Volume 12, Issue 2 (June 2025)
J. Food Qual. Hazards Control 2025, 12(2): 118-126 |
Back to browse issues page
Ethics code: Not applicable.
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ozabor T, Makanjuola M, Jejeniwa M, Adekomi I, Oni G, Oluwajide O et al . Effects of Fermentation on Proximate, Mineral Elements and Total Antioxidant Activity of Cloves-Cinnamon Formulated Cereal Gruels. J. Food Qual. Hazards Control 2025; 12 (2) :118-126
URL: http://jfqhc.ssu.ac.ir/article-1-1277-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1277-en.html
Faculty of Basic and Applied Sciences, Department of Microbiology, Osun State University, Osogbo, Faculty of Science, Food Microbiology and Biotechnology Unit, Department of Microbiology, University of Ibadan , praise.ozabor@uniosun.edu.ng
Full-Text [PDF 411 kb]
(154 Downloads)
| Abstract (HTML) (727 Views)
Full-Text: (116 Views)
Effects of Fermentation on Proximate, Mineral Elements and Total Antioxidant Activity of Cloves-Cinnamon Formulated Cereal Gruels
T. Ozabor 1,2[*]* , M. Makanjuola 1, M. Jejeniwa 1, I. Adekomi 1, G. Oni 1, O. Oluwajide 3, J. Olaitan 1
1. Faculty of Basic and Applied Sciences, Department of Microbiology, Osun State University, Osogbo
2. Faculty of Science, Food Microbiology and Biotechnology Unit, Department of Microbiology, University of Ibadan
3. Faculty of Basic and Applied Sciences, Department of Science Laboratory Technology, Osun State University, Osogbo
HIGHLIGHTS
To cite: Ozabor T., Makanjuola M., Jejeniwa M., Adekomi I., Oni G., Oluwajide O., Olaitan J. (2025). Effects of fermentation on proximate, mineral elements and total antioxidant activity of cloves-cinnamon formulated cereal gruels. Journal of Food Quality and Hazards Control. 12: 118-126.
Table 1: Physicochemical properties of clove-cinnamon formulated and un-formulated ogi
Table 2: Proximate compositions of clove-cinnamon formulated and un-formulated fermented ogi
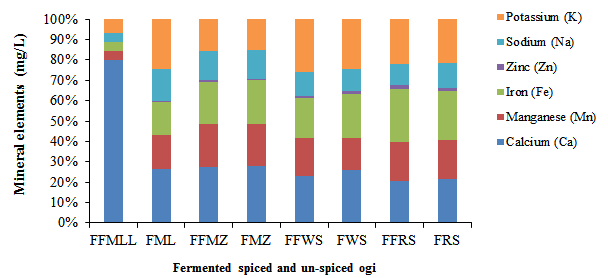
Figure 1: Mineral element compositions of fermented clove-cinnamon formulated and un-formulated ogi
FFML=Formulated Fermented Millet; FML=Fermented Millet only; FFMZ=Formulated Fermented Maize; FMZ=Fermented Maize only; FFWS=Formulated Fermented White Sorghum; FWS=Fermented White Sorghum only; FFRS=Formulated Fermented Red Sorghum; FRS=Fermented Red Sorghum only; FFOF=Formulated Fermented ogi blends. Values are mean±standard error (SE) at (p<0.05)
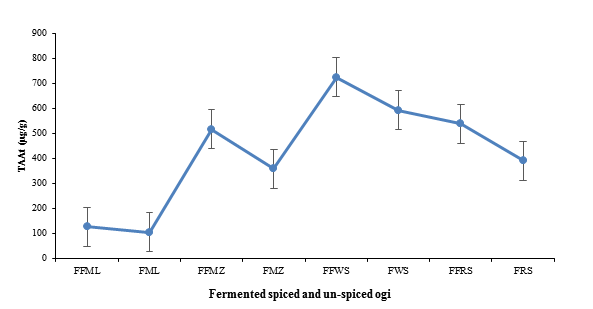
Figure 2: Total Antioxidant Activity (TAA) of fermented clove-cinnamon formulated and un-formulated ogi
FFML=Formulated Fermented Millet; FML=Fermented Millet only; FFMZ=Formulated Fermented Maize; FMZ=Fermented Maize only; FFWS=Formulated Fermented White Sorghum; FWS=Fermented White Sorghum only; FFRS=Formulated Fermented Red Sorghum; FRS=Fermented Red Sorghum only; FFOF=Formulated Fermented ogi blends. Values are mean±Standard Error (SE) at (p<0.05)
T. Ozabor 1,2[*]*
1. Faculty of Basic and Applied Sciences, Department of Microbiology, Osun State University, Osogbo
2. Faculty of Science, Food Microbiology and Biotechnology Unit, Department of Microbiology, University of Ibadan
3. Faculty of Basic and Applied Sciences, Department of Science Laboratory Technology, Osun State University, Osogbo
- Fermentation activities improved proximate composition as well as the release bond mineral elements in the fermented cereal substrates.
- The integration of clove and cinnamon enhanced the total antioxidant activity, a feature crucial for preventing cell damage.
- Formulated fermented grains (ogi) can provide the recommended dietary allowance of the desired mineral elements.
| Article type Original article |
ABSTRACT Background: Ogi is a popularly consumed fermented cereal in Nigeria. Concerns have arisen regarding the loss of essential nutrients during processing as pomace. Therefore, this study was designed to produce cereal gruels by taking the advantages of fermentation and formulations with cloves and cinnamon with the aim of enhancing its proximate, minerals, and total antioxidant potentials. Methods: Four different cereal grains were collected between May-June, 2022. Cereal grains, cloves, and cinnamon were formulated following established protocols. The fermenting organisms were isolated from the spontaneously formulated fermented blends using standard microbiological methods. Physicochemical parameters, such as temperature, pH, and Titratable Acidity, as well as proximate and mineral compositions, were assessed using standard procedures. The Total Antioxidant Activity was determined via the Trolox Equivalent Antioxidant Capacity method, and the obtained data were analyzed using Analysis of Variance (ANOVA) at p<0.05. Results: The temperature of the formulated fermented cereal ranged from 21.4 to 27.8 oC. The highest pH of 5.81 was recorded in formulated fermented white sorghum, while fermented red sorghum exhibited the lowest value of 3.53. The formulated cereal blends demonstrated reduced Titratable Acidity relative to controls. The isolated Lactic Acid Bacteria and yeasts genera were Lactococcus, Lactobacillus, Candida, Cryptococcus, and Trichosporon. The highest percentage of protein, crude fiber, and carbohydrate was recorded in formulated fermented white sorghum and formulated fermented millet with the corresponding values of 9.33, 3.6, and 81.57%, respectively. In addition, a significant increase in calcium (Ca), potassium (K), and zinc (Zn) was documented in formulated fermented millet and formulated fermented red sorghum with corresponding values of 33960, 2870, and 210 mg/L, respectively. Formulated fermented white sorghum showed the highest total antioxidant activity of 724.65 µg/g among fermented samples. Conclusion: This study suggests that usage of cloves and cinnamon with cereal grains during fermentation improved proximate, mineral elements, and total antioxidant activity. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Fermentation Antioxidants Lactobacillales Yeasts Nigeria |
||
| Article history Received: 19 Oct 2024 Revised: 26 Jan 2025 Accepted: 03 May 2025 |
||
| Abbreviations LAB=Lactic Acid Bacteria MRS=DeMan, Rogosa, and Sharpe agar TA=Titratable Acidity TAA=Total Antioxidant Activity YEA=Yeast Extract Agar |
To cite: Ozabor T., Makanjuola M., Jejeniwa M., Adekomi I., Oni G., Oluwajide O., Olaitan J. (2025). Effects of fermentation on proximate, mineral elements and total antioxidant activity of cloves-cinnamon formulated cereal gruels. Journal of Food Quality and Hazards Control. 12: 118-126.
Introduction
Cereals have been considered one of the most important food products for humans, and a major constituent of animal feeds globally (Los et al., 2018). The study of Koehler and Wieser (2013), documented that cereal grains are grown on approximately 60% of the cultivated land in the world with the aim of meeting the requirement of the ever-growing world population (Gabaza et al., 2019). According to the FAO (2017), cereal grains production has increased tremendously in the last 50 years. More so, the most commonly cultivated cereal grains in Africa includes: maize, sorghum, millet, wheat, rice, oats, rye, and barley.
The earlier study of Khoddami et al. (2023), reported that sorghum (Sorghum bicolor) is the fifth most cultivated cereal grain in the world, as they are mostly cultivated in hot arid lands that can tolerate hot temperature variations and drought as opposed to other cereal grains. Furthermore, Hussain et al. (2019) documented that millet (Pennisetum glaucum) is one of the major cereals consumed globally, most especially in the arid and semi-arid parts of Asia and Africa. The works of Zhu et al. (2018) documented that there are seven major types of millet, ranging from different sizes, colors, cultivation areas, and shapes, all of which belongs to the Poaceae family. However, maize on the other hand have also been reported as one of the mostly consumed staple foods in many homes all over the world, as Shah et al. (2016) referred to it as “the queen of cereals”.
The study of Yang et al. (2022) referred to fermented foods as foods or beverages that are produced under a spontaneous or controlled fermentation process in order to transform the raw substrates into new healthy food products. Traditional fermented cereal foods are widely consumed all around the world due to their highly perceived improved nutritional attributes. Among such traditional fermented food is the Nigerian “ogi” which is consumed by people of all ages and backgrounds in Africa as well as other continents of the world (Adelekan et al., 2021).
It has been well reported in literatures that these fermented cereal-based foods have functional health benefits and improved shelf-life (Banwo et al., 2021). Moving forward, a wide variety of cereal substrates, such as maize, sorghum, wheat, and millet can be used for the production of ogi. Based on the reports of Yepez et al. (2019), cereal substrates are sources of essential micro and macro nutrients associated with human functional health benefits (Ijarotimi et al., 2022), and apart from being fermented into gruels and flours, they can also be used for the production of non-alcoholic, alcoholic, and malted beverages (Ramashia et al., 2018). Furthermore, Noah and Alagamba (2020) documented that some of these grains have been bioengineered to be drought-resistant, enabling them to be cultivated year-round.
In recent times, scientists have paid much attention towards the integration of beneficial spices into fermented food products to enhance the food’s overall health benefits. The concept of integrating antioxidant-rich spices such as cloves and cinnamon helps to provide more nutrients than consuming the bland cereals (Paul et al., 2023). Therefore, in this study, cloves (Syzygium aromaticum) and cinnamon (Cinnamomum verum), which belong to the Myrtaceae and Lauraceae families, respectively, were integrated into four different cereal grains (millet, maize, white and red sorghum) for the production of ogi. These two spices were selected from among many others based on available literature indicating that they are rich in antioxidants and numerous phenolic compounds, which may have the potentials to mitigate and/or prevent cell damage (Hassan et al., 2021). Therefore, fermentation presents a medium for combining the blends of cloves, cinnamon, and cereal grains through the actions of the fermenting organisms (mostly lactic acid bacteria and yeasts) for the amplification of the potential health benefits (Houngbedji et al., 2018). Hence, this study was designed to produce cereal gruels with the advantages of fermentation and formulation with spices towards enhancing its antioxidant potentials.
Materials and methods
Collection of samples
Four different cereal grains namely: white and red sorghum (S. bicolor), maize (Zea mays), and millet (P. glaucum) were collected alongside cloves and cinnamon from Olu-ode market in Osogbo, Osun State, Nigeria between May-June, 2022. These samples were packaged inside low-density polythene bags and immediately transported to the Microbiology laboratory of Osun State University for further processing.
Preparations of cloves-cinnamon ogi
The cereal grains were cleaned to remove defective seeds and dirt, then portioned by weight into three groups labeled A-C (160:20:20; 180:10:10, and 200:00:00) for cereal grains, cloves, and cinnamon, respectively, while each raw grain served as a control (200 g).
