Volume 12, Issue 1 (March 2025)
J. Food Qual. Hazards Control 2025, 12(1): 46-60 |
Back to browse issues page
Ethics code: Not applicable
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Belkhir S, Abdessemed D, Refas I. Impact of Drying Methods on Physicochemical Properties, Bioactive content, and Antioxidant Activity of Opuntia ficus-indica Fruits. J. Food Qual. Hazards Control 2025; 12 (1) :46-60
URL: http://jfqhc.ssu.ac.ir/article-1-1266-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1266-en.html
Laboratory for the improvement of agricultural production and protection of ecosystems in arid zones (LAPAPEZA), Institute of Veterinary and Agronomic Sciences, Batna 1 University, Algeria , safia.belkhir@univ-batna.dz
Full-Text [PDF 1199 kb]
(564 Downloads)
| Abstract (HTML) (1014 Views)
To cite: Belkhir S., Abdessemed D., Refas I.. (2025). Impact of drying methods on physicochemical properties, bioactive content, and antioxidant activity of Opuntia ficus-indica fruits. Journal of Food Quality and Hazards Control. 12: 46-60.
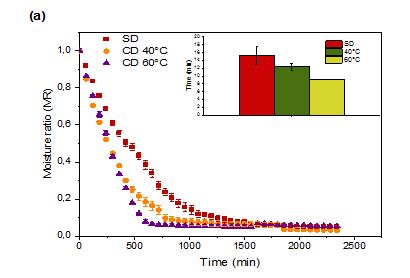
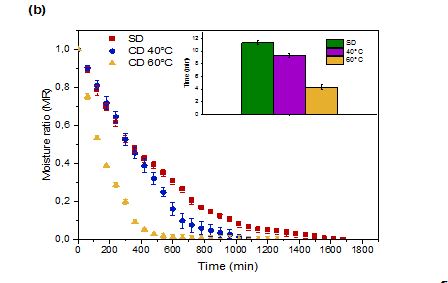
Figure 1: Moisture Ratio (MR) of prickly pear pulp (a) and peels (b) during Shade Drying (SD) and Convective Drying (CD)
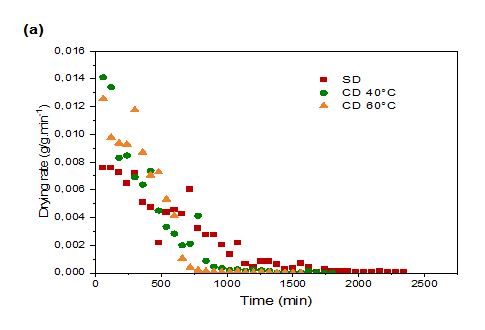
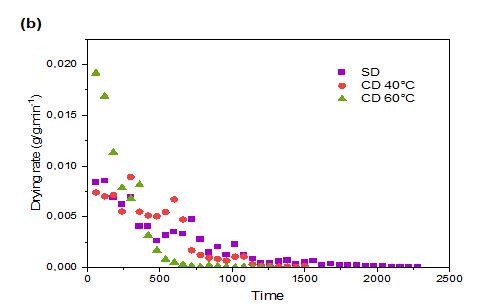
Figure 2: Drying Rate (DR) of prickly pear pulp (a) and peels (b) during Shade Drying (SD) and Convective Drying (CD) at different temperatures
Table 1: Determination of Effective Moisture Diffusivity (Deff) for convective and shade dried pulp and peel of prickly pear fruit
Table 2: Physicochemical and color characterisation of fresh and dried Pulp and Peels of Opuntia ficus-indica fruit

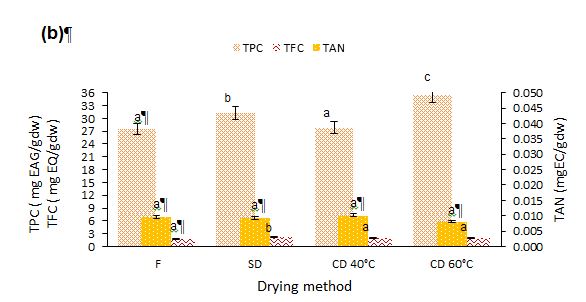
Figure 3: Effect of different drying methods on the phenolic components of prickly pear pulp (a) and peels (b).
Means with the different letter present a significant difference at (p<0.05). TPC=Total Phenolic Components; EAG=Equivalent of Gallic acid; TFC=Total Flavonoid Component; EQ=Equivalent of Quercetin; TAN=Tannin; EC=Equivalent of Catechin; F=Fresh; SD=Shade Drying; CD=Convective Drying at 40 and 60 °C
.JPG)
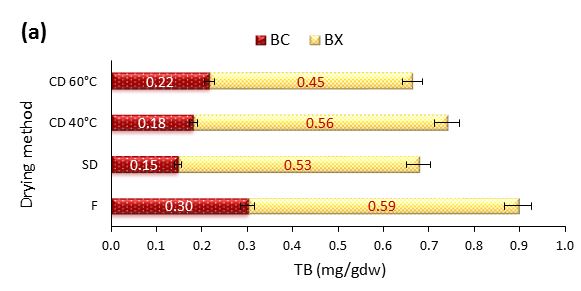
Figure 4: Effect of different drying methods on the betalain content of prickly pear fruit pulp (a) and peel (b). TB=Total Betalains; BC=Betacyanins; BX=Betaxanthins; F=Fresh; SD=Shade Drying; CD=Convective Drying at 40 and 60 °C
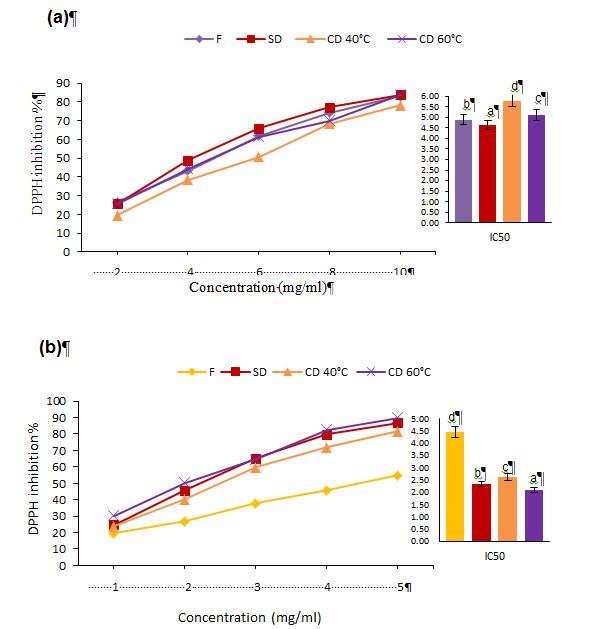
Figure 5: Effect of different drying methods on the antioxidant activity of various concentrations and Half Maximal Inhibitory Concentration (IC50) of prickly pear pulp (a) and peels (b) extracts.
Different latters in IC50 graphe barres present a significant difference at (p<0.05). F=Fresh; SD=Shade Drying; CD=Convective Drying at 40 and 60 °C
Table 3: Correlation of Bioactive content with Half Maximal Inhibitory Concentration (IC50) 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Inhibition of pulp and peels extracts
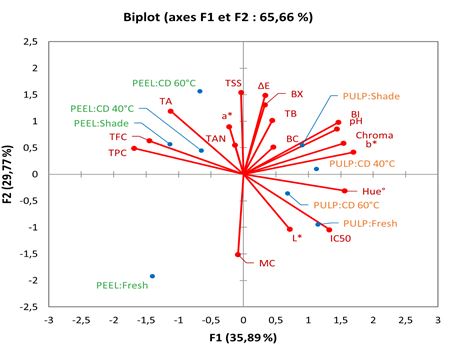
Figure 6: Principal Component Analysis (PCA) of different dried product of prickly pear pulp and peel on the physico-chemical proprieties, bioactive, and antioxidant content
E: Color change; a*: Redeness; b*: Yelewness; BC: Betacyanin; BI: Browning Index; BX: Betaxanthin; CD: Convective Drying; DR: Drying Rate; L*: Lightness; MC: Moisture Contenant; TA: Titratable Acidity; TAN: Tannins; TB: Total Betalains; TFC: Total Flavonoids Compounds; TPC: Total Phenolic Compounds; TSS: Total Soluble Solids
Full-Text: (253 Views)
Impact of Drying Methods on Physicochemical Properties, Bioactive content, and Antioxidant Activity of Opuntia ficus-indica Fruits
S. Belkhir [*]* , D. Abdessemed, I. Refas
Laboratory for the improvement of agricultural production and protection of ecosystems in arid zones (LAPAPEZA), Institute of Veterinary and Agronomic Sciences, Batna 1 University, Algeria.
S. Belkhir [*]*
Laboratory for the improvement of agricultural production and protection of ecosystems in arid zones (LAPAPEZA), Institute of Veterinary and Agronomic Sciences, Batna 1 University, Algeria.
HIGHLIGHTS
- Samples dried at 60 °C retained the highest antioxidant and bioactive content.
- Betalains are present in high concentrations in both fresh pulp and dried peels.
- Dried Peels exhibit favorable characteristics with strong bioactive qualities process.
| Article type Original article |
ABSTRACT Background: Opuntia ficus-indica (prickly pear), is a highly nutritious fruit known for its antioxidant and medicinal properties. However, its seasonal availability and high moisture content make it perishable, necessitating preservation methods like drying. This study aimed to assess the impact of conventional drying methods on the drying kinetics and quality characteristics of both pulp and peels of prickly pear fruit. Methods: Fruits harvested in August and September 2022 were subjected two drying methods: Shade drying and Convective drying (CD) at 40 and 60 °C using a hot air oven. Physicochemical and bioactive properties were analyzed., including Total Phenolic Content (TPC) quantified via the Folin-Ciocalteu assay, and antioxidant activity measured using radical scavenging assay. Data were analyzed using XLSTAT (version 14.5.03). One-way Analysis of Variance (ANOVA) followed by Duncan test (p<0.05), and Pearson’s correlation and Principal Component Analysis for data visualization. Results: Higher drying temperatures resulted in shorter drying times and lower moisture content. Drying also significantly increased the pH, while inducing changes in Total Soluble Solids and Titratable Acidity in both pulp and peels, along with a notable color change. Peels subjected to CD at 60 °C showed the highest Total Phenolic Content and Total Betalains content, at 35.54 mg Gallic Acid Equivalents (GAE)/g dry weight and 0.991 mg/g dry weight, respectively. Flavonoid and tannin content were highest in shade-dried pulp and at 40 °C, indicating the heat sensitivity of these compounds. Minor effects on antioxidant activity (IC50) were observed in pulp during drying, compared to the peels, where the lowest IC50 with values around 2.10 mg/ml, was recorded for convective dried peels at 60°C. Conclusion: Convective drying at 60 °C proved to be the most effective methods for drying and preserving the bioactive properties of prickly pear fruit, offering a balance between drying efficiency and retention of key nutrients and antioxidants. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Opuntia Phytochemicals Antioxidants Betalains. |
||
| Article history Received: 18 Sep 2024 Revised: 21 Dec 2024 Accept: 25 Mar 2025 |
||
| Abbreviations BI=Browning Index CD=Convective Drying Deff=Effective Moisture Diffusivity DPPH=2,2-Diphenyl-1-Picrylhydrazyl DR=Drying Rate IC50=Half Maximal Inhibitory Concentration MR=Moisture Ratio SD=Shade Drying TA=Titratable Acidity TB=Total Betalains TFC=Total Flavonoids Compounds TPC=Total Phenolic Compounds TSS=Total Soluble Solids |
To cite: Belkhir S., Abdessemed D., Refas I.. (2025). Impact of drying methods on physicochemical properties, bioactive content, and antioxidant activity of Opuntia ficus-indica fruits. Journal of Food Quality and Hazards Control. 12: 46-60.
