.46±0.06 b | 8.56±0.11 b | 8.32±0.52 a | 7.66±0.36 a | 7.54±0.24 b | 5.45±0.35 b | |
Values represent the Mean±Standard Deviation (SD). Different superscript letters indicate significant differences (p<0.05) within each column based on t-test.
Effect of essential oils on S. aureus with and without probiotic cultures
The effect of curry leaf essential oil and garlic essential oil on S. aureus was evaluated individually as a control and in the presence of probiotics strains at different time intervals (24 and 48 h) under different treatment conditions, as specified in the experimental design. The recorded measurements represent the mean values obtained from duplicate experimental setups, providing insights into each treatment’s effectiveness in influencing the growth dynamics of S. aureus over time.
RI and SF of essential oils of curry leaf and garlic (EO1 and EO2)
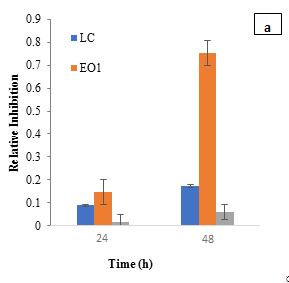
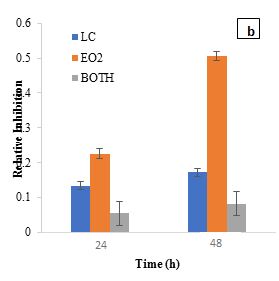
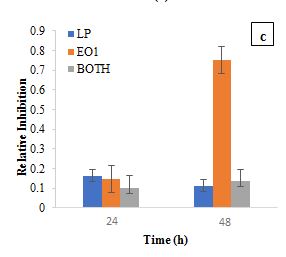
The RI metric assesses the inhibitory effect of a substance on microbial growth by comparing it to a control sample (Figure 2). RI values highlight the inhibitory effects of L. casei, EO1, and their combination on S. aureus. At 24 h, L. casei exhibited moderate inhibition (RI=0.0888), while EO1 showed a higher RI of 0.1479. The antibacterial activity of curry leaf oil has been attributed to its bioactive compounds, such as carbazole alkaloids, which inhibit Staphylococcus, Streptococcus, Escherichia coli, and Proteus (Patil et al., 2024). Similarly, Kumar et al. (2022) reported a dose-dependent antibacterial effect of curry leaf oil against E. coli, supporting its broad-spectrum antimicrobial activity. Additionally, tocopherol, beta-carotene, and lutein—bioactive compounds present in EO1 have also been associated with antibacterial properties (Shahidi et al., 2018). However, when L. casei was combined with EO1, the inhibitory effect was significantly reduced (RI=0.0154) compared to EO1 alone, which exhibited a much higher RI of 0.7528 at 48 h. The combination of L. casei and EO1 resulted in a lower RI of 0.0606.
According to De Montijo-Prieto et al. (2023), Lactic Acid Bacteria (LAB), including L. casei, produces enzymes that modify or degrade essential oils’ bioactive compounds. LAB is known to hydrolyze glycosidic bonds and decarboxylate certain compounds, leading to the transformation of phenolic compounds during fermentation. This enzymatic activity may metabolize alkaloids, phenolic compounds, and other antimicrobial constituents, potentially reducing the antibacterial efficacy of essential oils. The reduction in inhibitory effect when L. casei and EO1 were combined could be attributed to these enzymatic modifications, as LAB strains have been reported to degrade antimicrobial compounds, thereby reducing their availability for bacterial inhibition (De Montijo-Prieto et al., 2023).
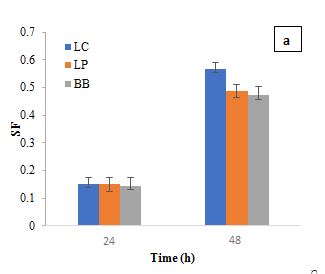
The SF evaluates interactions between substances, where values greater than one indicate synergy—meaning their combined effect exceeds the sum of their individual effects. The SF at 24 h (0.1501) and 48 h (0.5672) suggests a synergistic relationship between L. casei and EO1, where their combined effect exceeded expected contributions, indicating complementary mechanisms enhance antimicrobial activity. While some probiotic-essential oil combinations exhibited synergy, LAB-mediated degradation of bioactive compounds may have reduced antibacterial efficacy in certain cases (Figure 3). Furthermore, LAB strains are known to degrade substances such as alkylamides and alkaloids from essential oils, which can influence both antimicrobial properties and fermentation quality (Li et al., 2021). The co-administration of curry leaf or garlic essential oils with L. casei resulted in minimal reduction in S. aureus concentration compared to the oils alone, suggesting a complex interaction between the essential oils and probiotic formulation. Additionally, certain essential oil components may have inhibitory effects on L. casei at specific concentrations, further influencing antibacterial activity.