The portioned grains were thoroughly washed with portable water, and steeped together with the spices (cloves and cinnamon) in 1,000 ml of distilled water. The mixture was covered, securely taped at the edges, and allowed to ferment spontaneously for 48 h at room temperature. Following the initials fermentation, formulated steeped grains were blended and allowed to spontaneously
ferment again at room temperature for 72 h on the laboratory bench. To determine microbial qualities and physicochemical properties, a sample of the fermentate was collected after the 72 h fermentation process. Excess water was drained from the slurries, which then dried in a hot air oven at 60 oC for 2-3 h. The dried ogi flours were stored in clean, airtight zip-lock bags for further analysis, as described by (Ozabor et al., 2022).
Assessment of physicochemical parameters in clove-cinnamon formulated and un-formulated fermented cereal slurries
The method described by the AOAC (2012) was used to determine the temperature, pH, and Titratable Acidity (TA) of the fermentate. Ten ml of the fermented cereal slurries was taken aseptically with a pipetted into a beaker. Temperature was measured using a digital probe thermometer (Model DS18B20, China), while pH was determined using a glass electrode Orion pH meter (Model 330, Orion Research Inc, Beverly, MA). TA was determined by titrating 10 ml of the thoroughly homogenized fermented samples against 0.1 m sodium hydroxide (NaOH; AkzolNoble, Amsterdam), using phenolphthalein as an indicator. All analyses were performed in triplicate, and results were expressed as percentage of lactic acid.
Microbiological qualities of the clove-cinnamon formulated and un-formulated fermented cereal blends
The microbiological characteristics of the raw (control) and formulated ogi samples were determined using DeMan, Rogosa, and Sharpe agar (MRS; TM media, Rajasthan, India) for the isolations of Lactic Acid Bacteria (LAB), and Yeast Extract Agar (YEA; Berkshire RG, UK) for yeast isolation. One g of the fermented slurries was homogenized in nine ml of sterile 0.1% Buffered Peptone Water (BPW), followed by spread plating of appropriate dilutions (1/105 and 1/107) on sterile agar plates. The MRS plates used for the isolation of LAB were incubated anaerobically at 37 oC for 24-48 h, while the YEA used for the isolations of yeasts were incubated aerobically at 30±2 oC for 2-5 days. Representative, distinct colonies were randomly picked from the plates of the highest dilution factor for both MRS and YEA plates to determine the dominant LAB and yeasts involved in the fermentation process. The selected colonies were purified by repeated sub-culturing on MRS for LAB and YEA for yeast. Phenotypic and morphological characterizations, including shape, color, size, elevation, and Gram staining for LAB isolates, were performed on the distinct colonies. Biochemical tests such as catalase, oxidase, and sugar fermentations were also conducted. For yeast identification, the fungi compendium (Alexopoulos) was used (Banwo et al., 2021; Onipede et al., 2021).
Determination of proximate and mineral compositions of formulated fermented cereal blends
The proximate composition, including carbohydrate, crude protein, moisture, crude fibre, ash, and fat contents, was analyzed using the oven-dried formulated fermented ogi flours as described by AOAC (2012). The total carbohydrate content was calculated by subtracting the sum of the percentage of moisture, crude protein, fat, crude fiber, and ash from 100%. For mineral analysis, five g of the ogi flours were heated in a muffle furnace until it turned into a white-greyish ash powder. The ash was left to cool on the laboratory bench at room temperature for approximately 3-5 h. This was followed by adding 20 ml of distilled water and 10 ml of diluted hydrochloric acid (HCl; AkzolNoble, Amsterdam) into the ash powder. The ogi flour samples were analyzed for calcium (Ca), manganese (Mn), iron (Fe), zinc (Zn), sodium (Na), and potassium (K) using an Atomic Absorption Spectrophotometer (AAS; model VGB 210 system, Buck Scientific Instruments, USA) (Banwo et al., 2022).
Determination of Total Antioxidant Activity (TAA) using the Trolox Equivalent Antioxidant Capacity (TEAC)
The TAA was determined using the TEAC method, with slight modifications, as described by AOAC (2012). An aliquot (0.3 ml) of ogi flour samples were homogenized with 2.7 ml of reagent solution (0.6 m H2SO4, 2.8 mm sodium phosphate, and 4 mm ammonium molybdate). The mixtures were capped and incubated in a boiling water bath at 95 oC for 90 min. After incubation, the ogi flour extracts were cooled to room temperature, and absorbance was measured at 695 nm. A blank solution containing 2.7 ml of the reagent solution and 6 ml of ethanol was incubated as a control. Stock solutions of α-tocopherol were prepared in methanol (range 0-10 µmol/ml) and treated identically to the samples. The TAA of the ogi flour extracts was expressed as equivalents of α-tocopherol using an extinction coefficient of 4×103 1/m on a Jenway 6,000 UV-visible spectrophotometer (Caliber Scientific Company, UK). The TAA was calculated as:
TAA (µg/g)=Absorbance of sample×Gradient factor×Dilution factor (103, 105, and 107) (Li et al., 2012).
Statistical analysis
Values are presented as means±Standard Error (SE) of triplicate determinations and interpreted using Analysis of Variance (ANOVA) with MINITAB statistical software (MinitabeÒ Release 14.13, Minitab Inc, USA). Significant differences between means were separated using the lease significant difference (LSD) at p<0.05.
Results and discussion
Physicochemical properties of clove-cinnamon formulated and un-formulated ogi
There was no significant difference (p<0.05) in the temperature among ogi samples made from millet, maize, and red sorghum; however, the temperature of fermented white sorghum ogi differed, although all fermented grains maintained a temperature within the room temperature range of 25.0±2.0 oC. Moreover, the pH of all formulated fermented ogi slurries was statistically significant at (p<0.05) and higher than that of the fermented control samples (without cloves and cinnamon). The pH of all the formulated fermented ogi slurries fell within the acidic pH range (4.0-5.7). In addition, TA increased as the pH decreased. The TA of formulated fermented cereals was also recorded to be statistically different from those of the control samples (without the addition of cloves and cinnamon). The lowest TA was observed in the formulated fermented ogi slurries (Table 1).
The earlier study of Khoddami et al. (2023), reported that sorghum (Sorghum bicolor) is the fifth most cultivated cereal grain in the world, as they are mostly cultivated in hot arid lands that can tolerate hot temperature variations and drought as opposed to other cereal grains. Furthermore, Hussain et al. (2019) documented that millet (Pennisetum glaucum) is one of the major cereals consumed globally, most especially in the arid and semi-arid parts of Asia and Africa. The works of Zhu et al. (2018) documented that there are seven major types of millet, ranging from different sizes, colors, cultivation areas, and shapes, all of which belongs to the Poaceae family. However, maize on the other hand have also been reported as one of the mostly consumed staple foods in many homes all over the world, as Shah et al. (2016) referred to it as “the queen of cereals”.
The study of Yang et al. (2022) referred to fermented foods as foods or beverages that are produced under a spontaneous or controlled fermentation process in order to transform the raw substrates into new healthy food products. Traditional fermented cereal foods are widely consumed all around the world due to their highly perceived improved nutritional attributes. Among such traditional fermented food is the Nigerian “ogi” which is consumed by people of all ages and backgrounds in Africa as well as other continents of the world (Adelekan et al., 2021).
It has been well reported in literatures that these fermented cereal-based foods have functional health benefits and improved shelf-life (Banwo et al., 2021). Moving forward, a wide variety of cereal substrates, such as maize, sorghum, wheat, and millet can be used for the production of ogi. Based on the reports of Yepez et al. (2019), cereal substrates are sources of essential micro and macro nutrients associated with human functional health benefits (Ijarotimi et al., 2022), and apart from being fermented into gruels and flours, they can also be used for the production of non-alcoholic, alcoholic, and malted beverages (Ramashia et al., 2018). Furthermore, Noah and Alagamba (2020) documented that some of these grains have been bioengineered to be drought-resistant, enabling them to be cultivated year-round.
In recent times, scientists have paid much attention towards the integration of beneficial spices into fermented food products to enhance the food’s overall health benefits. The concept of integrating antioxidant-rich spices such as cloves and cinnamon helps to provide more nutrients than consuming the bland cereals (Paul et al., 2023). Therefore, in this study, cloves (Syzygium aromaticum) and cinnamon (Cinnamomum verum), which belong to the Myrtaceae and Lauraceae families, respectively, were integrated into four different cereal grains (millet, maize, white and red sorghum) for the production of ogi. These two spices were selected from among many others based on available literature indicating that they are rich in antioxidants and numerous phenolic compounds, which may have the potentials to mitigate and/or prevent cell damage (Hassan et al., 2021). Therefore, fermentation presents a medium for combining the blends of cloves, cinnamon, and cereal grains through the actions of the fermenting organisms (mostly lactic acid bacteria and yeasts) for the amplification of the potential health benefits (Houngbedji et al., 2018). Hence, this study was designed to produce cereal gruels with the advantages of fermentation and formulation with spices towards enhancing its antioxidant potentials.
Materials and methods
Collection of samples
Four different cereal grains namely: white and red sorghum (S. bicolor), maize (Zea mays), and millet (P. glaucum) were collected alongside cloves and cinnamon from Olu-ode market in Osogbo, Osun State, Nigeria between May-June, 2022. These samples were packaged inside low-density polythene bags and immediately transported to the Microbiology laboratory of Osun State University for further processing.
Preparations of cloves-cinnamon ogi
The cereal grains were cleaned to remove defective seeds and dirt, then portioned by weight into three groups labeled A-C (160:20:20; 180:10:10, and 200:00:00) for cereal grains, cloves, and cinnamon, respectively, while each raw grain served as a control (200 g).
The portioned grains were thoroughly washed with portable water, and steeped together with the spices (cloves and cinnamon) in 1,000 ml of distilled water. The mixture was covered, securely taped at the edges, and allowed to ferment spontaneously for 48 h at room temperature. Following the initials fermentation, formulated steeped grains were blended and allowed to spontaneously
ferment again at room temperature for 72 h on the laboratory bench. To determine microbial qualities and physicochemical properties, a sample of the fermentate was collected after the 72 h fermentation process. Excess water was drained from the slurries, which then dried in a hot air oven at 60 oC for 2-3 h. The dried ogi flours were stored in clean, airtight zip-lock bags for further analysis, as described by (Ozabor et al., 2022).
Assessment of physicochemical parameters in clove-cinnamon formulated and un-formulated fermented cereal slurries
The method described by the AOAC (2012) was used to determine the temperature, pH, and Titratable Acidity (TA) of the fermentate. Ten ml of the fermented cereal slurries was taken aseptically with a pipetted into a beaker. Temperature was measured using a digital probe thermometer (Model DS18B20, China), while pH was determined using a glass electrode Orion pH meter (Model 330, Orion Research Inc, Beverly, MA). TA was determined by titrating 10 ml of the thoroughly homogenized fermented samples against 0.1 m sodium hydroxide (NaOH; AkzolNoble, Amsterdam), using phenolphthalein as an indicator. All analyses were performed in triplicate, and results were expressed as percentage of lactic acid.