Introduction
In recent years, there has been a significant shift toward adopting integrative medicine and healthier dietary habits to minimize the risk of chronic diseases including diabetes, digestive disorders, cardiovascular illnesses, and cancer. This trend is driven by the high nutritional and bioactive value of natural products such as medicinal plants, fruits, and vegetables (Gallegos-Infante et al., 2009).
Opuntia ficus-indica is a cactus plant that thrives in arid and semiarid regions across the new and old worlds (El-Hawary et al., 2020). This species gradually gained economic importance due to its durability, resistance to harsh climatic conditions, and richness in bioactive components, as well as its nutritional value. This has led to its long-standing recognition as a functional food and medicinal plant (Barba et al., 2017). Moreover, Opuntia provides delicious and nutritious fruits that can be consumed fresh or processed. However, fruit processing generates substantial waste, such as prickly pear peels, which are a rich source of bioactive substances (Barba et al., 2017). Several research highlight that polyphenols and betalains extracted from prickly pear pulp and peels exhibit antioxidant, antibacterial, anti-inflammatory, and antidiabetic properties (Aruwa et al., 2018).
Like many other plants, Opuntia produces seasonal, perishable fruits that are susceptible to chemical and microbial deterioration due to their high moisture content, limiting their shelf life. However, employing various preservation methods such as freezing and drying can extend their shelf-life (Abrol et al., 2014).
Drying has been used since ancient times as a post-harvest preservation method for food and medicinal plants (Guine et al., 2018) to enhance their shelf life by removing most of the water content. This process significantly decreases the water activity, which in turn reduces the proliferation of germs and inhibits certain enzymatic activities. Furthermore, the reduction in moisture content decreases the overall mass of the product, making storage, packaging, and transportation more manageable and less expensive (Phuon et al., 2022). However, dehydration can negatively impact product quality by causing physicochemical damage, including Maillard reaction, color changes, oxidation, and shrinkage. Additionally, it affects the nutritional and functional components, such as phenolic compounds, vitamins, and pigments (Meng et al., 2018). Consequently, this may render the product unacceptable to consumers. The appearance and discoloration of dried products are critical physical attributes that affect consumer perceptions of food quality. Moreover, these changes can impact the availability of active compounds, such as flavonoids, carotenoids, and betalains, which are essential in natural food coloring and phytopharmaceutical industries (El-Hawary et al., 2020). These characteristics are often influenced by various dehydration factors including temperature, airflow, drying time, and the specific drying method employed (Gallegos-Infante et al., 2009).
Various drying technologies have been used based on the type and characteristics of the product. Selecting the most appropriate drying technology and optimizing the drying conditions are critical for preservation, enhancing the added value of food, and reducing operating costs (Mohammed et al., 2020). Moreover, traditional drying methods like sun and Shade Drying (SD) are widely used for ecological and socioeconomic considerations, including ease of use, basic equipment, and low capital costs. However, this process has some inherent problems, as it is highly weather-dependent, involves long dry periods, and is susceptible to dust contamination and insect infestation. Therefore, Convective Drying (CD), also known as hot air-oven drying, is considered the most common and efficient method on the industrial scale (Shi et al., 2019). This method is more cost-effective, reduces processing time, and provides uniformity and hygiene (Abrol et al., 2014; Refas et al., 2025). However, some disadvantages are associated with this process, such as prolonged exposure to high or low temperatures during drying. Consequently, product quality declines due to the degradation of nutritional elements, color, and essential flavor (Meng et al., 2018). Existing research on prickly pear drying methods is quite limited. This study aimed to assess the physicochemical and phytochemical characteristics, as well as the antioxidant activity of both the pulp and peels of O. ficus-indica fruits. Additionally, it examined the impact of conventional drying methods to identify the optimal conditions for preserving the key quality attributes of the dried pulp and peels.
Opuntia ficus-indica is a cactus plant that thrives in arid and semiarid regions across the new and old worlds (El-Hawary et al., 2020). This species gradually gained economic importance due to its durability, resistance to harsh climatic conditions, and richness in bioactive components, as well as its nutritional value. This has led to its long-standing recognition as a functional food and medicinal plant (Barba et al., 2017). Moreover, Opuntia provides delicious and nutritious fruits that can be consumed fresh or processed. However, fruit processing generates substantial waste, such as prickly pear peels, which are a rich source of bioactive substances (Barba et al., 2017). Several research highlight that polyphenols and betalains extracted from prickly pear pulp and peels exhibit antioxidant, antibacterial, anti-inflammatory, and antidiabetic properties (Aruwa et al., 2018).
Like many other plants, Opuntia produces seasonal, perishable fruits that are susceptible to chemical and microbial deterioration due to their high moisture content, limiting their shelf life. However, employing various preservation methods such as freezing and drying can extend their shelf-life (Abrol et al., 2014).
Drying has been used since ancient times as a post-harvest preservation method for food and medicinal plants (Guine et al., 2018) to enhance their shelf life by removing most of the water content. This process significantly decreases the water activity, which in turn reduces the proliferation of germs and inhibits certain enzymatic activities. Furthermore, the reduction in moisture content decreases the overall mass of the product, making storage, packaging, and transportation more manageable and less expensive (Phuon et al., 2022). However, dehydration can negatively impact product quality by causing physicochemical damage, including Maillard reaction, color changes, oxidation, and shrinkage. Additionally, it affects the nutritional and functional components, such as phenolic compounds, vitamins, and pigments (Meng et al., 2018). Consequently, this may render the product unacceptable to consumers. The appearance and discoloration of dried products are critical physical attributes that affect consumer perceptions of food quality. Moreover, these changes can impact the availability of active compounds, such as flavonoids, carotenoids, and betalains, which are essential in natural food coloring and phytopharmaceutical industries (El-Hawary et al., 2020). These characteristics are often influenced by various dehydration factors including temperature, airflow, drying time, and the specific drying method employed (Gallegos-Infante et al., 2009).
Various drying technologies have been used based on the type and characteristics of the product. Selecting the most appropriate drying technology and optimizing the drying conditions are critical for preservation, enhancing the added value of food, and reducing operating costs (Mohammed et al., 2020). Moreover, traditional drying methods like sun and Shade Drying (SD) are widely used for ecological and socioeconomic considerations, including ease of use, basic equipment, and low capital costs. However, this process has some inherent problems, as it is highly weather-dependent, involves long dry periods, and is susceptible to dust contamination and insect infestation. Therefore, Convective Drying (CD), also known as hot air-oven drying, is considered the most common and efficient method on the industrial scale (Shi et al., 2019). This method is more cost-effective, reduces processing time, and provides uniformity and hygiene (Abrol et al., 2014; Refas et al., 2025). However, some disadvantages are associated with this process, such as prolonged exposure to high or low temperatures during drying. Consequently, product quality declines due to the degradation of nutritional elements, color, and essential flavor (Meng et al., 2018). Existing research on prickly pear drying methods is quite limited. This study aimed to assess the physicochemical and phytochemical characteristics, as well as the antioxidant activity of both the pulp and peels of O. ficus-indica fruits. Additionally, it examined the impact of conventional drying methods to identify the optimal conditions for preserving the key quality attributes of the dried pulp and peels.
Materials and methods
Solvents and chemicals
2,2-diphenyl-1-picrylhydrazyl (DPPH; SP1D4313-1G, Prochima-Sigma, Asie), (+)-Catechin hydrate (C1251-5G, Sigma-Aldrich, China), aluminum chloride (AlCl3; 206911-1KG, Sigma-Aldrich, Germany), ethanol (BIOCHEM Chemopharma, France), Folin–Ciocalteu's phenol reagent (BIOCHEM Chemopharma, France), gallic acid (G7384-100G, Merck, China), hydrochloric acid (HCl; 07102-2,5L-GL, Honeywell, Austria), methanol (34860-2,5L, Honeywell, France), quercetin (Q4951-10G, Merck, China), sodium carbonate (13418-1KG-R, Sigma-Aldrich, Germany), sodium hydroxide (NaOH; 30620-1KG, Honeywell, Sweden), sodium nitrite (NaNO2; 563218-25G, Merck, Germany), vanillin (V1104-100G, Sigma-Aldrich, Germany). All chemicals used were of analytical grade.
Plant preparation and drying
O. ficus-indica fruits of the prickly pear were harvested from the Oulad Fadel region in Batna, Algeria, during August and September 2022. Fruits with consistent maturation, uniform size, and no defects were selected. First, the Opuntia fruits were cleaned with flowing tap water to remove dust and impurities, rinsed with distilled water, and then disinfected with ethanol (80%). Lastly, the residual moisture was removed by drying them with tissue paper. After manual peeling, the fruit pulp was cut into small cylindrical pieces each measuring 3.5 cm in diameter and 0.5 cm in thickness, and weighing 6±0.5 g. The peels were also cut into pieces of 2×4 cm and 3±0.5 g.
Two types of drying methods were examined to evaluate their impact on the product:
-SD: using a drying rack, the samples were placed in a shaded area at an ambient temperature (25-30 °C). The weight of the samples was monitored daily and hourly (from 09.00 to 17.00 h).
-CD: conducted using a hot-air oven (Memmert, Germany) at temperatures of 40 and 60 °C, with a constant drying air flow (1.5 m/s). The weight of the samples was monitored hourly. An analytical balance (KERN, Germany) was used to weigh samples until equilibrium was reached and a consistent weight was noted. Dehydrated samples were ground into powder, placed in an opaque airtight glass jar and kept at 4 °C until further analysis.
Two types of drying methods were examined to evaluate their impact on the product:
-SD: using a drying rack, the samples were placed in a shaded area at an ambient temperature (25-30 °C). The weight of the samples was monitored daily and hourly (from 09.00 to 17.00 h).
-CD: conducted using a hot-air oven (Memmert, Germany) at temperatures of 40 and 60 °C, with a constant drying air flow (1.5 m/s). The weight of the samples was monitored hourly. An analytical balance (KERN, Germany) was used to weigh samples until equilibrium was reached and a consistent weight was noted. Dehydrated samples were ground into powder, placed in an opaque airtight glass jar and kept at 4 °C until further analysis.
Drying curves
-Moisture Ratio (MR)
The MR of prickly pear is calculated according to the following equation
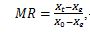 , (1)
, (1)
Where Xt: Moisture content at any time (kg water/kg dry solid), X0: initial moisture content (kg water/kg dry solid), and Xe: the moisture content at equilibrium (kg water/kg dry solid) (Refas et al., 2025)
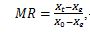 , (1)
, (1)Where Xt: Moisture content at any time (kg water/kg dry solid), X0: initial moisture content (kg water/kg dry solid), and Xe: the moisture content at equilibrium (kg water/kg dry solid) (Refas et al., 2025)
-Drying Rate (DR)
The DR of prickly pear was calculated as:
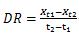 (2)
(2)
X t 1 and X t 2
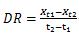 (2)
(2)-Effective Moisture Diffusivity (Deff)
Deff is a value that describes the rate of moisture removal. It could be expressed according to Fick’s second law of diffusion for unsteady state as shown in the following formula
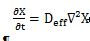 (3)
(3)
Where X is the moisture content (kg water/kg db.), t is the drying time, and Deff is the effective diffusivity (m2/s). Assuming the initial moisture content of the product at the beginning is unchanged, thermal equilibrium exists between the sample surface and the drying air, with only minimal shrinkage during drying.
The general solution of equation (3) can be obtained for common shapes such as slab for the peel (4) (Pinheiro and Castro, 2023), and a cylinder for the fruit pulp (5), using appropriate boundary conditions (Touil et al., 2014).
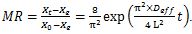 . (4)
. (4)
Where L is the thickness of the peel.