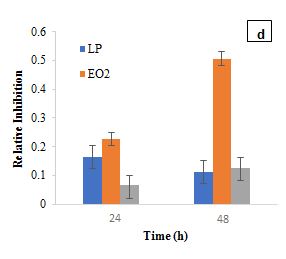
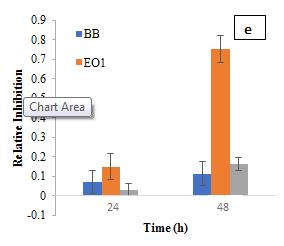
The RI of L. plantarum and B. bifidum alone was relatively low, indicating a limited direct antibacterial activity. However, when combined with EO1, the RI increased significantly, suggesting a synergistic interaction. At 24 h, L. plantarum and EO1 had an SF of 0.1489, while B. bifidum and EO1 had an SF of 0.1447. By 48 h, these values increased to 0.4861 and 0.4728, respectively, reinforcing the idea that certain essential oils can enhance their antimicrobial efficacy. This is consistent with previous studies (Amouei et al., 2021), which suggest that essential oils may act as prebiotics, promoting the growth and metabolic activity of beneficial probiotics, which in turn contributes to pathogen inhibition. The increased RI in combination treatments further indicates that the interactions between probiotics and essential oils are complex, with some strains (L. plantarum and B. bifidum) enhancing antimicrobial effects. Regarding EO2, L. casei exhibited a moderate inhibitory effect with an RI of 0.1337 at 24 h, while EO2 alone showed a higher RI of 0.2260. However, when L. casei was combined with EO2, the RI dropped significantly to 0.0544 at 24 h, increasing slightly to 0.0816 at 48 h. This reduction suggests that L. casei may have influenced EO2's antimicrobial efficacy, potentially through enzymatic degradation of its bioactive components. Garlic essential oil demonstrated notable inhibitory effects on S. aureus in the first 24 h, but this activity waned over time, likely due to the instability and degradation of its primary active compound, allicin (Booyens and Thantsha, 2013). These findings align with previous studies indicating that allicin's antimicrobial properties diminish due to oxidative degradation, reducing its long-term effectiveness. The observed decline in RI over time suggests that while EO2 has strong initial antibacterial activity, its instability could limit sustained efficacy in practical applications. The SF for L. casei and EO2 at 24 h (0.1669) and 48 h (0.2772) confirmed a synergistic interaction, where combined effects exceeded expected outcomes. L. plantarum and B. bifidum also showed synergistic effects when combined with EO2. At 24 h, the synergy factors for L. plantarum and EO2 were 0.1709, while for B. bifidum and EO2, it was 0.1469. At 48 h, these values increased to 0.2458 for L. plantarum and EO2 and 0.1900 for B. bifidum and EO2, further supporting the increased inhibitory effect observed when combined with EO2.
These findings underscore the complex interactions between essential oils and probiotics, where some combinations enhance antimicrobial efficacy through synergistic effects, while others, particularly those involving L. casei, may lead to bioactive compound degradations, thereby reducing their inhibitory efficacy. Similarly, L. plantarum and B. bifidum could diminish essential oil effectiveness, possibly due to the breakdown of antimicrobial compounds or resource competition. Interestingly, essential oils may also act as prebiotics, promoting the growth of beneficial LAB strains like Lactobacillus (Amouei et al., 2021). While this supports probiotic viability, it may also compromise antibacterial activity against pathogens.
The effect of curry leaf essential oil and garlic essential oil on S. aureus was evaluated individually as a control and in the presence of probiotics strains at different time intervals (24 and 48 h) under different treatment conditions, as specified in the experimental design. The recorded measurements represent the mean values obtained from duplicate experimental setups, providing insights into each treatment’s effectiveness in influencing the growth dynamics of S. aureus over time.
RI and SF of essential oils of curry leaf and garlic (EO1 and EO2)
The RI metric assesses the inhibitory effect of a substance on microbial growth by comparing it to a control sample (Figure 2). RI values highlight the inhibitory effects of L. casei, EO1, and their combination on S. aureus. At 24 h, L. casei exhibited moderate inhibition (RI=0.0888), while EO1 showed a higher RI of 0.1479. The antibacterial activity of curry leaf oil has been attributed to its bioactive compounds, such as carbazole alkaloids, which inhibit Staphylococcus, Streptococcus, Escherichia coli, and Proteus (Patil et al., 2024). Similarly, Kumar et al. (2022) reported a dose-dependent antibacterial effect of curry leaf oil against E. coli, supporting its broad-spectrum antimicrobial activity. Additionally, tocopherol, beta-carotene, and lutein—bioactive compounds present in EO1 have also been associated with antibacterial properties (Shahidi et al., 2018). However, when L. casei was combined with EO1, the inhibitory effect was significantly reduced (RI=0.0154) compared to EO1 alone, which exhibited a much higher RI of 0.7528 at 48 h. The combination of L. casei and EO1 resulted in a lower RI of 0.0606.