Microbiological qualities of the clove-cinnamon formulated and un-formulated fermented cereal blends
The microbiological characteristics of the raw (control) and formulated ogi samples were determined using DeMan, Rogosa, and Sharpe agar (MRS; TM media, Rajasthan, India) for the isolations of Lactic Acid Bacteria (LAB), and Yeast Extract Agar (YEA; Berkshire RG, UK) for yeast isolation. One g of the fermented slurries was homogenized in nine ml of sterile 0.1% Buffered Peptone Water (BPW), followed by spread plating of appropriate dilutions (1/105 and 1/107) on sterile agar plates. The MRS plates used for the isolation of LAB were incubated anaerobically at 37 oC for 24-48 h, while the YEA used for the isolations of yeasts were incubated aerobically at 30±2 oC for 2-5 days. Representative, distinct colonies were randomly picked from the plates of the highest dilution factor for both MRS and YEA plates to determine the dominant LAB and yeasts involved in the fermentation process. The selected colonies were purified by repeated sub-culturing on MRS for LAB and YEA for yeast. Phenotypic and morphological characterizations, including shape, color, size, elevation, and Gram staining for LAB isolates, were performed on the distinct colonies. Biochemical tests such as catalase, oxidase, and sugar fermentations were also conducted. For yeast identification, the fungi compendium (Alexopoulos) was used (Banwo et al., 2021; Onipede et al., 2021).
Determination of proximate and mineral compositions of formulated fermented cereal blends
The proximate composition, including carbohydrate, crude protein, moisture, crude fibre, ash, and fat contents, was analyzed using the oven-dried formulated fermented ogi flours as described by AOAC (2012). The total carbohydrate content was calculated by subtracting the sum of the percentage of moisture, crude protein, fat, crude fiber, and ash from 100%. For mineral analysis, five g of the ogi flours were heated in a muffle furnace until it turned into a white-greyish ash powder. The ash was left to cool on the laboratory bench at room temperature for approximately 3-5 h. This was followed by adding 20 ml of distilled water and 10 ml of diluted hydrochloric acid (HCl; AkzolNoble, Amsterdam) into the ash powder. The ogi flour samples were analyzed for calcium (Ca), manganese (Mn), iron (Fe), zinc (Zn), sodium (Na), and potassium (K) using an Atomic Absorption Spectrophotometer (AAS; model VGB 210 system, Buck Scientific Instruments, USA) (Banwo et al., 2022).
Determination of Total Antioxidant Activity (TAA) using the Trolox Equivalent Antioxidant Capacity (TEAC)
The TAA was determined using the TEAC method, with slight modifications, as described by AOAC (2012). An aliquot (0.3 ml) of ogi flour samples were homogenized with 2.7 ml of reagent solution (0.6 m H2SO4, 2.8 mm sodium phosphate, and 4 mm ammonium molybdate). The mixtures were capped and incubated in a boiling water bath at 95 oC for 90 min. After incubation, the ogi flour extracts were cooled to room temperature, and absorbance was measured at 695 nm. A blank solution containing 2.7 ml of the reagent solution and 6 ml of ethanol was incubated as a control. Stock solutions of α-tocopherol were prepared in methanol (range 0-10 µmol/ml) and treated identically to the samples. The TAA of the ogi flour extracts was expressed as equivalents of α-tocopherol using an extinction coefficient of 4×103 1/m on a Jenway 6,000 UV-visible spectrophotometer (Caliber Scientific Company, UK). The TAA was calculated as:
TAA (µg/g)=Absorbance of sample×Gradient factor×Dilution factor (103, 105, and 107) (Li et al., 2012).
Statistical analysis
Values are presented as means±Standard Error (SE) of triplicate determinations and interpreted using Analysis of Variance (ANOVA) with MINITAB statistical software (MinitabeÒ Release 14.13, Minitab Inc, USA). Significant differences between means were separated using the lease significant difference (LSD) at p<0.05.
Results and discussion
Physicochemical properties of clove-cinnamon formulated and un-formulated ogi
There was no significant difference (p<0.05) in the temperature among ogi samples made from millet, maize, and red sorghum; however, the temperature of fermented white sorghum ogi differed, although all fermented grains maintained a temperature within the room temperature range of 25.0±2.0 oC. Moreover, the pH of all formulated fermented ogi slurries was statistically significant at (p<0.05) and higher than that of the fermented control samples (without cloves and cinnamon). The pH of all the formulated fermented ogi slurries fell within the acidic pH range (4.0-5.7). In addition, TA increased as the pH decreased. The TA of formulated fermented cereals was also recorded to be statistically different from those of the control samples (without the addition of cloves and cinnamon). The lowest TA was observed in the formulated fermented ogi slurries (Table 1).
Table 1: Physicochemical properties of clove-cinnamon formulated and un-formulated ogi
| Sample codes | FFML | FML | FFMZ | FMZ | FFWS | FWS | FFRS | FRS |
| Temperature (0C) | 27.8±0.01 a | 27.5±0.01 a | 27.7±0.01 a | 27.5±0.00 a | 27.4±0.01 a | 21.4±0.01 b | 27.9±0.01 a | 27.8±0.00 a |
| pH | 4.12±0.01 a | 3.92±0.03 b | 4.46±0.01 a | 4.01±0.01 a | 5.81±0.04 | 4.3±0.03 a | 5.15±0.01c | 3.53±0.01 b |
| TA (%) | 0.02±0.01 a | 0.04±0.02 b | 0.01±0.01 | 0.03±0.00 d | 0.01±0.02 | 0.03±0.03 d | 0.01±0.01c | 0.03±0.01 d |
Values are Mean±Standard Error (SE). Mean values with the same letters in each row are not statistically different at 95% confidence level (p<0.05).
FFML=Formulated Fermented Millet; FML=Fermented Millet only; FFMZ=Formulated Fermented Maize; FMZ=Fermented Maize only; FFWS=Formulated Fermented White Sorghum; FWS=Fermented White Sorghum only; FFRS=Formulated Fermented Red Sorghum; FRS=Fermented Red Sorghum only; FFOF=Formulated Fermented Ogi Blends; TA=Titratable Acidity.
FFML=Formulated Fermented Millet; FML=Fermented Millet only; FFMZ=Formulated Fermented Maize; FMZ=Fermented Maize only; FFWS=Formulated Fermented White Sorghum; FWS=Fermented White Sorghum only; FFRS=Formulated Fermented Red Sorghum; FRS=Fermented Red Sorghum only; FFOF=Formulated Fermented Ogi Blends; TA=Titratable Acidity.
The acidic pH recorded in the fermented cereal blends in this study confers enhanced shelf-life and microbial stability as some of the organic acids produced during the fermentation process may have antimicrobial inhibitory properties against unwanted pathogenic microorganisms (Itaman and Nwachukwu, 2021). TA, a measure of the total acids produced by fermenting organisms, further contributes to these benefits. Similar pH and TA values were documented in studies by Banwo et al. (2022), Mohammed et al. (2017), and Ozabor et al. (2022) which analyzed fermented cereal grains enriched with Parkia biglobosa (African locust beans), fermented sorghum-cowpea flour blends, fermented Cyperus esculentus (tiger nuts) synergized cereal grains, and co-fermented Ipomoea batatas (sweet potato) with sprouted Glycine max (soybeans), respectively. In this study, it was recorded that the addition of cloves and cinnamon enhanced the acidification process, potentially due to the release of bioactive microbial compounds embedded in the spices. This observation is in line with the earlier study of Liu et al. (2023), who stated that integrating spices into food matrixes during fermentation improves microbial dynamics by enhancing the growth of beneficial microbes (mostly LAB and yeasts) over spoilage and/or pathogenic organisms, thus improving the physicochemical parameters.
Characterization and identification of LAB and yeasts isolated from spontaneously fermented clove-cinnamon formulated and un-formulated ogi
The genera of the isolated and characterized LAB and yeasts were: Lactococcus (30%) and Lactobacillus (70%) for LAB, and Candida (40%), Cryptococcus (45%), and Trichosporon (15%) for yeasts.
The isolation and characterization of Lactococcus, Lactobacillus, Candida, Cryptococcus, and Saccharomyces genera were documented in the studies by Adebo et al. (2022) and Fadahunsi et al. (2020) during cereal grains fermentation for the production of weaning and functional foods. Literatures have also documented that LAB enhances substrate acidification (Terefe et al., 2021) and produce flavor and volatile compounds in the fermenting medium such as diacetyl, lactic, formic, acetic, and formic acids (Omemu et al., 2007). Concurrently, yeasts have been reported to enhance saccharification of the fermenting medium due to their ability to tolerate high sugar concentrations (Turker et al., 2014), and producing inhibitory peptides such as hydrogen peroxide and enzymes such as laccase, amylase, cellulase, lipase, etc. (Fadahunsi and Olubodun, 2021).
Proximate and mineral compositions of clove-cinnamon formulated and un-formulated fermented ogi
Samples in group B (180:10:10) were analyzed for proximate composition (Table 2), minerals and TAA. This was done to determine the effect of the lowest ratio of cloves and cinnamon on the fermented cereal grains. Cloves and cinnamon are spices; hence they are only required in small quantities.
Characterization and identification of LAB and yeasts isolated from spontaneously fermented clove-cinnamon formulated and un-formulated ogi
The genera of the isolated and characterized LAB and yeasts were: Lactococcus (30%) and Lactobacillus (70%) for LAB, and Candida (40%), Cryptococcus (45%), and Trichosporon (15%) for yeasts.
The isolation and characterization of Lactococcus, Lactobacillus, Candida, Cryptococcus, and Saccharomyces genera were documented in the studies by Adebo et al. (2022) and Fadahunsi et al. (2020) during cereal grains fermentation for the production of weaning and functional foods. Literatures have also documented that LAB enhances substrate acidification (Terefe et al., 2021) and produce flavor and volatile compounds in the fermenting medium such as diacetyl, lactic, formic, acetic, and formic acids (Omemu et al., 2007). Concurrently, yeasts have been reported to enhance saccharification of the fermenting medium due to their ability to tolerate high sugar concentrations (Turker et al., 2014), and producing inhibitory peptides such as hydrogen peroxide and enzymes such as laccase, amylase, cellulase, lipase, etc. (Fadahunsi and Olubodun, 2021).
Proximate and mineral compositions of clove-cinnamon formulated and un-formulated fermented ogi
Samples in group B (180:10:10) were analyzed for proximate composition (Table 2), minerals and TAA. This was done to determine the effect of the lowest ratio of cloves and cinnamon on the fermented cereal grains. Cloves and cinnamon are spices; hence they are only required in small quantities.
Table 2: Proximate compositions of clove-cinnamon formulated and un-formulated fermented ogi
| Sample groups | Moisture content (%) | Ash content (%) | Crude protein (%) | Fat content (%) | Crude fiber (%) | CHO (%) |
| FFML | 10.420±0.00 d | 2.300±0.00 a | 7.560±0.00 c | 0.660±0.01 a | 3.600±0.00 a | 81.570±0.02 a |
| FML | 12.600±0.01 b | 1.650±0.05 b | 6.170±0.01 d | 0.550±0.01 b | 3.200±0.01 a | 75.830±0.00 c |
| FFMZ | 12.060±0.02 b | 1.300±0.00 c | 7.940±0.05 c | 0.300±0.00 d | 2.600±0.02 b | 75.800±0.00 c |
| FMZ | 10.090±0.00 d | 1.810±0.04 b | 4.060±0.01 e | 0.100±0.03 e | 3.110±0.00 a | 80.630±0.02 a |
| FFWS | 12.640±0.00 b | 1.840±0.01 b | 9.330±0.00 a | 0.500±0.02 b | 1.850±0.00 c | 73.840±0.01 d |
| FWS | 14.240±0.01 a | 2.620±0.00 a | 4.890±0.01 e | 0.600±0.00 a | 2.500±0.02 b | 75.150±0.00 c |
| FFRS | 11.140±0.02 c | 0.720±0.00 d | 8.160±0.02 b | 0.400±0.02 c | 2.040±0.00 b | 77.540±0.00 b |
| FRS | 12.220±0.01 b | 1.560±0.00 c | 4.010±0.01 e | 0.500±0.00 b | 2.750±0.02 b | 78.960±0.00 b |
Values are Mean±Standard Error (SE). Mean values with the same letters in each column are not statistically different at 95% confidence level (p<0.05).