 . (5)
. (5)
Where 𝜀𝑛 is roots of Bessel function.
The general solution of equation (3) can be obtained for common shapes such as slab for the peel (4) (Pinheiro and Castro, 2023), and a cylinder for the fruit pulp (5), using appropriate boundary conditions (Touil et al., 2014).
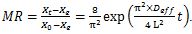 . (4)
. (4)Where L is the thickness of the peel.
 . (5)
. (5)Where 𝜀𝑛 is roots of Bessel function.
Physicochemical proprieties
Fresh and dried fruit pulp and peels were analysed for their physicochemical characteristics. Moisture content (MC) (g/g of wet basis (wb)), pH, Titratable Acidity (TA) (g of citric acid/100 g wb), and Total Soluble Solids (TSS) (Brix°) were determined according to AOAC methods 925.10, 981.12, 942.15, and 932.12, respectively (AOAC, 2000).
Color measurement
The chromatic properties of fresh and dried samples were quantified using a chroma meter (Color reader CR-10, Germany). Illuminant D65 served as the light source. The CIELab color values measured included Lightness (L*), redness/greenness (a*), and yellowness/blueness (b*). Three replicate measurements were performed. Furthermore, the following equations were used to compute color intensity (Chroma), Hue angle (H°), and the total color change (ΔE):
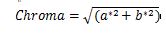 (6)
(6)
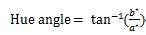 (7)
(7)
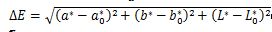 (8)
(8)
ΔE represents the color difference between fresh and dried samples. L*0, b*0, and a*0 correspond to the color parameters of the fresh samples, while L*, a*, and b* refer to those of the dried samples.
The Browning Index (BI) is cited as an essential measure in procedures related to both enzymatic or non-enzymatic browning (Pathare et al., 2013). The BI is calculated using L*, a*, and b* values when applying the following equation:
 (9)
(9)
Where
 (10)
(10)
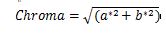 (6)
(6)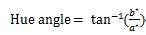 (7)
(7)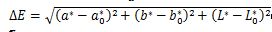 (8)
(8)ΔE represents the color difference between fresh and dried samples. L*0, b*0, and a*0 correspond to the color parameters of the fresh samples, while L*, a*, and b* refer to those of the dried samples.
The Browning Index (BI) is cited as an essential measure in procedures related to both enzymatic or non-enzymatic browning (Pathare et al., 2013). The BI is calculated using L*, a*, and b* values when applying the following equation:
 (9)
(9)Where
 (10)
(10)Determination of phytochemical properties
-Preparation of extracts
Ten g of fresh and dried pulp and peels of prickly pear fruits were combined with 100 ml of hydro ethanol solution (80% v/v) and stirred at room temperature for 24 h. The extraction was repeated twice under similar conditions. Afterwards, the filtrate was collected and the solvent was evaporated at 40±2 °C under decreased pressure using a rotavapor (Stuart, UK). The dried extracts were kept at 4 °C for further analysis.
-Total Phenolic Compounds (TPC)
The Folin–Ciocalteu method, described by Singleton and Rossi (1965), was employed to assess the TPC in the extracts. Five tenths ml of crude extract (3 mg/ml) was mixed with 2.5 ml of diluted Folin–Ciocalteu reagent. After five min in the dark, 0.8 ml of a 7.5% sodium carbonate solution was added. A spectrophotometer (UV-visible 7305– Jenway, UK) was used to detect the absorbance at 765 nm following a two h dark incubation. Triplicate measurements were taken and the results were expressed as mg Equivalent of Gallic Acid (EGA)/g of dry weight using gallic acid standard curve.
-Total Flavonoids Compounds (TFC)
TFC was assessed according to the method outlined by Zhishen et al. (1999). In this procedure, 0.5 ml aliquot of the crude extract solution was combined with 1.5 ml of distillated water and 150 µl of NaNO2. After 5 min, 150 µl of AlCl3 (10%) and 0.5 ml of NaOH were added. The mixture was then incubated for 30 min at room temperature in the dark. Absorbance was read at 430 nm by a spectrophotometer. Quercetin served as the standard, and TFC was expressed as mg equivalent of Quercetin (QE)/g of dry weight.
-Tannins compounds
The tannins content was measured using the vanillin/HCl method from Price et al. (1978). The vanillin reagent, freshly prepared before the experiment, consisted of equal parts of methanol solutions containing 8% HCl and 1% vanillin. An aliquot of three ml of the vanillin reagent was added to 0.6 ml of the sample. After a 20 min incubation at 30 °C, the mixture’s absorbance was measured at 500 nm. Catechin served as the reference standard, and the TFC was expressed as mg Equivalent of Catechin (EC)/100 g of dry weight.
-Betalains content
The quantification of betalains pigments, particularly betaxanthin and betacyanin, was performed using the methodology described by Nadia et al. (2013). This involved using 80% methanol as the extraction solvent. The samples were stirred for 6 h and then centrifuged at 4,000 rpm for 20 min. The betalains concentrations were determined by measuring spectrophotometer absorbance at 480 nm and 535 nm, respectively. The results were expressed according to the following equation:
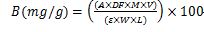 (11)
(11)
Where A: absorbance, V: extract volume, W: sample masse (g), L: cuvette length (one cm), DF: dilution factor, M: molecular weight molar, ε: extinction coefficient, (M: 550 g/mol, ε: 60,000 L/mol.cm for betacyanin), and (M: 308 g/mol, ε: 48,000 L/mol.cm for betaxanthin).
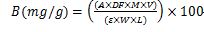 (11)
(11)Where A: absorbance, V: extract volume, W: sample masse (g), L: cuvette length (one cm), DF: dilution factor, M: molecular weight molar, ε: extinction coefficient, (M: 550 g/mol, ε: 60,000 L/mol.cm for betacyanin), and (M: 308 g/mol, ε: 48,000 L/mol.cm for betaxanthin).
DPPH antioxidant assay
The Free radical scavenging activity of prickly pear pulp and peels extracts was evaluated using the DPPH assay, as outlined by Brand-Williams et al. (1995). A one ml aliquot of various dilutions was mixed with two ml of DPPH (0.1 mmol). The process was performed in triplicate. The reaction mixture was vortexed for 30 s and left to stand in the dark for 20 min at room temperature. Using a spectrophotometer, the absorbance was measured at 517 nm against a blank. Total Antioxidant Capacity (TAC) was expressed as the percentage inhibition of the DPPH radical, calculated using the following equation:
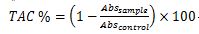 (12)
(12)
Where; Abs sample is the absorbance of the mixture of the extract and reagent and Abs control is the absorbance of the blank.
The half maximal Inhibitory Concentration (IC50), representing the concentration at which 50% of the DPPH radicals are scavenged by the diluted samples, was determined.
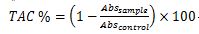 (12)
(12)Where; Abs sample is the absorbance of the mixture of the extract and reagent and Abs control is the absorbance of the blank.
The half maximal Inhibitory Concentration (IC50), representing the concentration at which 50% of the DPPH radicals are scavenged by the diluted samples, was determined.
Statistical analysis
The statistical analysis of all experiments was conducted using XLSTAT (version14.5.03.). The results were provided as Mean±SD. An analysis of variance ANOVA was conducted with Duncan’s post hoc test to assess the differences among means at a 95% confidence interval (p<0.05). The correlation between bioactive components and antioxidant activity assays was analysed using Pearson’s test. A biplot Principal Component Analysis (PCA) was used to visualise the physicochemical proprieties and bioactive components, as well as the IC50 DPPH of fresh and dried prickly pear fruit pulp and peels in a 2D representation.
Results and discussions
Drying curves of prickly pear pulp and Peels fruit
-MR
Figure 1 depicts the changes in MR during CD at 40 and 60 °C and SD of prickly pear fruit pulp and peels. The MR decreased exponentially throughout drying; a behaviour consistent with the dehydration of fruits and vegetables (Onwude et al., 2016). As anticipated, higher temperatures reduced drying times, primarily due to increased vapour pressure within the fruit, accelerating moisture removal (Touil et al., 2014). These findings align with previous reports by Refas et al.(2025). Hot air drying at varying temperatures shortened the total drying duration from 15 to 9 h for prickly pear pulp, and from 11 to 4 h for peels compared to SD. Notably, peel drying progressed more rapidly than pulp drying.


Figure 1: Moisture Ratio (MR) of prickly pear pulp (a) and peels (b) during Shade Drying (SD) and Convective Drying (CD)
-DR
Figure 2 illustrates the DR of prickly pear fruit pulp and peels subjected to CD and SD. Initially, the DR increased significantly, likely due to rapid heat absorption by the large amount of water within the fresh fruit and peels (Sadin et al., 2014). However, the rates gradually declined until the drying process concluded. Elevated temperatures improved both the DR and diffusion coefficient, thereby accelerating water evaporation compared to SD (Figure 2).


Figure 2: Drying Rate (DR) of prickly pear pulp (a) and peels (b) during Shade Drying (SD) and Convective Drying (CD) at different temperatures
-Deff
The Deff for convective and shade dried pulp and peels of prickly pear fruit were determined using Equation (4) and (5). The results, presented in Table 1, reveal an insignificant statistical difference in Deff values. Notably, lower Deff values were observed for SD, while higher values were found for CD, indicating slower moisture migration in SD due to the absence of forced airflow and lower drying temperatures. These values denote the longer drying time required for this conventional methods. During CD, the Deff increased with rising temperatures, especially for the pulp, as higher thermal energy enhanced water molecule mobility and accelerated moisture removal. Notably, the peels exhibited lower diffusivity values under convective conditions, suggesting a more resistant structure that retained moisture longer than the pulp. These findings align with previous reports by Aral and Beşe (2016) and support the faster dehydration of peels observed in the MR section.
Table 1: Determination of Effective Moisture Diffusivity (Deff) for convective and shade dried pulp and peel of prickly pear fruit
| Pulp | Peels | |||||
| SD | CD 40 °C | CD 60 °C | SD | CD 40 °C | CD 60 °C | |
| Deff (m2/s) | 1.37.10-9 ± 3.39.10-10 a |
1.57.10-9 ± 3.39.10-10 a |
1.96.10-9 ± 3.39.10-10 a |
5.39 .10-9 ± 1.55.10-11 a |
7.76.10-11 ± 5.84.10-12 a |
7.08.10-11 ± 2.68.10-11 a |
Values are the Mean±SD. Different letters present significant differences (p≤0.05).
SD=Shade Drying; CD=Convective Drying at 40 and 60 °C.
SD=Shade Drying; CD=Convective Drying at 40 and 60 °C.
Physicochemical proprieties
Table 2 shows the initial moisture content in O. ficus-indica fresh pulp and peels with values of 0.85 and 0.82 (g H2O/g dry weight), respectively. These values align with those reported by Nadia et al. (2013) for O. ficus-indica varieties grown in northern Algeria. Following drying, the moisture content significantly decreased (p<0.05). Notably, CD at 60 °C and SD were most effective in significantly reducing moisture due to high temperature and prolonged drying time, respectively. The pH values, Titrable Acidity (TA), and TSS all increased significantly during the drying process (p<0.05). The rise in TA can be attributed to the reduction in moisture, causing higher concentrations with the production of acids by converting sugars and other chemical reactions (Abrol et al., 2014). Lower moisture content also increased dry matter concentration (Bourhia et al., 2020), resulting in a marked TSS increase. The CD at 60 °C samples had the highest content of soluble solids, with 58.33 and 60 °Brix for pulp and peels, respectively. These values were lower than those measured by Ettalibi et al. (2020) for Moroccan varieties, and higher than those reported by Nadia et al. (2013) and García-Cayuela et al. (2019) in yellow prickly pear pulp of Algerian and Spanish varieties, respectively.