According to De Montijo-Prieto et al. (2023), Lactic Acid Bacteria (LAB), including L. casei, produces enzymes that modify or degrade essential oils’ bioactive compounds. LAB is known to hydrolyze glycosidic bonds and decarboxylate certain compounds, leading to the transformation of phenolic compounds during fermentation. This enzymatic activity may metabolize alkaloids, phenolic compounds, and other antimicrobial constituents, potentially reducing the antibacterial efficacy of essential oils. The reduction in inhibitory effect when L. casei and EO1 were combined could be attributed to these enzymatic modifications, as LAB strains have been reported to degrade antimicrobial compounds, thereby reducing their availability for bacterial inhibition (De Montijo-Prieto et al., 2023).
The SF evaluates interactions between substances, where values greater than one indicate synergy—meaning their combined effect exceeds the sum of their individual effects. The SF at 24 h (0.1501) and 48 h (0.5672) suggests a synergistic relationship between L. casei and EO1, where their combined effect exceeded expected contributions, indicating complementary mechanisms enhance antimicrobial activity. While some probiotic-essential oil combinations exhibited synergy, LAB-mediated degradation of bioactive compounds may have reduced antibacterial efficacy in certain cases (Figure 3). Furthermore, LAB strains are known to degrade substances such as alkylamides and alkaloids from essential oils, which can influence both antimicrobial properties and fermentation quality (Li et al., 2021). The co-administration of curry leaf or garlic essential oils with L. casei resulted in minimal reduction in S. aureus concentration compared to the oils alone, suggesting a complex interaction between the essential oils and probiotic formulation. Additionally, certain essential oil components may have inhibitory effects on L. casei at specific concentrations, further influencing antibacterial activity.
The RI of L. plantarum and B. bifidum alone was relatively low, indicating a limited direct antibacterial activity. However, when combined with EO1, the RI increased significantly, suggesting a synergistic interaction. At 24 h, L. plantarum and EO1 had an SF of 0.1489, while B. bifidum and EO1 had an SF of 0.1447. By 48 h, these values increased to 0.4861 and 0.4728, respectively, reinforcing the idea that certain essential oils can enhance their antimicrobial efficacy. This is consistent with previous studies (Amouei et al., 2021), which suggest that essential oils may act as prebiotics, promoting the growth and metabolic activity of beneficial probiotics, which in turn contributes to pathogen inhibition. The increased RI in combination treatments further indicates that the interactions between probiotics and essential oils are complex, with some strains (L. plantarum and B. bifidum) enhancing antimicrobial effects. Regarding EO2, L. casei exhibited a moderate inhibitory effect with an RI of 0.1337 at 24 h, while EO2 alone showed a higher RI of 0.2260. However, when L. casei was combined with EO2, the RI dropped significantly to 0.0544 at 24 h, increasing slightly to 0.0816 at 48 h. This reduction suggests that L. casei may have influenced EO2's antimicrobial efficacy, potentially through enzymatic degradation of its bioactive components. Garlic essential oil demonstrated notable inhibitory effects on S. aureus in the first 24 h, but this activity waned over time, likely due to the instability and degradation of its primary active compound, allicin (Booyens and Thantsha, 2013). These findings align with previous studies indicating that allicin's antimicrobial properties diminish due to oxidative degradation, reducing its long-term effectiveness. The observed decline in RI over time suggests that while EO2 has strong initial antibacterial activity, its instability could limit sustained efficacy in practical applications. The SF for L. casei and EO2 at 24 h (0.1669) and 48 h (0.2772) confirmed a synergistic interaction, where combined effects exceeded expected outcomes. L. plantarum and B. bifidum also showed synergistic effects when combined with EO2. At 24 h, the synergy factors for L. plantarum and EO2 were 0.1709, while for B. bifidum and EO2, it was 0.1469. At 48 h, these values increased to 0.2458 for L. plantarum and EO2 and 0.1900 for B. bifidum and EO2, further supporting the increased inhibitory effect observed when combined with EO2.