FFML=Formulated Fermented Millet; FML=Fermented Millet only; FFMZ=Formulated Fermented Maize; FMZ=Fermented Maize only; FFWS=Formulated Fermented White Sorghum; FWS=Fermented White Sorghum only; FFRS=Formulated Fermented Red Sorghum; FRS=Fermented Red Sorghum only; FFOF=Formulated Fermented ogi blends
FFML=Formulated Fermented Millet; FML=Fermented Millet only; FFMZ=Formulated Fermented Maize; FMZ=Fermented Maize only; FFWS=Formulated Fermented White Sorghum; FWS=Fermented White Sorghum only; FFRS=Formulated Fermented Red Sorghum; FRS=Fermented Red Sorghum only; FFOF=Formulated Fermented ogi blends
Moisture contents ranging from 0.83 to 42.65% in fermented sorghum-cowpea flour blends were documented by Ojokoh et al. (2020). The study of Ogodo et al. (2017) also reported moisture contents of 10.82 to 14.2% in fermented maize flours. Moisture content is a key factor in determining microbial stability and shelf-life. In this study, the moisture content of formulated fermented samples and their controls varied. These variations may be attributed to factors such as, the nature of the substrates and the microbial metabolic activity of the fermenting microorganisms. Notably, lower moisture contents help to improve the shelf-life of the fermented product.
The % ash content of the formulated fermented ogi blends was significantly lower than that of the control samples, except for the formulated fermented millet. The presence of ash in food is an indicator of how absorbable the food is in the body system, as fermenting microorganisms break down macromolecules into simpler and more absorbable forms. Hence, processed food products are expected to have lower ash contents than the unprocessed ones (Banik et al., 2020; Ozabor et al., 2022). The ash content obtained in this study aligns with the Recommended ash Dietary Allowance (RDA) of ≤5.0 mg/100 g according to the study of Okafor et al. (2018). Moreover, the % ash contents ranging from 2.12 to 3.73% in fermented maize flours were previously documented by Terefe et al. (2021).
The formulated fermented white sorghum demonstrated highest % protein content (9.33%) while the fermented red sorghum demonstrated the least (4.01%). Ozabor et al. (2022) stated that fermenting LAB and yeasts produces proteolytic enzymes that hydrolyze protein into simpler forms during fermentation processes. Anaemene and Fadupin (2020) and Banik et al. (2020) also reported protein contents of 18.4 and 13.68% from fermented cereal flour blends, respectively. In addition, Banwo et al. (2022) documented similar % protein contents of 3.92 to 735% in fermented tiger nuts synergized cereal grains. The high protein content recorded in this study may be attributed to the addition of cloves and cinnamon, as Wei et al. (2022) previously reported that these spices can help to stimulate microbial activity during the fermentation process.
The decrease in fat content suggests that fermenting microbes are able to utilize oxidized lipids for energy generation and cell metabolism (Ogodo et al., 2017). Olawale and Ojokoh. (2019) and Terefe et al. (2021) also documented % fat contents of 2.0 to 22.0% and 2.12 to 2.82% in extruded-fermented sweet potato beni seed flours and fermented maize flours, respectively.
Crude fibre improves digestion and stimulates the gut microbial organisms (Minnaar et al., 2017). The observed decrease in fibre contents could be the result of fermenting microbes releasing extracellular enzymes into the fermenting medium for hydrolyzing and metabolizing insoluble polysaccharides (Adegunloye and Oparinde, 2017). The study of Ozabor et al. (2022) reported crude fibre of 1.75 to 2.32% and 2.70 to 3.60% in unfermented and fermented sweet potato-soybeans flour blends, which aligns with the findings of this study. The % carbohydrate in this study ranged from 73.84 to 81.57% in formulated fermented ogi blends and 75.15 to 80.63% for the control samples. Carbohydrates are sugars required only in calculated amounts. According to Terefe et al. (2021), a lower % carbohydrate content of 65.59 to 67.11% was recorded in fermented maize flours, consistent with the results of this study. The fermenting microbes activate hydrolytic enzymes, such as a-maltase and a-amylase, which help to breakdown the starch present in cereals into simple sugars (Nkhata et al., 2018).
The % ash content of the formulated fermented ogi blends was significantly lower than that of the control samples, except for the formulated fermented millet. The presence of ash in food is an indicator of how absorbable the food is in the body system, as fermenting microorganisms break down macromolecules into simpler and more absorbable forms. Hence, processed food products are expected to have lower ash contents than the unprocessed ones (Banik et al., 2020; Ozabor et al., 2022). The ash content obtained in this study aligns with the Recommended ash Dietary Allowance (RDA) of ≤5.0 mg/100 g according to the study of Okafor et al. (2018). Moreover, the % ash contents ranging from 2.12 to 3.73% in fermented maize flours were previously documented by Terefe et al. (2021).
The formulated fermented white sorghum demonstrated highest % protein content (9.33%) while the fermented red sorghum demonstrated the least (4.01%). Ozabor et al. (2022) stated that fermenting LAB and yeasts produces proteolytic enzymes that hydrolyze protein into simpler forms during fermentation processes. Anaemene and Fadupin (2020) and Banik et al. (2020) also reported protein contents of 18.4 and 13.68% from fermented cereal flour blends, respectively. In addition, Banwo et al. (2022) documented similar % protein contents of 3.92 to 735% in fermented tiger nuts synergized cereal grains. The high protein content recorded in this study may be attributed to the addition of cloves and cinnamon, as Wei et al. (2022) previously reported that these spices can help to stimulate microbial activity during the fermentation process.
The decrease in fat content suggests that fermenting microbes are able to utilize oxidized lipids for energy generation and cell metabolism (Ogodo et al., 2017). Olawale and Ojokoh. (2019) and Terefe et al. (2021) also documented % fat contents of 2.0 to 22.0% and 2.12 to 2.82% in extruded-fermented sweet potato beni seed flours and fermented maize flours, respectively.
Crude fibre improves digestion and stimulates the gut microbial organisms (Minnaar et al., 2017). The observed decrease in fibre contents could be the result of fermenting microbes releasing extracellular enzymes into the fermenting medium for hydrolyzing and metabolizing insoluble polysaccharides (Adegunloye and Oparinde, 2017). The study of Ozabor et al. (2022) reported crude fibre of 1.75 to 2.32% and 2.70 to 3.60% in unfermented and fermented sweet potato-soybeans flour blends, which aligns with the findings of this study. The % carbohydrate in this study ranged from 73.84 to 81.57% in formulated fermented ogi blends and 75.15 to 80.63% for the control samples. Carbohydrates are sugars required only in calculated amounts. According to Terefe et al. (2021), a lower % carbohydrate content of 65.59 to 67.11% was recorded in fermented maize flours, consistent with the results of this study. The fermenting microbes activate hydrolytic enzymes, such as a-maltase and a-amylase, which help to breakdown the starch present in cereals into simple sugars (Nkhata et al., 2018).

Figure 1: Mineral element compositions of fermented clove-cinnamon formulated and un-formulated ogi
FFML=Formulated Fermented Millet; FML=Fermented Millet only; FFMZ=Formulated Fermented Maize; FMZ=Fermented Maize only; FFWS=Formulated Fermented White Sorghum; FWS=Fermented White Sorghum only; FFRS=Formulated Fermented Red Sorghum; FRS=Fermented Red Sorghum only; FFOF=Formulated Fermented ogi blends. Values are mean±standard error (SE) at (p<0.05)
The mineral contents (Ca, Mn, Fe, Zn, Na, and K) of the formulated fermented ogi blends are documented in Figure 1. The Ca content (mg/g) recorded in this study ranged from 2,106 to 3,695 mg/g for the formulated fermented ogi blends while that of control samples ranged from 2,675 to 4,037 mg/g. Ca enhances the proper formation and development of bones most especially in infants and children, while manganese helps to form clotting factors, bones, and connective tissues (Olojede et al., 2020). The Mn content ranged from 1,881 to 2,840 mg/g in the formulated fermented ogi blends, while the controls ranged from 1,732 to 3,003. The formulated fermented ogi blends demonstrated Fe contents in the range of 2,000 to 2,724 mg/g and 1,832 to 3,112 mg/g for the control samples. Zn content for the formulated fermented ogi blends ranged from 49 to 210 mg/g, while the control samples ranged from 38 to 188 mg/g. The formulated fermented ogi blends demonstrated Na content in the range of 1,013 to 1,948 mg/g, while the control samples ranged from 12,150 to 2,059 mg/g. Lastly, the K content for the formulated fermented ogi blends ranged from 2,067 to 2,870 mg/g, whereas the control samples ranged from 2,203 to 2,752 mg/g. Zn and Na are necessary for maintaining proper bodily functions (Gabaza et al., 2017). According to Banwo et al. (2021), the Ca, Fe, Zn, Na, and k contents (mg/100 g) were reported as 3.80-7.31, 5.27-38.47, 0.36-16.84, 2.82-5.86, and 86.10-212.13, respectively, in both spontaneously and starter culture fermented malted and unmalted cereal grains. Olaniran and Abiose (2019b) also reported Na and Zn contents of 4.69-6.55 and 1.37-1.78 mg/100 g, respectively, in fermented maize ogi fortified with garlic and ginger. They also documented Na, Zn, Fe, and Mn contents in the range of 12.51-16.15, 7.01-8.50, 0.68-1.77, and 0.45-0.85 mg/100 g, respectively, in fermented sorghum ogi fortified with garlic and ginger (Olaniran and Abiose, 2019a). In addition, Marcel et al. (2022) reported Na, Fe, Ca, and Zn contents of 3.0-30.30, 0.60-16.40, 24.40-300.36, and 0.30-4.80 mg/100 g, respectively, in a fermented weaning porridge made of orange-fleshed sweet potato, amaranth grains, pumpkin seeds, and soybeans flour blends. However, Hejazi and Orsat (2016) reported that LAB and yeasts enhance the bioavailability of mineral compounds during fermentation.

Figure 2: Total Antioxidant Activity (TAA) of fermented clove-cinnamon formulated and un-formulated ogi
FFML=Formulated Fermented Millet; FML=Fermented Millet only; FFMZ=Formulated Fermented Maize; FMZ=Fermented Maize only; FFWS=Formulated Fermented White Sorghum; FWS=Fermented White Sorghum only; FFRS=Formulated Fermented Red Sorghum; FRS=Fermented Red Sorghum only; FFOF=Formulated Fermented ogi blends. Values are mean±Standard Error (SE) at (p<0.05)
Antioxidant activity
The TAAs of the formulated fermented ogi blends are documented in Figure 2. The results indicate that the formulated fermented ogi blends had significantly higher TAA than the controls samples. The highest and lowest TAA (µg/g) were recorded as 724.65 for formulated fermented white sorghum and 105.23 for fermented millet.
According to the reports by Hejazi and Orsat (2016), Okafor et al. (2018), and Olojede et al. (2020), fermentation enhances the synthesis of bound phenolic compounds and mineral elements imbedded in the fermenting substrates by activating endogenous enzymes produced by the metabolic activities of LAB and yeasts. Previous studies by Lee and Kang (2018) documented antioxidant activities of 13.10-70.48 µg/g, whereas Banwo et al. (2021) documented 2,2-Diphenyl-1-picrylhydrazyl (DPPH) antioxidant activity values of 98.2, 72.3, 92.5, and 60.3% for malted starter culture fermented millet, malted spontaneously fermented millet, malted starter culture fermented sorghum, and unmalted spontaneously fermented sorghum slurries. Thus, the antioxidant activity of fermented products is usually higher than those found in the raw samples.
Conclusion
Fermentation of maize, sorghum, and millet integrated with cloves and cinnamon enhanced the physicochemical, proximate, mineral elements, and TAA relative to un-formulated samples (without cloves and cinnamon). Thus, the formulated gruels may be recommended as high-quality fermented cereal-based foods with enhanced TAA, offering protective benefits against cellular oxidative damage. Future research may focus on animal studies (in-vivo) to investigate the effects of the formulated fermented cereal gruels on antioxidant activity in experimental rats.