Table 2: Physicochemical and color characterisation of fresh and dried Pulp and Peels of Opuntia ficus-indica fruit
| Drying methods | ||||||||||
| *F | **SD | ***CD 40°C | CD 60°C | |||||||
| Physico-chemical characteristics | ||||||||||
| Moisture content (g H2O/g dry weight) | Pulp | 0.847±0.021 b | 0.050±0.015 a | 0.056±0.022 a | 0.049±0.012 a | |||||
| peels | 0.825±0.016 b | 0.036±0.021 a | 0.040±0.043 a | 0.025±0.006 a | ||||||
| pH | Pulp | 5.87±0.07 a | 6.59±0.05 c | 6.14±0.01 b | 5.91±0.06 a | |||||
| peels | 4.90±0.02 a | 5.37±0.03 b | 5.88±0.02 c | 5.73±0.16 c | ||||||
| TA (g citric acid/100 g wb) | Pulp | 0.16±0.02 a | 0.22±0.02 b | 0.28±0.03 c | 0.25±0.03 bc | |||||
| peels | 0.27±0.02 a | 0.35±0.01 b | 0.31±0.03 ab | 0.46±0.04 c | ||||||
| TSSs (Brix°) | Pulp | 13.50±0.5 a | 55±5 c | 45±5 b | 58.33±7.64 c | |||||
| peels | 13.17±0.5 a | 56.67±7.64 b | 55.00±5 b | 60.00±5 b | ||||||
| Color parameters | ||||||||||
| Lightness (L*) | Pulp | 49.13±0.42 c | 36.70±1.51 a | 38.23±1.58 ab | 40.37±0.57 b | |||||
| peels | 40.37±1.05 a | 36.63±4.57 a | 37.20±2.55 a | 38.03±1.66 a | ||||||
| Redness (a*) | Pulp | 18.97±7.05 b | 13.73±1.93 ab | 10.23±0.90 a | 12.87±0.15 ab | |||||
| peels | 10.43±0.38 a | 17.57±1.80 bc | 15.43±1.75 b | 19.66±2.20 c | ||||||
| Yellowness (b*) | Pulp | 43.13±7.48 a | 37.87±0.15 a | 39.57±3.65 a | 35.63±0.60 a | |||||
| peels | 19.30±0.25 a | 27.83±4.25 b | 29.63±2.51 b | 31.80±1.32 b | ||||||
| Chroma | Pulp | 47.39±8.24 b | 40.31±0.61 ab | 40.90±3.34 ab | 37.89±0.55 a | |||||
| peels | 21.80±0.11 a | 32.98±3.87 b | 33.45±2.22 b | 37.43±1.49 b | ||||||
| Hue angle (H°) | Pulp | 66.31±7.21 a | 70.10±2.63 ab | 75.37±2.40 b | 70.14±0.45 ab | |||||
| peels | 61.39±1.15 a | 57.53±4.29 a | 62.44±3.62 a | 58.30±3.22 a | ||||||
| Color change (ΔE) | Pulp | - | 16.46±3.37 a | 15.98±6.28 a | 14.97±5.05 a | |||||
| peels | - | 12.87±2.12 a | 12.22±2.38 a | 16.11±1.22 b | ||||||
| Browning index (BI) | Pulp | 192.34±49.92 b | 247.94±23.60 ab | 242.67±23.20 ab | 186.06±8.58 a | |||||
| peels | 81.96±2.60 a | 160.86±19.22 b | 168.95±34.24 b | 184.52±15.50 b | ||||||
Values are the Mean±SD. Different letters in row present significant differences (p≤0.05) for each parameter. TA=Titratable Acidity; TSS=Total Soluble Solid; *F=Frech; **SD=Shade Drying; ***CD=Convective Drying at 40 and 60°C.
Color characterisation
Color is a key quality indicator that affects the commercial value and consumer acceptance of food products, serving as a sign of ripeness and the impact of treatment. The CIELab colour parameters are commonly used to track color changes during the drying process of products. Table 2 shows a significant decrease (p<0.05) in Lightness (L*) for fresh pulp and peel, indicating a darker color. The L* value decreased by 25.30, 22.18, and 17.84% for pulp and 28.17, 19.28, and 12.38% for peel after SD, and CD at 40 and 60 °C, respectively; suggesting that higher temperatures increase lightness, making it closer to fresh samples, possibly due to the Maillard reaction, enzymatic browning, or pigments degradation (Guclu et al., 2022). Consequently, shorter drying times reduce the duration of oxidation of some phenolic components and the intensity of browning reactions (Guine et al., 2018).
The redness value (a*) revealed a significant reduction in dried pulp, with the CD at 40 °C sample being the lowest, showing a decrease of 46.06%. Conversely, the redness increased significantly (p<0.05) in dried peels, reaching a peak of 46.97% in the 60 °C sample. Changes in a* value reflect the degradation or concentration of red pigments, consistent with betacyanin levels in dried Opuntia fruit (Figure 4). The yellow pigment (b*) decreased significantly in pulp (17.38% at 60 °C), while it increased (p<0.05) by 39.84% in peels. These results align with the betaxanthin yellow pigment levels shown in Figure 4. The Chroma and Hue° values for fresh pulp were 47.39 and 66.31, respectively. Variations in drying temperature led to significant decreases in Chroma and increases in Hue angle, findings consistent with those reported by Bourhia et al. (2020), and Phuon et al. (2022). For the Opuntia fruit peels, there was no statistical difference in Hue° values between fresh and dried samples, but Chroma values increased significantly (p<0.05), indicating greater color intensity compared to fresh peels. The color change ΔE was most prominent in pulp dried with SD and peels dried with CD at 60 °C, illustrating the browning process. The BI was calculated to confirm these findings, with the highest values observed under the same conditions. This may be attributed to the high amount of reducing sugars (Hernández García et al., 2020), as well as the oxidation of ascorbic acid during heat drying (Ettalibi et al., 2020), which enhance the Maillard reaction. The BI values for fresh pulp and peels were 192.34 and 81.96, respectively; linked to high moisture content promoting enzymatic browning through oxidation of phenolic compounds to o-quinones by Polyphenolic Oxidase (PPO), which then polymerizes to generate dark-colored pigments (Yap et al., 2020). Pathare et al. (2013) noted that color changes during drying are often non-uniform due to varying moisture loss rates and chemical reactions in different food parts.
Phytochemical proprieties
Figure 3 shows the impact of drying methods on TPCs, TFCs, and tannin in the prickly pear pulp and peels. The TPC in fresh pulp and peels was 5.79 and 27.49 mg Gallic Acid Equivalents (GAE)/g dry weight, respectively; A significant decrease (p<0.05) in TPC was observed in the pulp after SD and convective drying at 40 °C. In contrast, TPC increased in both the pulp and peels convective dried at 60 °C, as well as in the peels after SD and CD at 40 °C. As reported by Farahmandfar et al. (2020) for dried orange peels and Kaur et al. (2020) for dried tomato and sweet pepper, the decline of the TPC can be attributed to enzymatic degradation and polyphenol-protein interactions. Furthermore, the prolonged drying duration in SD and CD 40 °C creates favorable conditions for enzymatic oxidation of polyphenol. Additionally, the TPC reduction may also be attributed to polyphenol binding to other compounds; such as proteins, or to changes in its chemical structure, making them unextractable or undetectable by the analytical methods employed (Vega-Gálvez et al., 2009). These results are similar to those reported by El Mannoubi (2021) and Ettalibi et al. (2020). The CD at 60 °C led to a higher content of total polyphenol in both pulp and peels of the Opuntia fruit at 6.44 and 35.54 mg GAE/g dry weight. These findings are consistent with those reported by Capar et al. (2023). As clarified by Capar et al. (2023) and Guclu et al. (2022), the increase in TPC throughout the drying process is linked to the generation of novel liberated phenolic fragments. This process involves the release of phenolic constituents from their bonded state, facilitated by heat-induced breakdown of ester bonds between phenolic and the cell wall.
The production of Maillard reaction products during heat exposure may contribute to the generation of new phenolic compounds from their precursors at elevated temperatures ( Sultana et al., 2012). Low TPC in the fresh sample may be attributed to its high moisture content, which increases enzymatic reactions that cause the degradation of phenolic components (Hossain et al., 2010). As described in Figure 3, SD showed the highest TFC of 1.615 and 2.303 mg QE/g dry weight for pulp and peels, respectively, followed by CD at 40 °C then at 60 °C. these findings indicate that lower drying temperatures may have advantages in preserving heat-sensitive bioactive compounds (Farahmandfar et al., 2020). Additionally, the tannin content significantly increased in samples dried at lower temperatures compared to fresh samples, consistent with the results reported by Najman et al. (2023) for dried quince fruit.
The redness value (a*) revealed a significant reduction in dried pulp, with the CD at 40 °C sample being the lowest, showing a decrease of 46.06%. Conversely, the redness increased significantly (p<0.05) in dried peels, reaching a peak of 46.97% in the 60 °C sample. Changes in a* value reflect the degradation or concentration of red pigments, consistent with betacyanin levels in dried Opuntia fruit (Figure 4). The yellow pigment (b*) decreased significantly in pulp (17.38% at 60 °C), while it increased (p<0.05) by 39.84% in peels. These results align with the betaxanthin yellow pigment levels shown in Figure 4. The Chroma and Hue° values for fresh pulp were 47.39 and 66.31, respectively. Variations in drying temperature led to significant decreases in Chroma and increases in Hue angle, findings consistent with those reported by Bourhia et al. (2020), and Phuon et al. (2022). For the Opuntia fruit peels, there was no statistical difference in Hue° values between fresh and dried samples, but Chroma values increased significantly (p<0.05), indicating greater color intensity compared to fresh peels. The color change ΔE was most prominent in pulp dried with SD and peels dried with CD at 60 °C, illustrating the browning process. The BI was calculated to confirm these findings, with the highest values observed under the same conditions. This may be attributed to the high amount of reducing sugars (Hernández García et al., 2020), as well as the oxidation of ascorbic acid during heat drying (Ettalibi et al., 2020), which enhance the Maillard reaction. The BI values for fresh pulp and peels were 192.34 and 81.96, respectively; linked to high moisture content promoting enzymatic browning through oxidation of phenolic compounds to o-quinones by Polyphenolic Oxidase (PPO), which then polymerizes to generate dark-colored pigments (Yap et al., 2020). Pathare et al. (2013) noted that color changes during drying are often non-uniform due to varying moisture loss rates and chemical reactions in different food parts.
Phytochemical proprieties
Figure 3 shows the impact of drying methods on TPCs, TFCs, and tannin in the prickly pear pulp and peels. The TPC in fresh pulp and peels was 5.79 and 27.49 mg Gallic Acid Equivalents (GAE)/g dry weight, respectively; A significant decrease (p<0.05) in TPC was observed in the pulp after SD and convective drying at 40 °C. In contrast, TPC increased in both the pulp and peels convective dried at 60 °C, as well as in the peels after SD and CD at 40 °C. As reported by Farahmandfar et al. (2020) for dried orange peels and Kaur et al. (2020) for dried tomato and sweet pepper, the decline of the TPC can be attributed to enzymatic degradation and polyphenol-protein interactions. Furthermore, the prolonged drying duration in SD and CD 40 °C creates favorable conditions for enzymatic oxidation of polyphenol. Additionally, the TPC reduction may also be attributed to polyphenol binding to other compounds; such as proteins, or to changes in its chemical structure, making them unextractable or undetectable by the analytical methods employed (Vega-Gálvez et al., 2009). These results are similar to those reported by El Mannoubi (2021) and Ettalibi et al. (2020). The CD at 60 °C led to a higher content of total polyphenol in both pulp and peels of the Opuntia fruit at 6.44 and 35.54 mg GAE/g dry weight. These findings are consistent with those reported by Capar et al. (2023). As clarified by Capar et al. (2023) and Guclu et al. (2022), the increase in TPC throughout the drying process is linked to the generation of novel liberated phenolic fragments. This process involves the release of phenolic constituents from their bonded state, facilitated by heat-induced breakdown of ester bonds between phenolic and the cell wall.