These findings underscore the complex interactions between essential oils and probiotics, where some combinations enhance antimicrobial efficacy through synergistic effects, while others, particularly those involving L. casei, may lead to bioactive compound degradations, thereby reducing their inhibitory efficacy. Similarly, L. plantarum and B. bifidum could diminish essential oil effectiveness, possibly due to the breakdown of antimicrobial compounds or resource competition. Interestingly, essential oils may also act as prebiotics, promoting the growth of beneficial LAB strains like Lactobacillus (Amouei et al., 2021). While this supports probiotic viability, it may also compromise antibacterial activity against pathogens.
 |
 |
 |
 |
 |
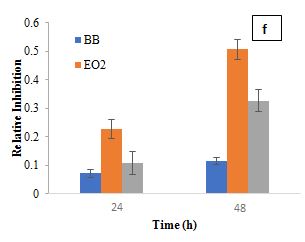 |
Figure 2: Relative Inhibition (RI) in presence of EO1 with and without a) Lactobacillus casei; c) Lactobacillus plantarum; e) Bifidobacterium bifidum. RI in presence of EO2 with and without b) Lactobacillus casei; d) Lactobacillus plantarum; f) Bifidobacterium bifidum
 |
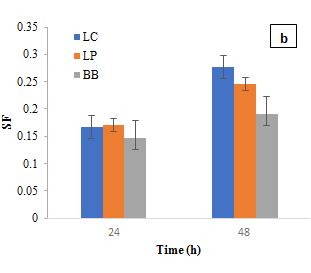 |
Figure 3: Synergistic Factor (SF) on Lactobacillus casei, Lactobacillus plantarum, and Bifidobacterium bifidum in the presence of Essential oil 1 (a); SF on L. casei, L. plantarum, and B. bifidum in the presence of Essential oil 2 (b).
Conclusion
This study demonstrated that essential oils exhibit strong antibacterial activity against S. aureus, though their efficacy is influenced by the presence of probiotics. Notably, curry leaf and garlic essential oils demonstrated significant inhibition of S. aureus when used alone. However, their combination with LAB, particularly L. casei, led to a reduced antibacterial activity, suggesting potential interactions between LAB enzymes and the bioactive compounds in essential oils. Conversely, combining L. plantarum and B. bifidum with essential oils enhanced antimicrobial effects, indicating a strain-dependent relationship. These findings underscore the complexity of probiotic-essential oil interactions, where certain probiotics may degrade antimicrobial compounds while others contribute to synergistic effects. While this study was conducted in culture media, further research is required to evaluate these interactions in food matrices, considering factors such as flavor stability, chemical modifications, and antimicrobial persistence. Understanding these mechanisms is essential for optimizing probiotic-essential oil formulations for food preservation and S. aureus control. In real food systems, multiple factors such as pH, moisture content, and interactions with food components can influence antimicrobial efficacy of probiotics and essential oils. Therefore, incorporating these bioactive substances into food formulations requires a careful balance between maintaining sensory properties (e.g., taste and aroma) and ensuring their stability during processing and storage.
Author contributions
H.P designed and performed the work; H.P. and U.S. wrote the manuscript; P.S.T. supervised the research; P.S.T., R.P., P.C., and R.D. reviewed and edited the manuscript; U.S., R.P., P.C, and R.D. conducted the data analysis. All authors read and approved the final manuscript.
Acknowledgments
The Authors would like to thank the Head of the Departments of Botany (Agriculture Sciences) at Dayalbagh Educational Institute.
Conflicts of interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Ethical consideration
Not applicable.
References
Abebe E., Gugsa G., Ahmed, M. (2020). Review on major food-borne zoonotic bacterial pathogens. Journal of Tropical Medicine. 2020: 1-9. [DOI: 10.1155/2020/4674235]
Alengebawy A., Abdelkhalek S.T., Qureshi S.R., Wang M.Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics. 9: 42. [DOI: 10.3390/toxics9030042]
Amouei H., Ferronato G., Qotbi A.A.A., Bouyeh, M., Dunne P.G., Prandini A., Seidavi A. (2021). Effect of essential oil of thyme (Thymus vulgaris L.) or increasing levels of a commercial prebiotic (TechnoMOS®) on growth performance and carcass characteristics of male broilers. Animals. 11: 3330. [DOI: 10.3390/ani11113330]
Booyens J., Thantsha M.S. (2013). Antibacterial effect of hydrosoluble extracts of garlic (Allium sativum) against Bifidobacterium spp. and Lactobacillus acidophilus. African Journal of Microbiology Research. 7: 669-677. [DOI: 10.5897/AJMR12.1616]
Darbandi A., Asadi A., Mahdizade Ari M., Ohadi E., Talebi M., Halaj Zadeh M., Darb Emamie A., Ghanavati R., Kakanj, M. (2022). Bacteriocins: properties and potential use as antimicrobials. Journal of Clinical Laboratory Analysis. 36: e24093. [DOI: 10.1002/jcla.24093]
Davies C.R., Wohlgemut F., Young T., Violet J., Dickinson M., Sanders J.W., Vallieres C., Avery S.V. (2021). Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biology Reviews. 36:15-26. [DOI:10.1016/j.fbr.2021.01.003]