Author contributions
T.O. and J.O. designed the study; T.O., M.M., M.J., I.A., G.O., and O.O. conducted the experimental work; T.O., M.M., M.J., I.A., and G.O. analyzed the data; T.O. wrote the manuscript; T.O., O.O., and J.O. supervised the research. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to the Department of Microbiology laboratory staff members for the technical assistance provided during this study
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
Not applicable.
Ethical consideration
Not applicable.
References
Adebo J.A., Njobeh P.B., Gbashi S., Oyedeji A.B., Ogundele O.M., Oyeyinka S.A., Adebo O.A. (2022). Fermentation of cereals and legumes: impact on nutritional constituents and nutrient bioavailability. Fermentation. 63: 1-57. [DOI: 10.3390/ fermentation8020063]
Adegunloye D.V., Oparinde T.C. (2017). Effects of fermentation on the proximate composition of Irish (Solanum tuberosum) and sweet potato (Ipomoea batatas) peels. Advances in Microbiology. 7: 565-574. [DOI: 10.4236/aim.2017.77044]
Adelekan A.O., Alamu O.E., Daramola B.E. (2021). Effect of enrichment with turmeric and ginger on some quality characteristics of fermented maize ogi. Croatian Journal of Food Science and Technology. 13: 210-220. [DOI: 10.17508/ CJFST.2021.13.2.11]
Anaemene D.I., Fadupin G.T. (2020). Effect of fermentation, germination, and combined germination-fermentation processing methods on the nutrient and anti-nutrient contents of quality protein maize (QPM) seeds. Journal of Applied Sciences and Environmental Management. 24: 1625-1630. [DOI: 10.4314/jasem.v24i9.21]
Association of Official Analytical Chemists (AOAC). (2012). Official methods of analysis, 22nd edition. URL: https://www.aoac.org/ official-methods-of-analysis/. Accessed 16 Novemebr 2023.
Banik A., Ghosh K., Pal S., Halder S.K., Ghosh C. Mondal K.C. (2020). Biofortification of multi-grain substrates by probiotic yeast. Food Biotechnology. 34: 283-305. [DOI: 10.1080/08905436.2020.1833913]
Banwo K., Asogwa F.C., Ogunremi O.R., Adesulu-Dahunsi A., Sanni A. (2021). Nutritional profile and antioxidant capacities of fermented millet and sorghum gruels using lactic acid bacteria and yeasts. Food Biotechnology. 35: 199-220. [DOI: 10.1080/ 08905436.2021.1940197]
Banwo K., Oyeyipo A., Mishra L., Sarkar D., Shetty K. (2022). Improving phenolic-linked functional qualities of traditional cereal-based fermented food (ogi) of Nigeria using compatible food synergies with underutilized edible plants. NFS Journal. 27: 1-12. [DOI: 10.1016/j.nfs.2022.03.001]
Fadahunsi I.F., Akoja A.D., Ozabor T.P. (2020). Characterization of indigenous yeast species isolated from fruits for pineapple wine production. Carpathian Journal of Food Science and Technology. 12: 109-121. [DOI: 10.34302/crpjfst/2020.12.5.8]
Fadahunsi I.F., Olubodun S. (2021). Antagonistic pattern of yeasts species against some selected food-borne pathogens. Bulletin of the National Research Centre. 45: 34. [DOI: 10.1186/s42269-020-00482-x]
Food and Agriculture Organization of the United Nations (FAO). (2017). FAOSTA database. URL: https://faostat.fao.org/site/567/ default.aspx#ancor/. Accessed 16th November, 2023.
Gabaza M., Joossens M., Cnockart M., Muchuweti M., Raes K., Vandamme P. (2019). Lactococci dominate the bacterial communities of fermented maize, sorghum and millet slurries in Zimbabwe. International Journal of Food Microbiology. 289: 77-87. [DOI: 10.1016/j.ijfoodmicro.2018.09.001]
Gabaza M., Muchuweti M., Vandamme P., Raes K. (2017) Can fermentation be used as a sustainable strategy to reduce iron and zinc binders in traditional African fermented cereal porridges or gruels? Food Reviews International. 33: 561–586. [DOI: 10.1080/87559129.2016.1196491]
Hassan Z.M., Sebola N.A., Mabelebele M. (2021). The nutritional uses of millet grain for food and feed: a review. Agriculture and Food Security. 10: 282-296. [DOI: 10.1186/s40066-020-00282-6]
Hejazi S.N., Orsat V. (2016). Malting process optimization for protein digestibility enhancement in finger millet grain. Journal of Food Science and Technology. 53: 1929-1938. [DOI: 10.1007/s13197-016-2188-x]
Houngbedji M., Johansen P., Podonou S.W., Akissoe N., Arneborg N., Nielsen D.S., Hounhouigan D.J., Jesperen L. (2018). Occurrence of lactic acid bacteria and yeasts at species and strains level during spontaneous fermentation of mawe, a cereal dough produced in West Africa. Food Microbiology. 76: 267-278. [DOI: 10.1016/j.fm.2018.06.005]
Hussain S., Mohammed A.A., Alamri M.S., Ibraheem M.A., Qasem A.A.A., El-Din M.F.S., Almaiman S.A.M. (2019). Wheat-millet flour cookies: physical, textural, sensory attributes and antioxidant potential. Food Science and Technology International. 26: 311-320. [DOI: 10.1177/1082013219894127]
Ijarotimi O.S., Oluwajuyitan T.D., Olugbuyi A.O., Makanjuola S.B. (2022). Comparative study on nutrient composition, functional property, and glycaemic index of “ogi” in healthy rats prepared from selected cereal grains. Journal of Future Foods. 2: 380-387. [DOI: 10.1016/j.jfutfo.2022.08.010]
Itaman V.O., Nwachukwu E. (2021). Bacteriological and nutritional assessment of fermented maize (ogi) fortified with ugba (Pentaclethra macrophylla). Nigerian Journal of Microbiology. 35: 5906-5917.
Khoddami A., Messina V., Venkata K.V., Farahnaky A., Blanchard C.L., Roberts T.H. (2023). Sorghum in foods: functionality and potentials in innovative products. Critical Reviews in Food Science and Nutrition. 63: 1170-1186. [DOI: 10.1080/10408398. 2021.1960793]
Koehler P., Wieser H. (2013). Chemistry of cereal grains. In: Gobetti M., Gaenzle M. (Editors) Handbook on sourdough biotechnology. Springer, New York. pp: 11-45. [DOI: 10.1007/978-1-4614-5425-0_2]
Lee N.Y., Kang C.S. (2018). Quality improvement and antioxidant activity of sugar-snap cookies prepared using blends of cereal flour. Preventive Nutrition and Food Science. 23: 160-165. [DOI: 10.3746/pnf.2018.23.2.160]
Li S., Zhao Y., Zhang L., Zhang X., Huang L., Li D., Niu C., Yang Z., Wang Q. (2012). Antioxidant activity of Lactobacillus Plantarum strains isolated from traditional Chinese fermented foods. Food Chemistry. 135: 1914-1919. [DOI: 10.1016/j.foodchem.2012. 06.048]
Liu Z., Cai S., Zhang S., Xiao Y., Devahastin S., Guo C., Wang Y., Wang T., Yi J. (2023). A systematic review on fermented chili pepper products: sensorial quality, health benefits, fermentation microbiomes, and metabolic pathways. Trends in Food Science and Technology. 141: 104189. [DOI: 10.1016/j.tifs.2023.104189]
Los A., Ziuzina D., Bourke P. (2018). Current and future technologies for microbiological decontamination of cereal grains. Journal of Food Science. 83: 1484-1493. [DOI: 10.1111/1750-3841.14181]
Marcel M.R., Chacha J.S., Ofoedu C.E. (2022). Nutritional evaluation of complementary porridge formulated from orange-fleshed sweet potato, amaranth grain, pumpkin seed, and soybean flours. Food Science and Nutrition. 10: 536-553. [DOI: 10.1002/fsn3.2675]
Minnaar P.P., Du Plessis H.W., Paulsen V., Ntushelo N., Jolly N.P., Du Toit M. (2017). Saccharomyces cerevisiae, non-saccharomyces yeasts and lactic acid bacteria in sequential fermentations: effect on phenolics and sensory attributes of South African Syrah wines. South African Journal of Enology and Viticulture. 38: 237-244. [DOI: 10.21548/38-2-1621]
Mohammed S.S.D., Orukotan A.A., Musa J. (2017). Effect of fermentation and malting on some cereal weaning foods enriched with African locust beans. Journal of Applied Sciences and Environmental Management. 21: 911-921. [DOI: 10.4314/jasem.v21i5.17]
Nkhata S.G., Ayua E., Kamau E.H., Shingiro J.B. (2018). Fermentation and germination improve the nutritional value of cereals and legumes through the activation of endogenous enzymes. Food Science and Nutrition. 6: 2446-2458. [DOI: 10.1002/fsn3.846]
Noah A.A., Alagamba E.A. (2020). Microbial, proximate, and sensory quality of pito beverage locally prepared and hawked in Ogun State, Nigeria. European Journal of Biotechnology and Biosciences. 8: 35-39.
Ogodo A.C., Ugbogu O.C., Onyeagba R.A., Okereke H.C. (2017). Effect of lactic acid bacteria consortium fermentation on the proximate composition and in-vitro starch/protein digestibility of maize (Zea mays) flour. American Journal of Microbiology and Biotechnology. 4: 35-43.
Ojokoh A.O., Alade R.A., Ozabor P.T., Fadahunsi I.F. (2020). Effect of fermentation on sorghum and cowpea flour blends. Journal of Agricultural Biotechnology and Sustainable Development. 12: 39-49. [DOI: 10.5897/JABSD2019.0365]
Okafor U.I., Omemu A.M., Obadina A.O., Bankole M.O., Adeyeye S.A.O. (2018). Nutritional composition and antinutritional properties of maize ogi co-fermented with pigeon pea. Food Science and Nutrition. 6: 424-439. [DOI: 10.1002/fsn3.571]
Olaniran A.F., Abiose S.H. (2019a). Nutritional evaluation of enhanced unsieved ogi paste with garlic and ginger. Preventive Nutrition and Food Science. 24: 348-356. [DOI: 10.3746/pnf.2019.24.3.348]
Olaniran A.F., Abiose S.H. (2019b). Proximate and antioxidant activities of biopreserved ogi flour with garlic and ginger. F1000 Research. 7: 1936. [DOI: 10.12688/f1000research.17059.2]
Olawale K.M., Ojokoh A.O. (2019). Effects of fermentation and extrusion on the proximate compositions and organoleptic properties of sweet potato (Ipomoea batatas) and beniseed (Sesamum indicum) blends. South Asian Journal of Research in Microbiology. 5: 1-12. [DOI: 10.9734/sajrm/2019/v5i430137]
Olojede A.O., Sanni A.I., Banwo K. (2020). Effect of legume addition on the physicochemical and sensorial attributes of sorghum-based sourdough bread. LWT-Food Science and Technology. 118: 108769. [DOI: 10.1016/j.lwt.2019.108769]
Omemu A.M., Oyewole O.B., Bankole M.O. (2007). Significance of yeasts in the fermentation of maize for ogi production. Food Microbiology. 24: 571-576. [DOI: 10.1016/j.fm.2007.01.006]
Onipede G.O., Odah B.C., Kolapo A.L., Ajayi A.A., Fawole A.O. (2021). Technological properties of lactic acid bacteria and yeasts isolated from ogi, a West African fermented cereal gruel. International Journal of Food Science and Nutrition. 6: 43-50.