The production of Maillard reaction products during heat exposure may contribute to the generation of new phenolic compounds from their precursors at elevated temperatures ( Sultana et al., 2012). Low TPC in the fresh sample may be attributed to its high moisture content, which increases enzymatic reactions that cause the degradation of phenolic components (Hossain et al., 2010). As described in Figure 3, SD showed the highest TFC of 1.615 and 2.303 mg QE/g dry weight for pulp and peels, respectively, followed by CD at 40 °C then at 60 °C. these findings indicate that lower drying temperatures may have advantages in preserving heat-sensitive bioactive compounds (Farahmandfar et al., 2020). Additionally, the tannin content significantly increased in samples dried at lower temperatures compared to fresh samples, consistent with the results reported by Najman et al. (2023) for dried quince fruit.

|
a
|

Figure 3: Effect of different drying methods on the phenolic components of prickly pear pulp (a) and peels (b).
Means with the different letter present a significant difference at (p<0.05). TPC=Total Phenolic Components; EAG=Equivalent of Gallic acid; TFC=Total Flavonoid Component; EQ=Equivalent of Quercetin; TAN=Tannin; EC=Equivalent of Catechin; F=Fresh; SD=Shade Drying; CD=Convective Drying at 40 and 60 °C

.JPG)
Figure 4: Effect of different drying methods on the betalain content of prickly pear fruit pulp (a) and peel (b). TB=Total Betalains; BC=Betacyanins; BX=Betaxanthins; F=Fresh; SD=Shade Drying; CD=Convective Drying at 40 and 60 °C
Betalains content
Figure 4 Illustrates the Total Betalains (TB) content which represents the sum of the betacyanins and betaxanthin. A statistically significant difference (p<0.05) was detected between fresh and dried samples. The fresh pulp contained the highest TB content (0,894 mg/g dry weight), which is higher than the values reported by García-Cayuela et al. (2019) and Nadia et al. (2013) but lower than findings of Cejudo-Bastante et al. (2014).
The drying process led to a reduction in the TB content of the pulp, with values of 0.747 mg/g dry weight for SD, 0.706 mg/g dry weight for CD at 40 °C, and 0.691 mg/g dry weight for CD at 60 °C. This may be attributed to thermal degradation and the loss of sensitive compounds during the drying process. Conversely, a significant increase in TB was observed in dried O. ficus-indica peels with the highest amount (0.991 mg/g dry weight) found in CD samples at 60 °C, followed by SD and CD at 40 °C (0.691 and 0.681 mg/ml, respectively), compared to fresh peels (0.549 mg/ml). Similar results were reported by Ettalibi et al. (2020). The increased TB content in dried peels, especially at 60 °C, suggests that higher drying temperatures can preserve and enhance these compounds. This may be due to the short exposure time to heat and the concentration effect as moisture is removed.
Figure 4 Illustrates the Total Betalains (TB) content which represents the sum of the betacyanins and betaxanthin. A statistically significant difference (p<0.05) was detected between fresh and dried samples. The fresh pulp contained the highest TB content (0,894 mg/g dry weight), which is higher than the values reported by García-Cayuela et al. (2019) and Nadia et al. (2013) but lower than findings of Cejudo-Bastante et al. (2014).
The drying process led to a reduction in the TB content of the pulp, with values of 0.747 mg/g dry weight for SD, 0.706 mg/g dry weight for CD at 40 °C, and 0.691 mg/g dry weight for CD at 60 °C. This may be attributed to thermal degradation and the loss of sensitive compounds during the drying process. Conversely, a significant increase in TB was observed in dried O. ficus-indica peels with the highest amount (0.991 mg/g dry weight) found in CD samples at 60 °C, followed by SD and CD at 40 °C (0.691 and 0.681 mg/ml, respectively), compared to fresh peels (0.549 mg/ml). Similar results were reported by Ettalibi et al. (2020). The increased TB content in dried peels, especially at 60 °C, suggests that higher drying temperatures can preserve and enhance these compounds. This may be due to the short exposure time to heat and the concentration effect as moisture is removed.
Antioxidant activity
The amount of sample required to achieve a 50% reduction in the initial DPPH concentration (IC50) serves as a critical criterion for evaluating antioxidant activity, with lower IC50 values indicating stronger antioxidant capacity. As illustrated in Figure 5, significant differences (p<0.05) were observed in the antioxidant capacities of fresh and dried Opuntia pulp and peels.
The lowest IC50 value for dried pulp was found in the shade-dried sample, with a value of 4.64 mg/ml, followed by 4.88 mg/ml in the fresh sample. Conversely, dried peels exhibited a marked increase in antioxidant activity compared to fresh peels. The best antioxidant activity for the dried peels was recorded in the CD samples at 60 °C, which had an IC50 of 2.10 mg/ml. Dehydration at elevated temperatures exhibited greater antioxidant activity compared to lower temperatures. These findings align with those reported by Sultana et al. (2012).
The lowest IC50 value for dried pulp was found in the shade-dried sample, with a value of 4.64 mg/ml, followed by 4.88 mg/ml in the fresh sample. Conversely, dried peels exhibited a marked increase in antioxidant activity compared to fresh peels. The best antioxidant activity for the dried peels was recorded in the CD samples at 60 °C, which had an IC50 of 2.10 mg/ml. Dehydration at elevated temperatures exhibited greater antioxidant activity compared to lower temperatures. These findings align with those reported by Sultana et al. (2012).
|
d
|
|
a
|
|
c
|
|
b
|
|
(a)
|

|
|
Figure 5: Effect of different drying methods on the antioxidant activity of various concentrations and Half Maximal Inhibitory Concentration (IC50) of prickly pear pulp (a) and peels (b) extracts.
Different latters in IC50 graphe barres present a significant difference at (p<0.05). F=Fresh; SD=Shade Drying; CD=Convective Drying at 40 and 60 °C
Nicoli et al. (1999) stated that the retention or loss of bioactive molecules after treatment, as a result of chemical, enzymatic, or thermal impact, can lead to either an increase or decrease in antioxidant activity. The findings suggest that prickly pear peels contains higher amounts of phenolic compounds and greater antioxidant activity than the pulp, aligning with previous reports by El Mannoubi (2021). Alkaltham et al. (2020) reported that plants commonly create secondary metabolites as response to stress. The synthesis of these substances in plants is known to occur in response to stress factors such as physical injury, pathogen invasion, extreme temperatures, and dehydration. Therefore, as a protective barrier, the peels accumulates higher levels of phenolic compounds than the pulp, enhancing its resistance to environmental stress.
Table 3: Correlation of Bioactive content with Half Maximal Inhibitory Concentration (IC50) 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Inhibition of pulp and peels extracts
| Pearson’s correlation Coefficient (r) | ||||||
| TPC | TFC | Tannins | Betacyanin | Betaxanthin | TB | |
| IC50 pulp | -0.423 | -0.543 | 0.296 | -0.6123* | 0.0759 | -0.3294 |
| IC50 peel | -0.691* | -0.576 | 0.193 | -0.405 | -0.898** | -0.730** |
*Correlation is significant at p<0.05
**Correlation is significant at p<0.01
TB=Total Betalains; TFC=Total Flavonoids Compounds; TPC=Total Phenolic Compounds
**Correlation is significant at p<0.01
TB=Total Betalains; TFC=Total Flavonoids Compounds; TPC=Total Phenolic Compounds
Table 3 demonstrates the correlation between bioactive components and the antioxidant capacity IC50 of O. ficus-indica pulp and peels. A negative correlation was observed between TPC, TFC, and betacyanin, with IC50 in pulp extracts with values of -0.423, -0.543, and -0.6123, respectively. This suggests that, in addition to TFC, betacyanin plays a significant role in influencing the antioxidant activity of various pulp extracts. For the peels extracts, a negative correlation is observed between TPC, TFC, betacyanin, and betaxanthin, with the strongest negative correlation found between betaxanthin and IC50 (coefficient of -0.898), followed by TPC (-0.691), TFC (-0.576), BC (-0.405). The lower correlation between tannin compounds and IC50 in both pulp, and peels extracts may be due to the the relatively low concentration of tannins in these extracts. Overall, correlation analysis indicated that TPC, TFC, and TB significantly influence the DPPH IC50 inhibition values of fruit pulp and peels extracts. This finding is consistent with the results reported by El Mannoubi (2021) and Sultana et al. (2012).

Figure 6: Principal Component Analysis (PCA) of different dried product of prickly pear pulp and peel on the physico-chemical proprieties, bioactive, and antioxidant content
E: Color change; a*: Redeness; b*: Yelewness; BC: Betacyanin; BI: Browning Index; BX: Betaxanthin; CD: Convective Drying; DR: Drying Rate; L*: Lightness; MC: Moisture Contenant; TA: Titratable Acidity; TAN: Tannins; TB: Total Betalains; TFC: Total Flavonoids Compounds; TPC: Total Phenolic Compounds; TSS: Total Soluble Solids
PCA illustrated in Figure 6, showes that the first two principal components, F1 and F2, account for 65.66% of the total variance. The distribution of samples along these components highlights significant compositional differences between fresh and dried samples, as well as between peels and pulp samples. The data indicate that Chroma, Hue°, IC50, and other variables on the right side are strongly associated with the F1 component. Whereas, TFC, TPC, and TA are negatively associated with F1 component. The close clustering of dried peels, particularly those dried by SD and CD at 40 °C, suggests that these samples share similar physic-chemical, phytochemical, and antioxidant properties. Furthermore, the dried peels samples show a strong positive correlation with TPC, TFC, and tannins, indicating higher retention of bioactive compounds; while their negative correlation with IC50 suggests enhanced antioxidant activity. The clear separation between fresh and dried peels underscores the significant impact of drying on peels composition. In contrast, pulp samples appear more dispersed, suggesting that different drying methods can alter their properties. These samples are primarily associated with IC50, pH, and color parameters (L*, Chroma, Hue°, and BI), as well as TB, reflecting their sensitivity to heat-induced changes in pigmentation and antioxidant capacity.
Conclusion
This study examined the effect of conventional drying methods on the physic-chemical and phytochemical properties, as well as the antioxidant activity of O. ficus-indica pulp and peels. The results indicate that CD at 60 °C and SD are the most effective methods for preserving these properties. Notably, the peels contains a higher concentration of phenolic compounds and exhibits greater antioxidant activity compared to the pulp. Moreover, the peels undergoes favourable transformations during drying, effectively retaining its beneficial properties. These findings highlight the potential for optimizing conventional drying techniques and leveraging prickly pear fruits, particularly peels, in the food and pharmaceutical industries.
While the drying methods employed in this study are effective and economically viable, further research is needed to optimize their application. Specifically, a more extensive exploration of drying techniques and conditions, including temperature and airflow variations, is required to gain a deeper understanding of their impact on the properties of O. ficus-indica fruit.
While the drying methods employed in this study are effective and economically viable, further research is needed to optimize their application. Specifically, a more extensive exploration of drying techniques and conditions, including temperature and airflow variations, is required to gain a deeper understanding of their impact on the properties of O. ficus-indica fruit.
Author contributions
S.B. designed the work, collected the samples, performed the experimental work, analysed and interpreted the data, and wrote the manuscript; D.A. designed and supervised the work; I.R. analysed and interpreted the drying kinetics data. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to the Laboratory of Chemistry and Environmental Chemistry (LCCE), Faculty of Material Science, Batna 1 University. Algeria, and to the Common Biological Science Educational Laboratory, Biology department, Batna 2 University, Algeria.