De Montijo-Prieto S., Razola-Díaz M.D.C., Barbieri F., Tabanelli G., Gardini F., Jiménez-Valera M., Ruiz-Bravo A., Verardo V., Gómez-Caravaca A.M. (2023). Impact of lactic acid bacteria fermentation on phenolic compounds and antioxidant activity of avocado leaf extracts. Antioxidants. 12: 298. [DOI: 10.3390/antiox12020298]
Kumar A., Joishy T., Das S., Kalita M.C., Mukherjee A.K., Khan M.R. (2022). A potential probiotic Lactobacillus plantarum JBC5 improves longevity and healthy aging by modulating antioxidative, innate immunity and serotonin-signaling pathways in Caenorhabditis elegans. Antioxidants. 11: 268. [DOI: 10.3390/antiox11020268]
Li C., Kong Q., Mou H., Jiang Y., Du Y., Zhang F. (2021). Biotransformation of alkylamides and alkaloids by lactic acid bacteria strains isolated from Zanthoxylum bungeanum meal. Bioresource Technology. 330: 124944. [DOI: 10.1016/j.biortech.2021.124944]
Liu Q., Yin X., Languino L.R., Altieri D.C. (2018). Evaluation of drug combination effect using a bliss independence dose–response surface model. Statistics in Biopharmaceutical Research. 10: 112-122. [DOI: 10.1080/19466315.2018.1437071]
Monika K., Malik T., Gehlot R., Rekha K., Kumari A., Sindhu R., Rohilla P. (2021). Antimicrobial properties of probiotics. Environment Conservation Journal. 22: 33-48. [DOI: 10.36953/ECJ.2021.SE.2204]
Narayana D.B., Brindavanam N.B., Shirsekar S. (2024). History of safe use of herbs - approaches for documenting evidence. Journal of Ayurveda and Integrative Medicine. 15: 100849. [DOI: 10.1016/j.jaim.2023.100849]
Pan I., Nanjundan K., Achuthan A., Issac P.K., Rajagopal R., Chang S.W., Bhat S.A., Ravindran B. (2023). Exploration of compost soil for the production of thermo-stable Bacillus protease to synthesize bioactive compounds through soy protein hydrolysis. Agronomy. 13: 1019. [DOI: 10.3390/agronomy13041019]
Parihar H., Pathak R., Tirumalai P.S. (2023). Individual and collective effect of lactic acid bacteria on Staphylococcus aureus. Journal of Bacteriology and Mycology. 11: 87-91. [DOI: 10.15406/jbmoa.2023.11.00350]
Patil R., Mandlik S., Mandlik D. (2024). Murraya koenigii (curry tree): a review of its phytochemistry, ethnomedicinal uses, and pharmacology with respect to molecular mechanisms. Current Traditional Medicine. 10: 73–97. [DOI: 10.2174/2215083810666230609163404]
Šalomskienė J., Abraitienė A., Jonkuviene D., Mačionienė I., Repečkienė J., Stankienė J., Vaičiulytė-Funk L. (2015). Changes in antagonistic activity of lactic acid bacteria induced by their response to technological factors. Agricultural and Food Science. 24: 289-299. [DOI: 10.23986/afsci.50936]
Shahidi F., Hossain A. (2018). Bioactives in spices, and spice oleoresins: phytochemicals and their beneficial effects in food preservation and health promotion. Journal of Food Bioactives. 3: 8-75. [DOI: 10.31665/JFB.2018.3149]
Todd E. (2020). Food-borne disease prevention and risk assessment. International Journal of Environmental Research and Public Health. 17: 5129. [DOI: 10.3390/ijerph17145129]
Zhao W., Sachsenmeier K., Zhang L., Sult E., Hollingsworth R.E., Yang H. (2014). A new bliss independence model to analyze drug combination data. Journal of Biomolecular Screening. 19:817-821. [DOI: 10.1177/1087057114521]
This study demonstrated that essential oils exhibit strong antibacterial activity against S. aureus, though their efficacy is influenced by the presence of probiotics. Notably, curry leaf and garlic essential oils demonstrated significant inhibition of S. aureus when used alone. However, their combination with LAB, particularly L. casei, led to a reduced antibacterial activity, suggesting potential interactions between LAB enzymes and the bioactive compounds in essential oils. Conversely, combining L. plantarum and B. bifidum with essential oils enhanced antimicrobial effects, indicating a strain-dependent relationship. These findings underscore the complexity of probiotic-essential oil interactions, where certain probiotics may degrade antimicrobial compounds while others contribute to synergistic effects. While this study was conducted in culture media, further research is required to evaluate these interactions in food matrices, considering factors such as flavor stability, chemical modifications, and antimicrobial persistence. Understanding these mechanisms is essential for optimizing probiotic-essential oil formulations for food preservation and S. aureus control. In real food systems, multiple factors such as pH, moisture content, and interactions with food components can influence antimicrobial efficacy of probiotics and essential oils. Therefore, incorporating these bioactive substances into food formulations requires a careful balance between maintaining sensory properties (e.g., taste and aroma) and ensuring their stability during processing and storage.