Ozabor T., Damilola A., Iyabobola F., Abideen W., Anthony O. (2022). Effect of fermentation on the physicochemical and microbiological characteristics of sweet potato (Ipomoea batatas) and sprouted soybean (Glycine max) flour blends. Nigerian Food Journal. 40: 31-43. [DOI: 10.4313/nifoj.v40i2.3]
Paul A.K., Lim C.L., Apu M.A.I., Dolma K.G., Gupta M., De Lourdes Pereira M., Wilairatana P., Rahmatullah M., Wiart C., Nissapatorn V. (2023). Are fermented foods effective against inflammatory diseases?. International Journal of Environmental Research and Public Health. 20: 2481. [DOI: 10.3390/ijerph20032481]
Ramashia S.E., Gwata E.T., Meddows-Taylor S., Anyasi T.A., Jideani A.I.O. (2018). Some physical and functional properties of finger millet (Eleusine coracana) obtained in sub-Saharan Africa. Food Research International. 104: 110-118. [DOI: 10.1016./j. foodres.2017.09.065]
Shah A., Masoodi F.A., Gani A., Ashwar B.A. (2016). Newly released oat varieties of Himalayan region-techno-functional, rheological, and nutraceutical properties of flour. LWT-Food Science and Technology. 70: 111-118. [DOI: 10.1016/j.lwt.2016.02.033]
Terefe Z.K., Omwamba M.N., Nduko J.M. (2021). Effect of solid-state fermentation on proximate composition, antinutritional factors and invitro protein digestibility of maize flour. Food Science and Nutrition. 9: 6343-6352. [DOI: 10.1002/fsn3.2599]
Turker M. (2014). Yeast biotechnology: diversity and applications. In Proceedings of 27th VH Yeast Conference. 1-26.
Wei G., Zhao Q., Wang D., Fan Y., Shi Y., Huang A. (2022). Novel ACE inhibitory, antioxidant and alpha-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking. LWT-Food Science and Technology. 159: 113196. [DOI: 10.1016/j.lwt.2022.113196]
Yang Q., Yao H., Liu S., Mao J. (2022). Interaction and application of molds and yeasts in Chinese fermented foods. Frontiers in Microbiology. 12: 1-12. [DOI: 10.3389/fmicb.2021.664850]
Yepez A., Russo P., Spano G., Khomenko I., Biasioli F., Capozzi V., Aznar R. (2019). In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiology. 77: 61-68. [DOI: 10.1016/j.fm.2018.08.008]
Zhu Y., Chu J., Lu Z., Lv F., Bie X., Zhang C., Zhao H. (2018). Physicochemical and functional properties of dietary fiber from foxtail millets (Setaria italic) bran. Journal of Cereal Science. 79: 456-461. [DOI: 10.1016/j.jcs.2017.12.011]
The TAAs of the formulated fermented ogi blends are documented in Figure 2. The results indicate that the formulated fermented ogi blends had significantly higher TAA than the controls samples. The highest and lowest TAA (µg/g) were recorded as 724.65 for formulated fermented white sorghum and 105.23 for fermented millet.
According to the reports by Hejazi and Orsat (2016), Okafor et al. (2018), and Olojede et al. (2020), fermentation enhances the synthesis of bound phenolic compounds and mineral elements imbedded in the fermenting substrates by activating endogenous enzymes produced by the metabolic activities of LAB and yeasts. Previous studies by Lee and Kang (2018) documented antioxidant activities of 13.10-70.48 µg/g, whereas Banwo et al. (2021) documented 2,2-Diphenyl-1-picrylhydrazyl (DPPH) antioxidant activity values of 98.2, 72.3, 92.5, and 60.3% for malted starter culture fermented millet, malted spontaneously fermented millet, malted starter culture fermented sorghum, and unmalted spontaneously fermented sorghum slurries. Thus, the antioxidant activity of fermented products is usually higher than those found in the raw samples.
Conclusion
Fermentation of maize, sorghum, and millet integrated with cloves and cinnamon enhanced the physicochemical, proximate, mineral elements, and TAA relative to un-formulated samples (without cloves and cinnamon). Thus, the formulated gruels may be recommended as high-quality fermented cereal-based foods with enhanced TAA, offering protective benefits against cellular oxidative damage. Future research may focus on animal studies (in-vivo) to investigate the effects of the formulated fermented cereal gruels on antioxidant activity in experimental rats.
Author contributions
T.O. and J.O. designed the study; T.O., M.M., M.J., I.A., G.O., and O.O. conducted the experimental work; T.O., M.M., M.J., I.A., and G.O. analyzed the data; T.O. wrote the manuscript; T.O., O.O., and J.O. supervised the research. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to the Department of Microbiology laboratory staff members for the technical assistance provided during this study
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
Not applicable.
Ethical consideration
Not applicable.
References
Adebo J.A., Njobeh P.B., Gbashi S., Oyedeji A.B., Ogundele O.M., Oyeyinka S.A., Adebo O.A. (2022). Fermentation of cereals and legumes: impact on nutritional constituents and nutrient bioavailability. Fermentation. 63: 1-57. [DOI: 10.3390/ fermentation8020063]
Adegunloye D.V., Oparinde T.C. (2017). Effects of fermentation on the proximate composition of Irish (Solanum tuberosum) and sweet potato (Ipomoea batatas) peels. Advances in Microbiology. 7: 565-574. [DOI: 10.4236/aim.2017.77044]
Adelekan A.O., Alamu O.E., Daramola B.E. (2021). Effect of enrichment with turmeric and ginger on some quality characteristics of fermented maize ogi. Croatian Journal of Food Science and Technology. 13: 210-220. [DOI: 10.17508/ CJFST.2021.13.2.11]
Anaemene D.I., Fadupin G.T. (2020). Effect of fermentation, germination, and combined germination-fermentation processing methods on the nutrient and anti-nutrient contents of quality protein maize (QPM) seeds. Journal of Applied Sciences and Environmental Management. 24: 1625-1630. [DOI: 10.4314/jasem.v24i9.21]
Association of Official Analytical Chemists (AOAC). (2012). Official methods of analysis, 22nd edition. URL: https://www.aoac.org/ official-methods-of-analysis/. Accessed 16 Novemebr 2023.
Banik A., Ghosh K., Pal S., Halder S.K., Ghosh C. Mondal K.C. (2020). Biofortification of multi-grain substrates by probiotic yeast. Food Biotechnology. 34: 283-305. [DOI: 10.1080/08905436.2020.1833913]
Banwo K., Asogwa F.C., Ogunremi O.R., Adesulu-Dahunsi A., Sanni A. (2021). Nutritional profile and antioxidant capacities of fermented millet and sorghum gruels using lactic acid bacteria and yeasts. Food Biotechnology. 35: 199-220. [DOI: 10.1080/ 08905436.2021.1940197]
Banwo K., Oyeyipo A., Mishra L., Sarkar D., Shetty K. (2022). Improving phenolic-linked functional qualities of traditional cereal-based fermented food (ogi) of Nigeria using compatible food synergies with underutilized edible plants. NFS Journal. 27: 1-12. [DOI: 10.1016/j.nfs.2022.03.001]
Fadahunsi I.F., Akoja A.D., Ozabor T.P. (2020). Characterization of indigenous yeast species isolated from fruits for pineapple wine production. Carpathian Journal of Food Science and Technology. 12: 109-121. [DOI: 10.34302/crpjfst/2020.12.5.8]
Fadahunsi I.F., Olubodun S. (2021). Antagonistic pattern of yeasts species against some selected food-borne pathogens. Bulletin of the National Research Centre. 45: 34. [DOI: 10.1186/s42269-020-00482-x]
Food and Agriculture Organization of the United Nations (FAO). (2017). FAOSTA database. URL: https://faostat.fao.org/site/567/ default.aspx#ancor/. Accessed 16th November, 2023.
Gabaza M., Joossens M., Cnockart M., Muchuweti M., Raes K., Vandamme P. (2019). Lactococci dominate the bacterial communities of fermented maize, sorghum and millet slurries in Zimbabwe. International Journal of Food Microbiology. 289: 77-87. [DOI: 10.1016/j.ijfoodmicro.2018.09.001]
Gabaza M., Muchuweti M., Vandamme P., Raes K. (2017) Can fermentation be used as a sustainable strategy to reduce iron and zinc binders in traditional African fermented cereal porridges or gruels? Food Reviews International. 33: 561–586. [DOI: 10.1080/87559129.2016.1196491]
Hassan Z.M., Sebola N.A., Mabelebele M. (2021). The nutritional uses of millet grain for food and feed: a review. Agriculture and Food Security. 10: 282-296. [DOI: 10.1186/s40066-020-00282-6]
Hejazi S.N., Orsat V. (2016). Malting process optimization for protein digestibility enhancement in finger millet grain. Journal of Food Science and Technology. 53: 1929-1938. [DOI: 10.1007/s13197-016-2188-x]
Houngbedji M., Johansen P., Podonou S.W., Akissoe N., Arneborg N., Nielsen D.S., Hounhouigan D.J., Jesperen L. (2018). Occurrence of lactic acid bacteria and yeasts at species and strains level during spontaneous fermentation of mawe, a cereal dough produced in West Africa. Food Microbiology. 76: 267-278. [DOI: 10.1016/j.fm.2018.06.005]
Hussain S., Mohammed A.A., Alamri M.S., Ibraheem M.A., Qasem A.A.A., El-Din M.F.S., Almaiman S.A.M. (2019). Wheat-millet flour cookies: physical, textural, sensory attributes and antioxidant potential. Food Science and Technology International. 26: 311-320. [DOI: 10.1177/1082013219894127]
Ijarotimi O.S., Oluwajuyitan T.D., Olugbuyi A.O., Makanjuola S.B. (2022). Comparative study on nutrient composition, functional property, and glycaemic index of “ogi” in healthy rats prepared from selected cereal grains. Journal of Future Foods. 2: 380-387. [DOI: 10.1016/j.jfutfo.2022.08.010]
Itaman V.O., Nwachukwu E. (2021). Bacteriological and nutritional assessment of fermented maize (ogi) fortified with ugba (Pentaclethra macrophylla). Nigerian Journal of Microbiology. 35: 5906-5917.
Khoddami A., Messina V., Venkata K.V., Farahnaky A., Blanchard C.L., Roberts T.H. (2023). Sorghum in foods: functionality and potentials in innovative products. Critical Reviews in Food Science and Nutrition. 63: 1170-1186. [DOI: 10.1080/10408398. 2021.1960793]
Koehler P., Wieser H. (2013). Chemistry of cereal grains. In: Gobetti M., Gaenzle M. (Editors) Handbook on sourdough biotechnology. Springer, New York. pp: 11-45. [DOI: 10.1007/978-1-4614-5425-0_2]
Lee N.Y., Kang C.S. (2018). Quality improvement and antioxidant activity of sugar-snap cookies prepared using blends of cereal flour. Preventive Nutrition and Food Science. 23: 160-165. [DOI: 10.3746/pnf.2018.23.2.160]
Li S., Zhao Y., Zhang L., Zhang X., Huang L., Li D., Niu C., Yang Z., Wang Q. (2012). Antioxidant activity of Lactobacillus Plantarum strains isolated from traditional Chinese fermented foods. Food Chemistry. 135: 1914-1919. [DOI: 10.1016/j.foodchem.2012. 06.048]
Liu Z., Cai S., Zhang S., Xiao Y., Devahastin S., Guo C., Wang Y., Wang T., Yi J. (2023). A systematic review on fermented chili pepper products: sensorial quality, health benefits, fermentation microbiomes, and metabolic pathways. Trends in Food Science and Technology. 141: 104189. [DOI: 10.1016/j.tifs.2023.104189]
Los A., Ziuzina D., Bourke P. (2018). Current and future technologies for microbiological decontamination of cereal grains. Journal of Food Science. 83: 1484-1493. [DOI: 10.1111/1750-3841.14181]
Marcel M.R., Chacha J.S., Ofoedu C.E. (2022). Nutritional evaluation of complementary porridge formulated from orange-fleshed sweet potato, amaranth grain, pumpkin seed, and soybean flours. Food Science and Nutrition. 10: 536-553. [DOI: 10.1002/fsn3.2675]
Minnaar P.P., Du Plessis H.W., Paulsen V., Ntushelo N., Jolly N.P., Du Toit M. (2017). Saccharomyces cerevisiae, non-saccharomyces yeasts and lactic acid bacteria in sequential fermentations: effect on phenolics and sensory attributes of South African Syrah wines. South African Journal of Enology and Viticulture. 38: 237-244. [DOI: 10.21548/38-2-1621]
Mohammed S.S.D., Orukotan A.A., Musa J. (2017). Effect of fermentation and malting on some cereal weaning foods enriched with African locust beans. Journal of Applied Sciences and Environmental Management. 21: 911-921. [DOI: 10.4314/jasem.v21i5.17]
Nkhata S.G., Ayua E., Kamau E.H., Shingiro J.B. (2018). Fermentation and germination improve the nutritional value of cereals and legumes through the activation of endogenous enzymes. Food Science and Nutrition. 6: 2446-2458. [DOI: 10.1002/fsn3.846]
Noah A.A., Alagamba E.A. (2020). Microbial, proximate, and sensory quality of pito beverage locally prepared and hawked in Ogun State, Nigeria. European Journal of Biotechnology and Biosciences. 8: 35-39.