Conflicts of interest
The authors declare no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Ethical consideration
Not applicable.
References
Abrol G.S., Vaidya D., Sharma A., Sharma S. (2014). Effect of solar drying on physico-chemical and antioxidant properties of mango, banana and papaya. National Academy Science Letters. 37: 51-57. [DOI: 10.1007/s40009-013-0196-1]
Alkaltham M.S., Salamatullah A., Hayat K. (2020). Determination of coffee fruit antioxidants cultivated in Saudi Arabia under different drying conditions. Journal of Food Measurement and Characterization. 14: 1306-1313. [DOI: 10.1007/s11694-020-00378-4]
Aral S., Beşe A.V. (2016). Convective drying of hawthorn fruit (Crataegus spp.): effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chemistry. 210: 577-584. [DOI: 10.1016/j.foodchem. 2016.04.128]
Aruwa C.E., Amoo S.O., Kudanga T. (2018). Opuntia (Cactaceae) plant compounds, biological activities and prospects – a comprehensive review. Food Research International. 112: 328-344. [DOI: 10.1016/j.foodres.2018.06.047]
Association of Official Analytical Chemists (AOAC). (2000). Official methods of analysis, 17th Edition. Methods 925.10, 932.12, 942.15, 981.12. The Association of Official Analytical Chemists, Gaithersburg, MD, USA.
Barba F.J., Putnik P., Bursać Kovačević D., Poojary M.M., Roohinejad S., Lorenzo J.M., Koubaa M. (2017). Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: from preservation of beverages to valorization of by-products. Trends in Food Science and Technology. 67: 260-270. [DOI: 10.1016/j.tifs.2017.07.012]
Bourhia M., Elmahdaoui H., Moussa S.I., Ullah R., Bari A. (2020). Potential natural dyes food from the powder of prickly pear fruit peels (Opuntia spp.) growing in the mediterranean basin under climate stress. BioMed Research International. 2020. [DOI: 10.1155/2020/7579430]
Brand-Williams W., Cuvelier M.E., Berset C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 28: 25-30. [DOI: 10.1016/S0023-6438(95)80008-5]
Capar T.D., Dedebas T., Kavuncuoglu H., Karatas S.M., Ekici L., Yalcin H. (2023). Phenolic components, mineral composition, physicochemical, and bioactive properties of Opuntia ficus-indica with different drying methods. Erwerbs-Obstbau. 65: 347-353. [DOI: 10.1007/s10341-022-00807-2]
Cejudo-Bastante M.J., Chaalal M., Louaileche H., Parrado J., Heredia F.J. (2014). Betalain profile, phenolic content, and color characterization of different parts and varieties of opuntia ficus-indica. Journal of Agricultural and Food Chemistry. 62: 8491-8499. [DOI: 10.1021/jf502465g]
El-Hawary S.S., Sobeh M., Badr W.K., Abdelfattah M.A.O., Ali Z.Y., El-Tantawy M.E., Rabeh M.A., Wink M. (2020). HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi Journal of Biological Sciences. 27: 2829-2838. [DOI: 10.1016/j.sjbs.2020.07.003]
El Mannoubi I. (2021). Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow–orange Opuntia ficus indica fruits. Journal of Food Measurement and Characterization. 15: 643-651. [DOI: 10.1007/s11694-020-00673-0]
Ettalibi F., Elmahdaoui H., Amzil J., Gadhi C., Harrak H. (2020). Drying impact on physicochemical and biochemical criteria of prickly pear fruit peels of three varieties of Opuntia spp. Materials Today: Proceedings. 27: 3243-3248. [DOI: 10.1016/j.matpr.2020.04.726]
Farahmandfar R., Tirgarian B., Dehghan B., Nemati A. (2020). Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. Journal of Food Measurement and Characterization. 14: 862-875. [DOI: 10.1007/s11694-019-00334-x]
Gallegos-Infante J.A., Rocha-Guzman N.E., González-Laredo R.F., Reynoso-Camacho R., Medina-Torres L., Cervantes-Cardozo V. (2009). Effect of air flow rate on the polyphenols content and antioxidant capacity of convective dried cactus pear cladodes (Opuntia ficus indica). International Journal of Food Sciences and Nutrition. 60: 80-87. [DOI: 10.1080/09637480802477691]
García-Cayuela T., Gómez-Maqueo A., Guajardo-Flores D., Welti-Chanes J., Cano M.P. (2019). Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: a comparative study. Journal of Food Composition and Analysis. 76: 1-13. [DOI: 10.1016/j.jfca.2018.11.002]
Guclu G., Polat S., Kelebek H., Capanoglu E., Selli S. (2022). Elucidation of the impact of four different drying methods on the phenolics, volatiles, and color properties of the peels of four types of citrus fruits. Journal of the Science of Food and Agriculture. 102: 6036-6046. [DOI: 10.1002/jsfa.11956]
Guine R.P.F., Correia P.M.R., Correia A.C., Goncalves F., Brito M.F.S., Ribeiro J.R.P. (2018). Effect of drying temperature on the physical-chemical and sensorial properties of eggplant (Solanum melongena L.). Current Nutrition and Food Science. 14: 28-39. [DOI: 10.2174/1573401313666170316113359]
Hernández García F., Andreu Coll L., Cano-Lamadrid M., López Lluch D., A. Carbonell Barrachina Á., Legua Murcia P. (2020). Valorization of prickly pear [Opuntia ficus-indica (L.) mill]: nutritional composition, functional properties and economic aspects. In: El-Shafie H. (Editors). Invasive species—introduction pathways, economic impact, and possible management options. IntechOpen, London, UK. pp: 127-142. [DOI: 10.5772/intechopen.92009]
Hossain M.B., Barry-ryan C., Martin-diana A.B., Brunton N.P. (2010). Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chemistry. 123: 85-91. [DOI: 10.1016/j.foodchem.2010.04.003]
Kaur R., Kaur K., Ahluwalia P. (2020). Effect of drying temperatures and storage on chemical and bioactive attributes of dried tomato and sweet pepper. LWT. 117: 108604. [DOI: 10.1016/j.lwt.2019.108604]
Meng Q., Fan H., Li Y., Zhang L. (2018). Effect of drying methods on physico-chemical properties and antioxidant activity of Dendrobium officinale. Journal of Food Measurement and Characterization. 12: 1-10. [DOI: 10.1007/s11694-017-9611-5]
Mohammed S., Edna M., Siraj K. (2020). The effect of traditional and improved solar drying methods on the sensory quality and nutritional composition of fruits: a case of mangoes and pineapples. Heliyon. 6: e04163. [DOI: 10.1016/j.heliyon.2020.e04163]
Nadia C., Hayette L., Safia M., Yasmina M., Yasmina H., Abderezak T. (2013). Physico-chemical characterisation and antioxidant activity of some Opuntia ficus-indica varieties grown in North Algeria. African Journal of Biotechnology. 12: 299-307. [DOI: 10.5897/ajb12.1946]
Najman K., Adrian S., Sadowska A., Świąder K., Hallmann E., Buczak K., Waszkiewicz-Robak B., Szterk A. (2023). Changes in physicochemical and bioactive properties of quince (Cydonia oblonga mill.) and its products. Molecules. 28: 3066. [DOI: 10.3390/molecules28073066]
Nicoli M.C., Anese M., Parpinel M. (1999). Influence of processing on the antioxidant properties of fruit and vegetables. Trends in Food Science and Technology. 10: 94-100. [DOI: 10.1016/S0924-2244(99)00023-0]
Onwude D.I., Hashim N., Janius R.B., Nawi N.M., Abdan K. (2016). Modeling the thin-layer drying of fruits and vegetables: a review. Comprehensive Reviews in Food Science and Food Safety. 15: 599-618. [DOI: 10.1111/1541-4337.12196]
Pathare P.B., Opara U.L., Al-Said F.A.J. (2013). Colour measurement and analysis in fresh and processed foods: a review. Food and Bioprocess Technology. 6: 36-60. [DOI: 10.1007/s11947-012-0867-9]
Phuon V., Ramos I.N., Brandão T.R.S., Silva C.L.M. (2022). Assessment of the impact of drying processes on orange peel quality characteristics. Journal of Food Process Engineering. 45: e13794. [DOI: 10.1111/jfpe.13794]
Pinheiro M.N.C., Castro L.M.M.N. (2023). Effective moisture diffusivity prediction in two Portuguese fruit cultivars (Bravo de Esmolfe apple and Madeira banana) using drying kinetics data. Heliyon. 9: e17741. [DOI: 10.1016/j.heliyon.2023.e17741]
Price M.L., Scoyoc S.V., Butler L.G. (1978). A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. Journal of Agricultural and Food Chemistry. 26: 1214-1218. [DOI: 10.1021/jf60219a031]
Refas I., Amiali M., George O.A., Le K.H., Malekjani N., Kharaghani A. (2025). Bioactive composition, microstructure, and physicochemical properties of Arbutus unedo berries dried using different techniques. Journal of Stored Products Research. 111: 102501. [DOI: 10.1016/j.jspr.2024.102501]
Sadin R., Chegini G.-R., Sadin H. (2014). The effect of temperature and slice thickness on drying kinetics tomato in the infrared dryer. Heat and Mass Transfer. 50: 501-507. [DOI: 10.1007/s00231-013-1255-3]
Shi L., Gu Y., Wu D., Wu X., Grierson D., Tu Y., Wu, Y. (2019). Hot air drying of tea flowers: effect of experimental temperatures on drying kinetics, bioactive compounds and quality attributes. International Journal of Food Science and Technology. 54: 526-535. [DOI: 10.1111/ijfs.13967]
Singleton V.L., Rossi J.A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16: 144-158. [DOI: 10.5344/ajev.1965.16.3.144]
Sultana B., Anwar F., Ashraf M., Saari N. (2012). Effect of drying techniques on the total phenolic contents and antioxidant activity of selected fruits. Journal of Medicinal Plants Research. 6: 161-167. [DOI: 10.5897/jmpr11.916]
Touil A., Chemkhi S., Zagrouba F. (2014). Moisture diffusivity and shrinkage of fruit and cladode of Opuntia ficus-indica during infrared drying. Journal of Food Processing. 2014: 175402. [DOI: 10.1155/2014/175402]
Vega-Gálvez A., Di Scala K., Rodríguez K., Lemus-Mondaca R., Miranda M., López J., Perez-Won M. (2009). Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chemistry. 117: 647-653. [DOI: 10.1016/j.foodchem.2009.04.066]
Yap J.Y., Hii C.L., Ong S.P., Lim K.H., Abas F., Pin K.Y. (2020). Effects of drying on total polyphenols content and antioxidant properties of Carica papaya leaves. Journal of the Science of Food and Agriculture. 100: 2932-2937. [DOI: 10.1002/jsfa.10320]
Zhishen J., Mengcheng T., Jianming W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 64: 555-559. [DOI: 10.1016/S0308-8146(98)00102-2]
Alkaltham M.S., Salamatullah A., Hayat K. (2020). Determination of coffee fruit antioxidants cultivated in Saudi Arabia under different drying conditions. Journal of Food Measurement and Characterization. 14: 1306-1313. [DOI: 10.1007/s11694-020-00378-4]
Aral S., Beşe A.V. (2016). Convective drying of hawthorn fruit (Crataegus spp.): effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chemistry. 210: 577-584. [DOI: 10.1016/j.foodchem. 2016.04.128]
Aruwa C.E., Amoo S.O., Kudanga T. (2018). Opuntia (Cactaceae) plant compounds, biological activities and prospects – a comprehensive review. Food Research International. 112: 328-344. [DOI: 10.1016/j.foodres.2018.06.047]
Association of Official Analytical Chemists (AOAC). (2000). Official methods of analysis, 17th Edition. Methods 925.10, 932.12, 942.15, 981.12. The Association of Official Analytical Chemists, Gaithersburg, MD, USA.