Author contributions
H.P designed and performed the work; H.P. and U.S. wrote the manuscript; P.S.T. supervised the research; P.S.T., R.P., P.C., and R.D. reviewed and edited the manuscript; U.S., R.P., P.C, and R.D. conducted the data analysis. All authors read and approved the final manuscript.
Acknowledgments
The Authors would like to thank the Head of the Departments of Botany (Agriculture Sciences) at Dayalbagh Educational Institute.
Conflicts of interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Ethical consideration
Not applicable.
References
Abebe E., Gugsa G., Ahmed, M. (2020). Review on major food-borne zoonotic bacterial pathogens. Journal of Tropical Medicine. 2020: 1-9. [DOI: 10.1155/2020/4674235]
Alengebawy A., Abdelkhalek S.T., Qureshi S.R., Wang M.Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics. 9: 42. [DOI: 10.3390/toxics9030042]
Amouei H., Ferronato G., Qotbi A.A.A., Bouyeh, M., Dunne P.G., Prandini A., Seidavi A. (2021). Effect of essential oil of thyme (Thymus vulgaris L.) or increasing levels of a commercial prebiotic (TechnoMOS®) on growth performance and carcass characteristics of male broilers. Animals. 11: 3330. [DOI: 10.3390/ani11113330]
Booyens J., Thantsha M.S. (2013). Antibacterial effect of hydrosoluble extracts of garlic (Allium sativum) against Bifidobacterium spp. and Lactobacillus acidophilus. African Journal of Microbiology Research. 7: 669-677. [DOI: 10.5897/AJMR12.1616]
Darbandi A., Asadi A., Mahdizade Ari M., Ohadi E., Talebi M., Halaj Zadeh M., Darb Emamie A., Ghanavati R., Kakanj, M. (2022). Bacteriocins: properties and potential use as antimicrobials. Journal of Clinical Laboratory Analysis. 36: e24093. [DOI: 10.1002/jcla.24093]
Davies C.R., Wohlgemut F., Young T., Violet J., Dickinson M., Sanders J.W., Vallieres C., Avery S.V. (2021). Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biology Reviews. 36:15-26. [DOI:10.1016/j.fbr.2021.01.003]
De Montijo-Prieto S., Razola-Díaz M.D.C., Barbieri F., Tabanelli G., Gardini F., Jiménez-Valera M., Ruiz-Bravo A., Verardo V., Gómez-Caravaca A.M. (2023). Impact of lactic acid bacteria fermentation on phenolic compounds and antioxidant activity of avocado leaf extracts. Antioxidants. 12: 298. [DOI: 10.3390/antiox12020298]
Kumar A., Joishy T., Das S., Kalita M.C., Mukherjee A.K., Khan M.R. (2022). A potential probiotic Lactobacillus plantarum JBC5 improves longevity and healthy aging by modulating antioxidative, innate immunity and serotonin-signaling pathways in Caenorhabditis elegans. Antioxidants. 11: 268. [DOI: 10.3390/antiox11020268]
Li C., Kong Q., Mou H., Jiang Y., Du Y., Zhang F. (2021). Biotransformation of alkylamides and alkaloids by lactic acid bacteria strains isolated from Zanthoxylum bungeanum meal. Bioresource Technology. 330: 124944. [DOI: 10.1016/j.biortech.2021.124944]
Liu Q., Yin X., Languino L.R., Altieri D.C. (2018). Evaluation of drug combination effect using a bliss independence dose–response surface model. Statistics in Biopharmaceutical Research. 10: 112-122. [DOI: 10.1080/19466315.2018.1437071]
Monika K., Malik T., Gehlot R., Rekha K., Kumari A., Sindhu R., Rohilla P. (2021). Antimicrobial properties of probiotics. Environment Conservation Journal. 22: 33-48. [DOI: 10.36953/ECJ.2021.SE.2204]
Narayana D.B., Brindavanam N.B., Shirsekar S. (2024). History of safe use of herbs - approaches for documenting evidence. Journal of Ayurveda and Integrative Medicine. 15: 100849. [DOI: 10.1016/j.jaim.2023.100849]
Pan I., Nanjundan K., Achuthan A., Issac P.K., Rajagopal R., Chang S.W., Bhat S.A., Ravindran B. (2023). Exploration of compost soil for the production of thermo-stable Bacillus protease to synthesize bioactive compounds through soy protein hydrolysis. Agronomy. 13: 1019. [DOI: 10.3390/agronomy13041019]