Ogodo A.C., Ugbogu O.C., Onyeagba R.A., Okereke H.C. (2017). Effect of lactic acid bacteria consortium fermentation on the proximate composition and in-vitro starch/protein digestibility of maize (Zea mays) flour. American Journal of Microbiology and Biotechnology. 4: 35-43.
Ojokoh A.O., Alade R.A., Ozabor P.T., Fadahunsi I.F. (2020). Effect of fermentation on sorghum and cowpea flour blends. Journal of Agricultural Biotechnology and Sustainable Development. 12: 39-49. [DOI: 10.5897/JABSD2019.0365]
Okafor U.I., Omemu A.M., Obadina A.O., Bankole M.O., Adeyeye S.A.O. (2018). Nutritional composition and antinutritional properties of maize ogi co-fermented with pigeon pea. Food Science and Nutrition. 6: 424-439. [DOI: 10.1002/fsn3.571]
Olaniran A.F., Abiose S.H. (2019a). Nutritional evaluation of enhanced unsieved ogi paste with garlic and ginger. Preventive Nutrition and Food Science. 24: 348-356. [DOI: 10.3746/pnf.2019.24.3.348]
Olaniran A.F., Abiose S.H. (2019b). Proximate and antioxidant activities of biopreserved ogi flour with garlic and ginger. F1000 Research. 7: 1936. [DOI: 10.12688/f1000research.17059.2]
Olawale K.M., Ojokoh A.O. (2019). Effects of fermentation and extrusion on the proximate compositions and organoleptic properties of sweet potato (Ipomoea batatas) and beniseed (Sesamum indicum) blends. South Asian Journal of Research in Microbiology. 5: 1-12. [DOI: 10.9734/sajrm/2019/v5i430137]
Olojede A.O., Sanni A.I., Banwo K. (2020). Effect of legume addition on the physicochemical and sensorial attributes of sorghum-based sourdough bread. LWT-Food Science and Technology. 118: 108769. [DOI: 10.1016/j.lwt.2019.108769]
Omemu A.M., Oyewole O.B., Bankole M.O. (2007). Significance of yeasts in the fermentation of maize for ogi production. Food Microbiology. 24: 571-576. [DOI: 10.1016/j.fm.2007.01.006]
Onipede G.O., Odah B.C., Kolapo A.L., Ajayi A.A., Fawole A.O. (2021). Technological properties of lactic acid bacteria and yeasts isolated from ogi, a West African fermented cereal gruel. International Journal of Food Science and Nutrition. 6: 43-50.
Ozabor T., Damilola A., Iyabobola F., Abideen W., Anthony O. (2022). Effect of fermentation on the physicochemical and microbiological characteristics of sweet potato (Ipomoea batatas) and sprouted soybean (Glycine max) flour blends. Nigerian Food Journal. 40: 31-43. [DOI: 10.4313/nifoj.v40i2.3]
Paul A.K., Lim C.L., Apu M.A.I., Dolma K.G., Gupta M., De Lourdes Pereira M., Wilairatana P., Rahmatullah M., Wiart C., Nissapatorn V. (2023). Are fermented foods effective against inflammatory diseases?. International Journal of Environmental Research and Public Health. 20: 2481. [DOI: 10.3390/ijerph20032481]
Ramashia S.E., Gwata E.T., Meddows-Taylor S., Anyasi T.A., Jideani A.I.O. (2018). Some physical and functional properties of finger millet (Eleusine coracana) obtained in sub-Saharan Africa. Food Research International. 104: 110-118. [DOI: 10.1016./j. foodres.2017.09.065]
Shah A., Masoodi F.A., Gani A., Ashwar B.A. (2016). Newly released oat varieties of Himalayan region-techno-functional, rheological, and nutraceutical properties of flour. LWT-Food Science and Technology. 70: 111-118. [DOI: 10.1016/j.lwt.2016.02.033]
Terefe Z.K., Omwamba M.N., Nduko J.M. (2021). Effect of solid-state fermentation on proximate composition, antinutritional factors and invitro protein digestibility of maize flour. Food Science and Nutrition. 9: 6343-6352. [DOI: 10.1002/fsn3.2599]
Turker M. (2014). Yeast biotechnology: diversity and applications. In Proceedings of 27th VH Yeast Conference. 1-26.
Wei G., Zhao Q., Wang D., Fan Y., Shi Y., Huang A. (2022). Novel ACE inhibitory, antioxidant and alpha-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking. LWT-Food Science and Technology. 159: 113196. [DOI: 10.1016/j.lwt.2022.113196]
Yang Q., Yao H., Liu S., Mao J. (2022). Interaction and application of molds and yeasts in Chinese fermented foods. Frontiers in Microbiology. 12: 1-12. [DOI: 10.3389/fmicb.2021.664850]
Yepez A., Russo P., Spano G., Khomenko I., Biasioli F., Capozzi V., Aznar R. (2019). In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiology. 77: 61-68. [DOI: 10.1016/j.fm.2018.08.008]
Zhu Y., Chu J., Lu Z., Lv F., Bie X., Zhang C., Zhao H. (2018). Physicochemical and functional properties of dietary fiber from foxtail millets (Setaria italic) bran. Journal of Cereal Science. 79: 456-461. [DOI: 10.1016/j.jcs.2017.12.011]
[*] Corresponding author (T. Ozabor)
* E-mail: praise.ozabor@uniosun.edu.ng
ORCID ID: https://orcid.org/0000-0003-0047-8242
* E-mail: praise.ozabor@uniosun.edu.ng
ORCID ID: https://orcid.org/0000-0003-0047-8242
Type of Study: Original article |
Subject:
Special
Received: 24/10/19 | Accepted: 25/05/03 | Published: 25/06/22
Received: 24/10/19 | Accepted: 25/05/03 | Published: 25/06/22
References
1. Adebo J.A., Njobeh P.B., Gbashi S., Oyedeji A.B., Ogundele O.M., Oyeyinka S.A., Adebo O.A. (2022). Fermentation of cereals and legumes: impact on nutritional constituents and nutrient bioavailability. Fermentation. 63: 1-57. [DOI: 10.3390/ fermentation8020063] [DOI:10.3390/fermentation8020063]
2. Adegunloye D.V., Oparinde T.C. (2017). Effects of fermentation on the proximate composition of Irish (Solanum tuberosum) and sweet potato (Ipomoea batatas) peels. Advances in Microbiology. 7: 565-574. [DOI: 10.4236/aim.2017.77044] [DOI:10.4236/aim.2017.77044]
3. Adelekan A.O., Alamu O.E., Daramola B.E. (2021). Effect of enrichment with turmeric and ginger on some quality characteristics of fermented maize ogi. Croatian Journal of Food Science and Technology. 13: 210-220. [DOI: 10.17508/ CJFST.2021.13.2.11] [DOI:10.17508/CJFST.2021.13.2.11]
4. Anaemene D.I., Fadupin G.T. (2020). Effect of fermentation, germination, and combined germination-fermentation processing methods on the nutrient and anti-nutrient contents of quality protein maize (QPM) seeds. Journal of Applied Sciences and Environmental Management. 24: 1625-1630. [DOI: 10.4314/jasem.v24i9.21] [DOI:10.4314/jasem.v24i9.21]
5. Association of Official Analytical Chemists (AOAC). (2012). Official methods of analysis, 22nd edition. URL: https://www.aoac.org/ official-methods-of-analysis/. Accessed 16 Novemebr 2023.
6. Banik A., Ghosh K., Pal S., Halder S.K., Ghosh C. Mondal K.C. (2020). Biofortification of multi-grain substrates by probiotic yeast. Food Biotechnology. 34: 283-305. [DOI: 10.1080/08905436.2020.1833913] [DOI:10.1080/08905436.2020.1833913]
7. Banwo K., Asogwa F.C., Ogunremi O.R., Adesulu-Dahunsi A., Sanni A. (2021). Nutritional profile and antioxidant capacities of fermented millet and sorghum gruels using lactic acid bacteria and yeasts. Food Biotechnology. 35: 199-220. [DOI: 10.1080/ 08905436.2021.1940197] [DOI:10.1080/08905436.2021.1940197]
8. Banwo K., Oyeyipo A., Mishra L., Sarkar D., Shetty K. (2022). Improving phenolic-linked functional qualities of traditional cereal-based fermented food (ogi) of Nigeria using compatible food synergies with underutilized edible plants. NFS Journal. 27: 1-12. [DOI: 10.1016/j.nfs.2022.03.001] [DOI:10.1016/j.nfs.2022.03.001]
9. Fadahunsi I.F., Akoja A.D., Ozabor T.P. (2020). Characterization of indigenous yeast species isolated from fruits for pineapple wine production. Carpathian Journal of Food Science and Technology. 12: 109-121. [DOI: 10.34302/crpjfst/2020.12.5.8] [DOI:10.34302/crpjfst/2020.12.5.8]
10. Fadahunsi I.F., Olubodun S. (2021). Antagonistic pattern of yeasts species against some selected food-borne pathogens. Bulletin of the National Research Centre. 45: 34. [DOI: 10.1186/s42269-020-00482-x] [DOI:10.1186/s42269-020-00482-x]
11. Food and Agriculture Organization of the United Nations (FAO). (2017). FAOSTA database. URL: https://faostat.fao.org/site/567/ default.aspx#ancor/. Accessed 16th November, 2023.