Barba F.J., Putnik P., Bursać Kovačević D., Poojary M.M., Roohinejad S., Lorenzo J.M., Koubaa M. (2017). Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: from preservation of beverages to valorization of by-products. Trends in Food Science and Technology. 67: 260-270. [DOI: 10.1016/j.tifs.2017.07.012]
Bourhia M., Elmahdaoui H., Moussa S.I., Ullah R., Bari A. (2020). Potential natural dyes food from the powder of prickly pear fruit peels (Opuntia spp.) growing in the mediterranean basin under climate stress. BioMed Research International. 2020. [DOI: 10.1155/2020/7579430]
Brand-Williams W., Cuvelier M.E., Berset C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 28: 25-30. [DOI: 10.1016/S0023-6438(95)80008-5]
Capar T.D., Dedebas T., Kavuncuoglu H., Karatas S.M., Ekici L., Yalcin H. (2023). Phenolic components, mineral composition, physicochemical, and bioactive properties of Opuntia ficus-indica with different drying methods. Erwerbs-Obstbau. 65: 347-353. [DOI: 10.1007/s10341-022-00807-2]
Cejudo-Bastante M.J., Chaalal M., Louaileche H., Parrado J., Heredia F.J. (2014). Betalain profile, phenolic content, and color characterization of different parts and varieties of opuntia ficus-indica. Journal of Agricultural and Food Chemistry. 62: 8491-8499. [DOI: 10.1021/jf502465g]
El-Hawary S.S., Sobeh M., Badr W.K., Abdelfattah M.A.O., Ali Z.Y., El-Tantawy M.E., Rabeh M.A., Wink M. (2020). HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi Journal of Biological Sciences. 27: 2829-2838. [DOI: 10.1016/j.sjbs.2020.07.003]
El Mannoubi I. (2021). Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow–orange Opuntia ficus indica fruits. Journal of Food Measurement and Characterization. 15: 643-651. [DOI: 10.1007/s11694-020-00673-0]
Ettalibi F., Elmahdaoui H., Amzil J., Gadhi C., Harrak H. (2020). Drying impact on physicochemical and biochemical criteria of prickly pear fruit peels of three varieties of Opuntia spp. Materials Today: Proceedings. 27: 3243-3248. [DOI: 10.1016/j.matpr.2020.04.726]
Farahmandfar R., Tirgarian B., Dehghan B., Nemati A. (2020). Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. Journal of Food Measurement and Characterization. 14: 862-875. [DOI: 10.1007/s11694-019-00334-x]
Gallegos-Infante J.A., Rocha-Guzman N.E., González-Laredo R.F., Reynoso-Camacho R., Medina-Torres L., Cervantes-Cardozo V. (2009). Effect of air flow rate on the polyphenols content and antioxidant capacity of convective dried cactus pear cladodes (Opuntia ficus indica). International Journal of Food Sciences and Nutrition. 60: 80-87. [DOI: 10.1080/09637480802477691]
García-Cayuela T., Gómez-Maqueo A., Guajardo-Flores D., Welti-Chanes J., Cano M.P. (2019). Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: a comparative study. Journal of Food Composition and Analysis. 76: 1-13. [DOI: 10.1016/j.jfca.2018.11.002]
Guclu G., Polat S., Kelebek H., Capanoglu E., Selli S. (2022). Elucidation of the impact of four different drying methods on the phenolics, volatiles, and color properties of the peels of four types of citrus fruits. Journal of the Science of Food and Agriculture. 102: 6036-6046. [DOI: 10.1002/jsfa.11956]
Guine R.P.F., Correia P.M.R., Correia A.C., Goncalves F., Brito M.F.S., Ribeiro J.R.P. (2018). Effect of drying temperature on the physical-chemical and sensorial properties of eggplant (Solanum melongena L.). Current Nutrition and Food Science. 14: 28-39. [DOI: 10.2174/1573401313666170316113359]
Hernández García F., Andreu Coll L., Cano-Lamadrid M., López Lluch D., A. Carbonell Barrachina Á., Legua Murcia P. (2020). Valorization of prickly pear [Opuntia ficus-indica (L.) mill]: nutritional composition, functional properties and economic aspects. In: El-Shafie H. (Editors). Invasive species—introduction pathways, economic impact, and possible management options. IntechOpen, London, UK. pp: 127-142. [DOI: 10.5772/intechopen.92009]
Hossain M.B., Barry-ryan C., Martin-diana A.B., Brunton N.P. (2010). Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chemistry. 123: 85-91. [DOI: 10.1016/j.foodchem.2010.04.003]
Kaur R., Kaur K., Ahluwalia P. (2020). Effect of drying temperatures and storage on chemical and bioactive attributes of dried tomato and sweet pepper. LWT. 117: 108604. [DOI: 10.1016/j.lwt.2019.108604]
Meng Q., Fan H., Li Y., Zhang L. (2018). Effect of drying methods on physico-chemical properties and antioxidant activity of Dendrobium officinale. Journal of Food Measurement and Characterization. 12: 1-10. [DOI: 10.1007/s11694-017-9611-5]
Mohammed S., Edna M., Siraj K. (2020). The effect of traditional and improved solar drying methods on the sensory quality and nutritional composition of fruits: a case of mangoes and pineapples. Heliyon. 6: e04163. [DOI: 10.1016/j.heliyon.2020.e04163]
Nadia C., Hayette L., Safia M., Yasmina M., Yasmina H., Abderezak T. (2013). Physico-chemical characterisation and antioxidant activity of some Opuntia ficus-indica varieties grown in North Algeria. African Journal of Biotechnology. 12: 299-307. [DOI: 10.5897/ajb12.1946]
Najman K., Adrian S., Sadowska A., Świąder K., Hallmann E., Buczak K., Waszkiewicz-Robak B., Szterk A. (2023). Changes in physicochemical and bioactive properties of quince (Cydonia oblonga mill.) and its products. Molecules. 28: 3066. [DOI: 10.3390/molecules28073066]
Nicoli M.C., Anese M., Parpinel M. (1999). Influence of processing on the antioxidant properties of fruit and vegetables. Trends in Food Science and Technology. 10: 94-100. [DOI: 10.1016/S0924-2244(99)00023-0]
Onwude D.I., Hashim N., Janius R.B., Nawi N.M., Abdan K. (2016). Modeling the thin-layer drying of fruits and vegetables: a review. Comprehensive Reviews in Food Science and Food Safety. 15: 599-618. [DOI: 10.1111/1541-4337.12196]
Pathare P.B., Opara U.L., Al-Said F.A.J. (2013). Colour measurement and analysis in fresh and processed foods: a review. Food and Bioprocess Technology. 6: 36-60. [DOI: 10.1007/s11947-012-0867-9]
Phuon V., Ramos I.N., Brandão T.R.S., Silva C.L.M. (2022). Assessment of the impact of drying processes on orange peel quality characteristics. Journal of Food Process Engineering. 45: e13794. [DOI: 10.1111/jfpe.13794]
Pinheiro M.N.C., Castro L.M.M.N. (2023). Effective moisture diffusivity prediction in two Portuguese fruit cultivars (Bravo de Esmolfe apple and Madeira banana) using drying kinetics data. Heliyon. 9: e17741. [DOI: 10.1016/j.heliyon.2023.e17741]
Price M.L., Scoyoc S.V., Butler L.G. (1978). A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. Journal of Agricultural and Food Chemistry. 26: 1214-1218. [DOI: 10.1021/jf60219a031]
Refas I., Amiali M., George O.A., Le K.H., Malekjani N., Kharaghani A. (2025). Bioactive composition, microstructure, and physicochemical properties of Arbutus unedo berries dried using different techniques. Journal of Stored Products Research. 111: 102501. [DOI: 10.1016/j.jspr.2024.102501]
Sadin R., Chegini G.-R., Sadin H. (2014). The effect of temperature and slice thickness on drying kinetics tomato in the infrared dryer. Heat and Mass Transfer. 50: 501-507. [DOI: 10.1007/s00231-013-1255-3]
Shi L., Gu Y., Wu D., Wu X., Grierson D., Tu Y., Wu, Y. (2019). Hot air drying of tea flowers: effect of experimental temperatures on drying kinetics, bioactive compounds and quality attributes. International Journal of Food Science and Technology. 54: 526-535. [DOI: 10.1111/ijfs.13967]
Singleton V.L., Rossi J.A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16: 144-158. [DOI: 10.5344/ajev.1965.16.3.144]
Sultana B., Anwar F., Ashraf M., Saari N. (2012). Effect of drying techniques on the total phenolic contents and antioxidant activity of selected fruits. Journal of Medicinal Plants Research. 6: 161-167. [DOI: 10.5897/jmpr11.916]
Touil A., Chemkhi S., Zagrouba F. (2014). Moisture diffusivity and shrinkage of fruit and cladode of Opuntia ficus-indica during infrared drying. Journal of Food Processing. 2014: 175402. [DOI: 10.1155/2014/175402]
Vega-Gálvez A., Di Scala K., Rodríguez K., Lemus-Mondaca R., Miranda M., López J., Perez-Won M. (2009). Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chemistry. 117: 647-653. [DOI: 10.1016/j.foodchem.2009.04.066]
Yap J.Y., Hii C.L., Ong S.P., Lim K.H., Abas F., Pin K.Y. (2020). Effects of drying on total polyphenols content and antioxidant properties of Carica papaya leaves. Journal of the Science of Food and Agriculture. 100: 2932-2937. [DOI: 10.1002/jsfa.10320]
Zhishen J., Mengcheng T., Jianming W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 64: 555-559. [DOI: 10.1016/S0308-8146(98)00102-2]
* Corresponding author (S. Belkhir)
* E-mail: safia.belkhir@univ-batna.dz
ORCID ID: https://orcid.org/0000-0002-2692-0919
* E-mail: safia.belkhir@univ-batna.dz
ORCID ID: https://orcid.org/0000-0002-2692-0919
Type of Study: Original article |
Subject:
Special
Received: 24/09/18 | Accepted: 25/03/25 | Published: 25/03/30
Received: 24/09/18 | Accepted: 25/03/25 | Published: 25/03/30
References
1. Abrol G.S., Vaidya D., Sharma A., Sharma S. (2014). Effect of solar drying on physico-chemical and antioxidant properties of mango, banana and papaya. National Academy Science Letters. 37: 51-57. [DOI: 10.1007/s40009-013-0196-1] [DOI:10.1007/s40009-013-0196-1]
2. Alkaltham M.S., Salamatullah A., Hayat K. (2020). Determination of coffee fruit antioxidants cultivated in Saudi Arabia under different drying conditions. Journal of Food Measurement and Characterization. 14: 1306-1313. [DOI: 10.1007/s11694-020-00378-4] [DOI:10.1007/s11694-020-00378-4]
3. Aral S., Beşe A.V. (2016). Convective drying of hawthorn fruit (Crataegus spp.): effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chemistry. 210: 577-584. [DOI: 10.1016/j.foodchem. 2016.04.128] [DOI:10.1016/j.foodchem.2016.04.128] [PMID]
4. Aruwa C.E., Amoo S.O., Kudanga T. (2018). Opuntia (Cactaceae) plant compounds, biological activities and prospects - a comprehensive review. Food Research International. 112: 328-344. [DOI: 10.1016/j.foodres.2018.06.047] [DOI:10.1016/j.foodres.2018.06.047] [PMID]
5. Association of Official Analytical Chemists (AOAC). (2000). Official methods of analysis, 17th Edition. Methods 925.10, 932.12, 942.15, 981.12. The Association of Official Analytical Chemists, Gaithersburg, MD, USA.