Parihar H., Pathak R., Tirumalai P.S. (2023). Individual and collective effect of lactic acid bacteria on Staphylococcus aureus. Journal of Bacteriology and Mycology. 11: 87-91. [DOI: 10.15406/jbmoa.2023.11.00350]
Patil R., Mandlik S., Mandlik D. (2024). Murraya koenigii (curry tree): a review of its phytochemistry, ethnomedicinal uses, and pharmacology with respect to molecular mechanisms. Current Traditional Medicine. 10: 73–97. [DOI: 10.2174/2215083810666230609163404]
Šalomskienė J., Abraitienė A., Jonkuviene D., Mačionienė I., Repečkienė J., Stankienė J., Vaičiulytė-Funk L. (2015). Changes in antagonistic activity of lactic acid bacteria induced by their response to technological factors. Agricultural and Food Science. 24: 289-299. [DOI: 10.23986/afsci.50936]
Shahidi F., Hossain A. (2018). Bioactives in spices, and spice oleoresins: phytochemicals and their beneficial effects in food preservation and health promotion. Journal of Food Bioactives. 3: 8-75. [DOI: 10.31665/JFB.2018.3149]
Todd E. (2020). Food-borne disease prevention and risk assessment. International Journal of Environmental Research and Public Health. 17: 5129. [DOI: 10.3390/ijerph17145129]
Zhao W., Sachsenmeier K., Zhang L., Sult E., Hollingsworth R.E., Yang H. (2014). A new bliss independence model to analyze drug combination data. Journal of Biomolecular Screening. 19:817-821. [DOI: 10.1177/1087057114521]
* Corresponding author (P.S. Tirumalai)
* E-mail: premtsaran@dei.ac.in
ORCID ID: https://orcid.org/0000-0002-9060-5024
* E-mail: premtsaran@dei.ac.in
ORCID ID: https://orcid.org/0000-0002-9060-5024
Type of Study: Original article |
Subject:
Special
Received: 24/09/07 | Accepted: 25/03/12 | Published: 25/03/30
Received: 24/09/07 | Accepted: 25/03/12 | Published: 25/03/30
References
1. Abebe E., Gugsa G., Ahmed, M. (2020). Review on major food-borne zoonotic bacterial pathogens. Journal of Tropical Medicine. 2020: 1-9. [DOI: 10.1155/2020/4674235] [DOI:10.1155/2020/4674235] [PMID] [PMCID]
2. Alengebawy A., Abdelkhalek S.T., Qureshi S.R., Wang M.Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics. 9: 42. [DOI: 10.3390/toxics9030042] [DOI:10.3390/toxics9030042] [PMID] [PMCID]
3. Amouei H., Ferronato G., Qotbi A.A.A., Bouyeh, M., Dunne P.G., Prandini A., Seidavi A. (2021). Effect of essential oil of thyme (Thymus vulgaris L.) or increasing levels of a commercial prebiotic (TechnoMOS®) on growth performance and carcass characteristics of male broilers. Animals. 11: 3330. [DOI: 10.3390/ani11113330] [DOI:10.3390/ani11113330] [PMID] [PMCID]
4. Booyens J., Thantsha M.S. (2013). Antibacterial effect of hydrosoluble extracts of garlic (Allium sativum) against Bifidobacterium spp. and Lactobacillus acidophilus. African Journal of Microbiology Research. 7: 669-677. [DOI: 10.5897/AJMR12.1616]
5. Darbandi A., Asadi A., Mahdizade Ari M., Ohadi E., Talebi M., Halaj Zadeh M., Darb Emamie A., Ghanavati R., Kakanj, M. (2022). Bacteriocins: properties and potential use as antimicrobials. Journal of Clinical Laboratory Analysis. 36: e24093. [DOI: 10.1002/jcla.24093] [DOI:10.1002/jcla.24093] [PMID] [PMCID]
6. Davies C.R., Wohlgemut F., Young T., Violet J., Dickinson M., Sanders J.W., Vallieres C., Avery S.V. (2021). Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biology Reviews. 36:15-26. [DOI:10.1016/j.fbr.2021.01.003] [DOI:10.1016/j.fbr.2021.01.003] [PMID] [PMCID]