12. Gabaza M., Joossens M., Cnockart M., Muchuweti M., Raes K., Vandamme P. (2019). Lactococci dominate the bacterial communities of fermented maize, sorghum and millet slurries in Zimbabwe. International Journal of Food Microbiology. 289: 77-87. [DOI: 10.1016/j.ijfoodmicro.2018.09.001] [DOI:10.1016/j.ijfoodmicro.2018.09.001] [PMID]
13. Gabaza M., Muchuweti M., Vandamme P., Raes K. (2017) Can fermentation be used as a sustainable strategy to reduce iron and zinc binders in traditional African fermented cereal porridges or gruels? Food Reviews International. 33: 561-586. [DOI: 10.1080/87559129.2016.1196491] [DOI:10.1080/87559129.2016.1196491]
14. Hassan Z.M., Sebola N.A., Mabelebele M. (2021). The nutritional uses of millet grain for food and feed: a review. Agriculture and Food Security. 10: 282-296. [DOI: 10.1186/s40066-020-00282-6] [DOI:10.1186/s40066-020-00282-6] [PMID] [PMCID]
15. Hejazi S.N., Orsat V. (2016). Malting process optimization for protein digestibility enhancement in finger millet grain. Journal of Food Science and Technology. 53: 1929-1938. [DOI: 10.1007/s13197-016-2188-x] [DOI:10.1007/s13197-016-2188-x] [PMID] [PMCID]
16. Houngbedji M., Johansen P., Podonou S.W., Akissoe N., Arneborg N., Nielsen D.S., Hounhouigan D.J., Jesperen L. (2018). Occurrence of lactic acid bacteria and yeasts at species and strains level during spontaneous fermentation of mawe, a cereal dough produced in West Africa. Food Microbiology. 76: 267-278. [DOI: 10.1016/j.fm.2018.06.005] [DOI:10.1016/j.fm.2018.06.005] [PMID]
17. Hussain S., Mohammed A.A., Alamri M.S., Ibraheem M.A., Qasem A.A.A., El-Din M.F.S., Almaiman S.A.M. (2019). Wheat-millet flour cookies: physical, textural, sensory attributes and antioxidant potential. Food Science and Technology International. 26: 311-320. [DOI: 10.1177/1082013219894127] [DOI:10.1177/1082013219894127] [PMID]
18. Ijarotimi O.S., Oluwajuyitan T.D., Olugbuyi A.O., Makanjuola S.B. (2022). Comparative study on nutrient composition, functional property, and glycaemic index of "ogi" in healthy rats prepared from selected cereal grains. Journal of Future Foods. 2: 380-387. [DOI: 10.1016/j.jfutfo.2022.08.010] [DOI:10.1016/j.jfutfo.2022.08.010]
19. Itaman V.O., Nwachukwu E. (2021). Bacteriological and nutritional assessment of fermented maize (ogi) fortified with ugba (Pentaclethra macrophylla). Nigerian Journal of Microbiology. 35: 5906-5917.
20. Khoddami A., Messina V., Venkata K.V., Farahnaky A., Blanchard C.L., Roberts T.H. (2023). Sorghum in foods: functionality and potentials in innovative products. Critical Reviews in Food Science and Nutrition. 63: 1170-1186. [DOI: 10.1080/10408398. 2021.1960793] [DOI:10.1080/10408398.2021.1960793] [PMID]
21. Koehler P., Wieser H. (2013). Chemistry of cereal grains. In: Gobetti M., Gaenzle M. (Editors) Handbook on sourdough biotechnology. Springer, New York. pp: 11-45. [DOI: 10.1007/978-1-4614-5425-0_2] [DOI:10.1007/978-1-4614-5425-0_2]
22. Lee N.Y., Kang C.S. (2018). Quality improvement and antioxidant activity of sugar-snap cookies prepared using blends of cereal flour. Preventive Nutrition and Food Science. 23: 160-165. [DOI: 10.3746/pnf.2018.23.2.160] [DOI:10.3746/pnf.2018.23.2.160] [PMID] [PMCID]
23. Li S., Zhao Y., Zhang L., Zhang X., Huang L., Li D., Niu C., Yang Z., Wang Q. (2012). Antioxidant activity of Lactobacillus Plantarum strains isolated from traditional Chinese fermented foods. Food Chemistry. 135: 1914-1919. [DOI: 10.1016/j.foodchem.2012. 06.048] [DOI:10.1016/j.foodchem.2012.06.048] [PMID]
24. Liu Z., Cai S., Zhang S., Xiao Y., Devahastin S., Guo C., Wang Y., Wang T., Yi J. (2023). A systematic review on fermented chili pepper products: sensorial quality, health benefits, fermentation microbiomes, and metabolic pathways. Trends in Food Science and Technology. 141: 104189. [DOI: 10.1016/j.tifs.2023.104189] [DOI:10.1016/j.tifs.2023.104189]
25. Los A., Ziuzina D., Bourke P. (2018). Current and future technologies for microbiological decontamination of cereal grains. Journal of Food Science. 83: 1484-1493. [DOI: 10.1111/1750-3841.14181] [DOI:10.1111/1750-3841.14181] [PMID]
26. Marcel M.R., Chacha J.S., Ofoedu C.E. (2022). Nutritional evaluation of complementary porridge formulated from orange-fleshed sweet potato, amaranth grain, pumpkin seed, and soybean flours. Food Science and Nutrition. 10: 536-553. [DOI: 10.1002/fsn3.2675] [DOI:10.1002/fsn3.2675] [PMID] [PMCID]
27. Minnaar P.P., Du Plessis H.W., Paulsen V., Ntushelo N., Jolly N.P., Du Toit M. (2017). Saccharomyces cerevisiae, non-saccharomyces yeasts and lactic acid bacteria in sequential fermentations: effect on phenolics and sensory attributes of South African Syrah wines. South African Journal of Enology and Viticulture. 38: 237-244. [DOI: 10.21548/38-2-1621] [DOI:10.21548/38-2-1621]
28. Mohammed S.S.D., Orukotan A.A., Musa J. (2017). Effect of fermentation and malting on some cereal weaning foods enriched with African locust beans. Journal of Applied Sciences and Environmental Management. 21: 911-921. [DOI: 10.4314/jasem.v21i5.17] [DOI:10.4314/jasem.v21i5.17]
29. Nkhata S.G., Ayua E., Kamau E.H., Shingiro J.B. (2018). Fermentation and germination improve the nutritional value of cereals and legumes through the activation of endogenous enzymes. Food Science and Nutrition. 6: 2446-2458. [DOI: 10.1002/fsn3.846] [DOI:10.1002/fsn3.846] [PMID] [PMCID]
30. Noah A.A., Alagamba E.A. (2020). Microbial, proximate, and sensory quality of pito beverage locally prepared and hawked in Ogun State, Nigeria. European Journal of Biotechnology and Biosciences. 8: 35-39.
31. Ogodo A.C., Ugbogu O.C., Onyeagba R.A., Okereke H.C. (2017). Effect of lactic acid bacteria consortium fermentation on the proximate composition and in-vitro starch/protein digestibility of maize (Zea mays) flour. American Journal of Microbiology and Biotechnology. 4: 35-43.
32. Ojokoh A.O., Alade R.A., Ozabor P.T., Fadahunsi I.F. (2020). Effect of fermentation on sorghum and cowpea flour blends. Journal of Agricultural Biotechnology and Sustainable Development. 12: 39-49. [DOI: 10.5897/JABSD2019.0365]
33. Okafor U.I., Omemu A.M., Obadina A.O., Bankole M.O., Adeyeye S.A.O. (2018). Nutritional composition and antinutritional properties of maize ogi co-fermented with pigeon pea. Food Science and Nutrition. 6: 424-439. [DOI: 10.1002/fsn3.571] [DOI:10.1002/fsn3.571] [PMID] [PMCID]
34. Olaniran A.F., Abiose S.H. (2019a). Nutritional evaluation of enhanced unsieved ogi paste with garlic and ginger. Preventive Nutrition and Food Science. 24: 348-356. [DOI: 10.3746/pnf.2019.24.3.348] [DOI:10.3746/pnf.2019.24.3.348] [PMID] [PMCID]
35. Olaniran A.F., Abiose S.H. (2019b). Proximate and antioxidant activities of biopreserved ogi flour with garlic and ginger. F1000 Research. 7: 1936. [DOI: 10.12688/f1000research.17059.2] [DOI:10.12688/f1000research.17059.2] [PMID] [PMCID]
36. Olawale K.M., Ojokoh A.O. (2019). Effects of fermentation and extrusion on the proximate compositions and organoleptic properties of sweet potato (Ipomoea batatas) and beniseed (Sesamum indicum) blends. South Asian Journal of Research in Microbiology. 5: 1-12. [DOI: 10.9734/sajrm/2019/v5i430137] [DOI:10.9734/sajrm/2019/v5i430137]
37. Olojede A.O., Sanni A.I., Banwo K. (2020). Effect of legume addition on the physicochemical and sensorial attributes of sorghum-based sourdough bread. LWT-Food Science and Technology. 118: 108769. [DOI: 10.1016/j.lwt.2019.108769] [DOI:10.1016/j.lwt.2019.108769]
38. Omemu A.M., Oyewole O.B., Bankole M.O. (2007). Significance of yeasts in the fermentation of maize for ogi production. Food Microbiology. 24: 571-576. [DOI: 10.1016/j.fm.2007.01.006] [DOI:10.1016/j.fm.2007.01.006] [PMID]
39. Onipede G.O., Odah B.C., Kolapo A.L., Ajayi A.A., Fawole A.O. (2021). Technological properties of lactic acid bacteria and yeasts isolated from ogi, a West African fermented cereal gruel. International Journal of Food Science and Nutrition. 6: 43-50.
40. Ozabor T., Damilola A., Iyabobola F., Abideen W., Anthony O. (2022). Effect of fermentation on the physicochemical and microbiological characteristics of sweet potato (Ipomoea batatas) and sprouted soybean (Glycine max) flour blends. Nigerian Food Journal. 40: 31-43. [DOI: 10.4313/nifoj.v40i2.3]
41. Paul A.K., Lim C.L., Apu M.A.I., Dolma K.G., Gupta M., De Lourdes Pereira M., Wilairatana P., Rahmatullah M., Wiart C., Nissapatorn V. (2023). Are fermented foods effective against inflammatory diseases?. International Journal of Environmental Research and Public Health. 20: 2481. [DOI: 10.3390/ijerph20032481] [DOI:10.3390/ijerph20032481] [PMID] [PMCID]
42. Ramashia S.E., Gwata E.T., Meddows-Taylor S., Anyasi T.A., Jideani A.I.O. (2018). Some physical and functional properties of finger millet (Eleusine coracana) obtained in sub-Saharan Africa. Food Research International. 104: 110-118. [DOI: 10.1016./j. foodres.2017.09.065] [DOI:10.1016/j.foodres.2017.09.065] [PMID]
43. Shah A., Masoodi F.A., Gani A., Ashwar B.A. (2016). Newly released oat varieties of Himalayan region-techno-functional, rheological, and nutraceutical properties of flour. LWT-Food Science and Technology. 70: 111-118. [DOI: 10.1016/j.lwt.2016.02.033] [DOI:10.1016/j.lwt.2016.02.033]
44. Terefe Z.K., Omwamba M.N., Nduko J.M. (2021). Effect of solid-state fermentation on proximate composition, antinutritional factors and invitro protein digestibility of maize flour. Food Science and Nutrition. 9: 6343-6352. [DOI: 10.1002/fsn3.2599] [DOI:10.1002/fsn3.2599] [PMID] [PMCID]
45. Turker M. (2014). Yeast biotechnology: diversity and applications. In Proceedings of 27th VH Yeast Conference. 1-26.
46. Wei G., Zhao Q., Wang D., Fan Y., Shi Y., Huang A. (2022). Novel ACE inhibitory, antioxidant and alpha-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking. LWT-Food Science and Technology. 159: 113196. [DOI: 10.1016/j.lwt.2022.113196] [DOI:10.1016/j.lwt.2022.113196]
47. Yang Q., Yao H., Liu S., Mao J. (2022). Interaction and application of molds and yeasts in Chinese fermented foods. Frontiers in Microbiology. 12: 1-12. [DOI: 10.3389/fmicb.2021.664850] [DOI:10.3389/fmicb.2021.664850] [PMID] [PMCID]
48. Yepez A., Russo P., Spano G., Khomenko I., Biasioli F., Capozzi V., Aznar R. (2019). In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiology. 77: 61-68. [DOI: 10.1016/j.fm.2018.08.008] [DOI:10.1016/j.fm.2018.08.008] [PMID]
49. Zhu Y., Chu J., Lu Z., Lv F., Bie X., Zhang C., Zhao H. (2018). Physicochemical and functional properties of dietary fiber from foxtail millets (Setaria italic) bran. Journal of Cereal Science. 79: 456-461. [DOI: 10.1016/j.jcs.2017.12.011] [DOI:10.1016/j.jcs.2017.12.011]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