6. Barba F.J., Putnik P., Bursać Kovačević D., Poojary M.M., Roohinejad S., Lorenzo J.M., Koubaa M. (2017). Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: from preservation of beverages to valorization of by-products. Trends in Food Science and Technology. 67: 260-270. [DOI: 10.1016/j.tifs.2017.07.012] [DOI:10.1016/j.tifs.2017.07.012]
7. Bourhia M., Elmahdaoui H., Moussa S.I., Ullah R., Bari A. (2020). Potential natural dyes food from the powder of prickly pear fruit peels (Opuntia spp.) growing in the mediterranean basin under climate stress. BioMed Research International. 2020. [DOI: 10.1155/2020/7579430] [DOI:10.1155/2020/7579430] [PMID] [PMCID]
8. Brand-Williams W., Cuvelier M.E., Berset C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 28: 25-30. [DOI: 10.1016/S0023-6438(95)80008-5] [DOI:10.1016/S0023-6438(95)80008-5]
9. Capar T.D., Dedebas T., Kavuncuoglu H., Karatas S.M., Ekici L., Yalcin H. (2023). Phenolic components, mineral composition, physicochemical, and bioactive properties of Opuntia ficus-indica with different drying methods. Erwerbs-Obstbau. 65: 347-353. [DOI: 10.1007/s10341-022-00807-2] [DOI:10.1007/s10341-022-00807-2]
10. Cejudo-Bastante M.J., Chaalal M., Louaileche H., Parrado J., Heredia F.J. (2014). Betalain profile, phenolic content, and color characterization of different parts and varieties of opuntia ficus-indica. Journal of Agricultural and Food Chemistry. 62: 8491-8499. [DOI: 10.1021/jf502465g] [DOI:10.1021/jf502465g] [PMID]
11. El-Hawary S.S., Sobeh M., Badr W.K., Abdelfattah M.A.O., Ali Z.Y., El-Tantawy M.E., Rabeh M.A., Wink M. (2020). HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi Journal of Biological Sciences. 27: 2829-2838. [DOI: 10.1016/j.sjbs.2020.07.003] [DOI:10.1016/j.sjbs.2020.07.003] [PMID] [PMCID]
12. El Mannoubi I. (2021). Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow-orange Opuntia ficus indica fruits. Journal of Food Measurement and Characterization. 15: 643-651. [DOI: 10.1007/s11694-020-00673-0] [DOI:10.1007/s11694-020-00673-0]
13. Ettalibi F., Elmahdaoui H., Amzil J., Gadhi C., Harrak H. (2020). Drying impact on physicochemical and biochemical criteria of prickly pear fruit peels of three varieties of Opuntia spp. Materials Today: Proceedings. 27: 3243-3248. [DOI: 10.1016/j.matpr.2020.04.726] [DOI:10.1016/j.matpr.2020.04.726]
14. Farahmandfar R., Tirgarian B., Dehghan B., Nemati A. (2020). Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. Journal of Food Measurement and Characterization. 14: 862-875. [DOI: 10.1007/s11694-019-00334-x] [DOI:10.1007/s11694-019-00334-x]
15. Gallegos-Infante J.A., Rocha-Guzman N.E., González-Laredo R.F., Reynoso-Camacho R., Medina-Torres L., Cervantes-Cardozo V. (2009). Effect of air flow rate on the polyphenols content and antioxidant capacity of convective dried cactus pear cladodes (Opuntia ficus indica). International Journal of Food Sciences and Nutrition. 60: 80-87. [DOI: 10.1080/09637480802477691] [DOI:10.1080/09637480802477691] [PMID]
16. García-Cayuela T., Gómez-Maqueo A., Guajardo-Flores D., Welti-Chanes J., Cano M.P. (2019). Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: a comparative study. Journal of Food Composition and Analysis. 76: 1-13. [DOI: 10.1016/j.jfca.2018.11.002] [DOI:10.1016/j.jfca.2018.11.002]
17. Guclu G., Polat S., Kelebek H., Capanoglu E., Selli S. (2022). Elucidation of the impact of four different drying methods on the phenolics, volatiles, and color properties of the peels of four types of citrus fruits. Journal of the Science of Food and Agriculture. 102: 6036-6046. [DOI: 10.1002/jsfa.11956] [DOI:10.1002/jsfa.11956] [PMID]
18. Guine R.P.F., Correia P.M.R., Correia A.C., Goncalves F., Brito M.F.S., Ribeiro J.R.P. (2018). Effect of drying temperature on the physical-chemical and sensorial properties of eggplant (Solanum melongena L.). Current Nutrition and Food Science. 14: 28-39. [DOI: 10.2174/1573401313666170316113359] [DOI:10.2174/1573401313666170316113359]
19. Hernández García F., Andreu Coll L., Cano-Lamadrid M., López Lluch D., A. Carbonell Barrachina Á., Legua Murcia P. (2020). Valorization of prickly pear [Opuntia ficus-indica (L.) mill]: nutritional composition, functional properties and economic aspects. In: El-Shafie H. (Editors). Invasive species-introduction pathways, economic impact, and possible management options. IntechOpen, London, UK. pp: 127-142. [DOI: 10.5772/intechopen.92009] [DOI:10.5772/intechopen.92009]
20. Hossain M.B., Barry-ryan C., Martin-diana A.B., Brunton N.P. (2010). Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chemistry. 123: 85-91. [DOI: 10.1016/j.foodchem.2010.04.003] [DOI:10.1016/j.foodchem.2010.04.003]
21. Kaur R., Kaur K., Ahluwalia P. (2020). Effect of drying temperatures and storage on chemical and bioactive attributes of dried tomato and sweet pepper. LWT. 117: 108604. [DOI: 10.1016/j.lwt.2019.108604] [DOI:10.1016/j.lwt.2019.108604]
22. Meng Q., Fan H., Li Y., Zhang L. (2018). Effect of drying methods on physico-chemical properties and antioxidant activity of Dendrobium officinale. Journal of Food Measurement and Characterization. 12: 1-10. [DOI: 10.1007/s11694-017-9611-5] [DOI:10.1007/s11694-017-9611-5]
23. Mohammed S., Edna M., Siraj K. (2020). The effect of traditional and improved solar drying methods on the sensory quality and nutritional composition of fruits: a case of mangoes and pineapples. Heliyon. 6: e04163. [DOI: 10.1016/j.heliyon.2020.e04163] [DOI:10.1016/j.heliyon.2020.e04163] [PMID] [PMCID]
24. Nadia C., Hayette L., Safia M., Yasmina M., Yasmina H., Abderezak T. (2013). Physico-chemical characterisation and antioxidant activity of some Opuntia ficus-indica varieties grown in North Algeria. African Journal of Biotechnology. 12: 299-307. [DOI: 10.5897/ajb12.1946] [DOI:10.5897/AJB12.1946]
25. Najman K., Adrian S., Sadowska A., Świąder K., Hallmann E., Buczak K., Waszkiewicz-Robak B., Szterk A. (2023). Changes in physicochemical and bioactive properties of quince (Cydonia oblonga mill.) and its products. Molecules. 28: 3066. [DOI: 10.3390/molecules28073066] [DOI:10.3390/molecules28073066] [PMID] [PMCID]
26. Nicoli M.C., Anese M., Parpinel M. (1999). Influence of processing on the antioxidant properties of fruit and vegetables. Trends in Food Science and Technology. 10: 94-100. [DOI: 10.1016/S0924-2244(99)00023-0] [DOI:10.1016/S0924-2244(99)00023-0]
27. Onwude D.I., Hashim N., Janius R.B., Nawi N.M., Abdan K. (2016). Modeling the thin-layer drying of fruits and vegetables: a review. Comprehensive Reviews in Food Science and Food Safety. 15: 599-618. [DOI: 10.1111/1541-4337.12196] [DOI:10.1111/1541-4337.12196] [PMID]
28. Pathare P.B., Opara U.L., Al-Said F.A.J. (2013). Colour measurement and analysis in fresh and processed foods: a review. Food and Bioprocess Technology. 6: 36-60. [DOI: 10.1007/s11947-012-0867-9] [DOI:10.1007/s11947-012-0867-9]
29. Phuon V., Ramos I.N., Brandão T.R.S., Silva C.L.M. (2022). Assessment of the impact of drying processes on orange peel quality characteristics. Journal of Food Process Engineering. 45: e13794. [DOI: 10.1111/jfpe.13794] [DOI:10.1111/jfpe.13794]
30. Pinheiro M.N.C., Castro L.M.M.N. (2023). Effective moisture diffusivity prediction in two Portuguese fruit cultivars (Bravo de Esmolfe apple and Madeira banana) using drying kinetics data. Heliyon. 9: e17741. [DOI: 10.1016/j.heliyon.2023.e17741] [DOI:10.1016/j.heliyon.2023.e17741] [PMID] [PMCID]
31. Price M.L., Scoyoc S.V., Butler L.G. (1978). A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. Journal of Agricultural and Food Chemistry. 26: 1214-1218. [DOI: 10.1021/jf60219a031] [DOI:10.1021/jf60219a031]
32. Refas I., Amiali M., George O.A., Le K.H., Malekjani N., Kharaghani A. (2025). Bioactive composition, microstructure, and physicochemical properties of Arbutus unedo berries dried using different techniques. Journal of Stored Products Research. 111: 102501. [DOI: 10.1016/j.jspr.2024.102501] [DOI:10.1016/j.jspr.2024.102501]
33. Sadin R., Chegini G.-R., Sadin H. (2014). The effect of temperature and slice thickness on drying kinetics tomato in the infrared dryer. Heat and Mass Transfer. 50: 501-507. [DOI: 10.1007/s00231-013-1255-3] [DOI:10.1007/s00231-013-1255-3]
34. Shi L., Gu Y., Wu D., Wu X., Grierson D., Tu Y., Wu, Y. (2019). Hot air drying of tea flowers: effect of experimental temperatures on drying kinetics, bioactive compounds and quality attributes. International Journal of Food Science and Technology. 54: 526-535. [DOI: 10.1111/ijfs.13967] [DOI:10.1111/ijfs.13967]
35. Singleton V.L., Rossi J.A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16: 144-158. [DOI: 10.5344/ajev.1965.16.3.144] [DOI:10.5344/ajev.1965.16.3.144]
36. Sultana B., Anwar F., Ashraf M., Saari N. (2012). Effect of drying techniques on the total phenolic contents and antioxidant activity of selected fruits. Journal of Medicinal Plants Research. 6: 161-167. [DOI: 10.5897/jmpr11.916] [DOI:10.5897/JMPR11.916]
37. Touil A., Chemkhi S., Zagrouba F. (2014). Moisture diffusivity and shrinkage of fruit and cladode of Opuntia ficus-indica during infrared drying. Journal of Food Processing. 2014: 175402. [DOI: 10.1155/2014/175402] [DOI:10.1155/2014/175402]
38. Vega-Gálvez A., Di Scala K., Rodríguez K., Lemus-Mondaca R., Miranda M., López J., Perez-Won M. (2009). Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chemistry. 117: 647-653. [DOI: 10.1016/j.foodchem.2009.04.066] [DOI:10.1016/j.foodchem.2009.04.066]
39. Yap J.Y., Hii C.L., Ong S.P., Lim K.H., Abas F., Pin K.Y. (2020). Effects of drying on total polyphenols content and antioxidant properties of Carica papaya leaves. Journal of the Science of Food and Agriculture. 100: 2932-2937. [DOI: 10.1002/jsfa.10320] [DOI:10.1002/jsfa.10320] [PMID]
40. Zhishen J., Mengcheng T., Jianming W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 64: 555-559. [DOI: 10.1016/S0308-8146(98)00102-2] [DOI:10.1016/S0308-8146(98)00102-2]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