7. De Montijo-Prieto S., Razola-Díaz M.D.C., Barbieri F., Tabanelli G., Gardini F., Jiménez-Valera M., Ruiz-Bravo A., Verardo V., Gómez-Caravaca A.M. (2023). Impact of lactic acid bacteria fermentation on phenolic compounds and antioxidant activity of avocado leaf extracts. Antioxidants. 12: 298. [DOI: 10.3390/antiox12020298] [DOI:10.3390/antiox12020298] [PMID] [PMCID]
8. Kumar A., Joishy T., Das S., Kalita M.C., Mukherjee A.K., Khan M.R. (2022). A potential probiotic Lactobacillus plantarum JBC5 improves longevity and healthy aging by modulating antioxidative, innate immunity and serotonin-signaling pathways in Caenorhabditis elegans. Antioxidants. 11: 268. [DOI: 10.3390/antiox11020268] [DOI:10.3390/antiox11020268] [PMID] [PMCID]
9. Li C., Kong Q., Mou H., Jiang Y., Du Y., Zhang F. (2021). Biotransformation of alkylamides and alkaloids by lactic acid bacteria strains isolated from Zanthoxylum bungeanum meal. Bioresource Technology. 330: 124944. [DOI: 10.1016/j.biortech.2021.124944] [DOI:10.1016/j.biortech.2021.124944] [PMID]
10. Liu Q., Yin X., Languino L.R., Altieri D.C. (2018). Evaluation of drug combination effect using a bliss independence dose-response surface model. Statistics in Biopharmaceutical Research. 10: 112-122. [DOI: 10.1080/19466315.2018.1437071] [DOI:10.1080/19466315.2018.1437071] [PMID] [PMCID]
11. Monika K., Malik T., Gehlot R., Rekha K., Kumari A., Sindhu R., Rohilla P. (2021). Antimicrobial properties of probiotics. Environment Conservation Journal. 22: 33-48. [DOI: 10.36953/ECJ.2021.SE.2204] [DOI:10.36953/ECJ.2021.SE.2204]
12. Narayana D.B., Brindavanam N.B., Shirsekar S. (2024). History of safe use of herbs - approaches for documenting evidence. Journal of Ayurveda and Integrative Medicine. 15: 100849. [DOI: 10.1016/j.jaim.2023.100849] [DOI:10.1016/j.jaim.2023.100849] [PMID] [PMCID]
13. Pan I., Nanjundan K., Achuthan A., Issac P.K., Rajagopal R., Chang S.W., Bhat S.A., Ravindran B. (2023). Exploration of compost soil for the production of thermo-stable Bacillus protease to synthesize bioactive compounds through soy protein hydrolysis. Agronomy. 13: 1019. [DOI: 10.3390/agronomy13041019] [DOI:10.3390/agronomy13041019]
14. Parihar H., Pathak R., Tirumalai P.S. (2023). Individual and collective effect of lactic acid bacteria on Staphylococcus aureus. Journal of Bacteriology and Mycology. 11: 87-91. [DOI: 10.15406/jbmoa.2023.11.00350] [DOI:10.15406/jbmoa.2023.11.00350]
15. Patil R., Mandlik S., Mandlik D. (2024). Murraya koenigii (curry tree): a review of its phytochemistry, ethnomedicinal uses, and pharmacology with respect to molecular mechanisms. Current Traditional Medicine. 10: 73-97. [DOI: 10.2174/2215083810666230609163404] [DOI:10.2174/2215083810666230609163404]
16. Šalomskienė J., Abraitienė A., Jonkuviene D., Mačionienė I., Repečkienė J., Stankienė J., Vaičiulytė-Funk L. (2015). Changes in antagonistic activity of lactic acid bacteria induced by their response to technological factors. Agricultural and Food Science. 24: 289-299. [DOI: 10.23986/afsci.50936] [DOI:10.23986/afsci.50936]
17. Shahidi F., Hossain A. (2018). Bioactives in spices, and spice oleoresins: phytochemicals and their beneficial effects in food preservation and health promotion. Journal of Food Bioactives. 3: 8-75. [DOI: 10.31665/JFB.2018.3149] [DOI:10.31665/JFB.2018.3149]
18. Todd E. (2020). Food-borne disease prevention and risk assessment. International Journal of Environmental Research and Public Health. 17: 5129. [DOI: 10.3390/ijerph17145129] [DOI:10.3390/ijerph17145129] [PMID] [PMCID]
19. Zhao W., Sachsenmeier K., Zhang L., Sult E., Hollingsworth R.E., Yang H. (2014). A new bliss independence model to analyze drug combination data. Journal of Biomolecular Screening. 19:817-821. [DOI: 10.1177/1087057114521] [DOI:10.1177/1087057114521867] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







