Volume 12, Issue 3 (September 2025)
J. Food Qual. Hazards Control 2025, 12(3): 218-229 |
Back to browse issues page
Ethics code: 03.10.1/UN32.14.2.8/LT/2024
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kusmiyati N, Kusnadi J, Septiana S, Zahroh N, Utami U. Identification of Volatile Compounds and Yeast Species Derived from Salak Pondoh for Bread Aroma Enhancement. J. Food Qual. Hazards Control 2025; 12 (3) :218-229
URL: http://jfqhc.ssu.ac.ir/article-1-1301-en.html
URL: http://jfqhc.ssu.ac.ir/article-1-1301-en.html
Department of Food Science and Biotechnology, Faculty of Agricultural Technology, Universitas Brawijaya, Malang, Indonesia , nurkusmiyati@ub.ac.id
Full-Text [PDF 996 kb]
(306 Downloads)
| Abstract (HTML) (345 Views)
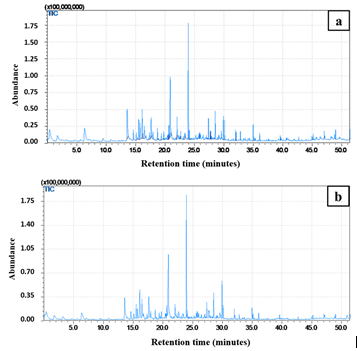
Figure 1: Total Ion Chromatogram (TIC) of volatile compounds detected in bread samples fermented with yeast (a): isolates YIS-3 and (b): YIS-4
Table 1: List of the top 10 volatile compounds with the widest area percentages

Figure 2: Visualization of Polymerase Chain Reaction (PCR) results: (M)=1 kb marker; (A)=YIS-3 replicate 1; (B)=YIS-3 replicate 2; (C)=YIS-4 replicate 1; (D)=YIS-4 replicate 2
Tabel 3: Results of Basic Local Alignment Search Tool (BLAST) yeast isolates YIS-3 and YIS-4
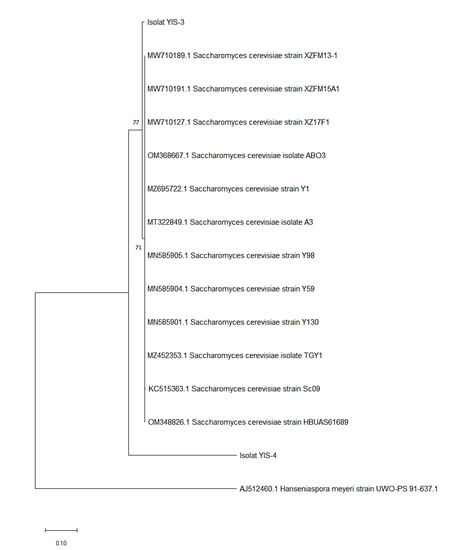
Figure 3: Reconstruction of phylogenetic tree for the yeast isolates YIS-3 and YIS-4
Full-Text: (7 Views)
Identification of Volatile Compounds and Yeast Species Derived from Salak Pondoh for Bread Aroma Enhancement
N. Kusmiyati *1, J. Kusnadi 1, S. Septiana 1, N. Zahroh 2, U. Utami 2
1. Department of Food Science and Biotechnology, Faculty of Agricultural Technology, Universitas Brawijaya, Malang, Indonesia
2. Departemen of Biology, Faculty of Science dan Technology, Universitas Islam Negeri Maulana Malik Ibrahim, Malang, Indonesia
HIGHLIGHT
N. Kusmiyati *1, J. Kusnadi 1, S. Septiana 1, N. Zahroh 2, U. Utami 2
1. Department of Food Science and Biotechnology, Faculty of Agricultural Technology, Universitas Brawijaya, Malang, Indonesia
2. Departemen of Biology, Faculty of Science dan Technology, Universitas Islam Negeri Maulana Malik Ibrahim, Malang, Indonesia
- The characteristic compound of isolate YIS-3 was benzeneethanamine, while that of YIS-4 was o-nitrostyrene.
- Both isolates produced a similar bread-like aroma as they originated from the same species, Saccharomyces cerevisiae.
- Isolate YIS-3 shared 95.88% genetic similarity with strain XZFM13-1, and YIS-4 shared 95.25% similarity with strain HBUAS61689.
| Article type Original article |
ABSTRACT Background: Yeast is a widely utilized microorganism in the fermentation industry, particularly as a leavening agent for bread. The bread-making process yields a number of metabolites, particularly volatile compounds, which influence the end product quality. This study sought to identify volatile compounds and yeast species involved in the leavening process of bread derived from salak pondoh fruit (Salacca edulis Reinw.) to enhance bread aroma. Methods: This study was conducted in between January and September 2024. Yeast isolates were selected based on their dough-leavening ability and were designated as YIS-3 and YIS-4. A descriptive experimental design was applied to evaluate the fermentation performance of both strains in bread making. Two yeast isolates were used in this study, and each isolate was used to produce bread. Volatile compounds in the bread were analyzed using Gas Chromatography–Mass Spectrometry, and sensory evaluation was conducted through organoleptic testing by 30 semi-trained panelists aged 20-35 years. Molecular identification was performed by sequencing the Internal Transcribed Spacer region. The Kruskal–Wallis test was used to identify significant differences among sample groups. When significant differences were observed (p<0.05), post hoc pairwise comparisons were conducted using the Mann–Whitney U test to determine which groups differed significantly. Results: Gas Chromatography–Mass Spectrometry analysis identified 254 volatile compounds in bread fermented with YIS-3 and 231 compounds in bread fermented with YIS-4. The dominant volatile compound in YIS-3 bread was benzeneethanamine, while o-nitrostyrene was predominant in YIS-4 bread. Sensory evaluation revealed no significant difference in aroma preference between the two samples (p>0.05). Molecular identification showed that isolate YIS-3 shared 95.88% sequence similarity with Saccharomyces cerevisiae strain XZFM13-1, while YIS-4 shared 95.25% similarity with S. cerevisiae strain HBUAS61689. Conclusion: Both species, identified as S. cerevisiae, contributed distinctive and sensorially acceptable aroma profiles in bread fermentation. © 2025, Shahid Sadoughi University of Medical Sciences. This is an open access article under the Creative Commons Attribution 4.0 International License. |
|
| Keywords Bread Odorants Sensation Volatile Organic Compounds Saccharomyces cerevisiae |
||
| Article history Received: 4 Dec 2024 Revised: 11 Apr 2025 Accepted: 25 Aug 2025 |
||
| Abbreviations BLAST=Basic Local Alignment Search Tool GC-MS=Gas Chromatography-Mass Spectrometry ITS=Internal Transcribed Spacer PCR=Polymerase Chain Reaction SPME=Solid Phase Microextraction |
To cite: Kusmiyati N., Kusnadi J., Septiana S., Zahroh N., Utami U. (2025). Identification of volatile compounds and yeast species derived from salak pondoh for bread aroma enhancement. Journal of Food Quality and Hazards Control. 12: 218-229.
Introduction
Introduction
Bread is a widely consumed food product, primarily due to its convenience. In Indonesia, the bread industry has experienced continuous growth, driven by increasing consumer demand. According to Dong et al. (2018), food safety has become a key concern for consumers, who are increasingly aware of the relationship between food, nutrition, and health. Among the factors influencing food quality, volatile compounds are regarded by consumers as critical contributors. A recent review highlights that volatile compounds are essential in defining the characteristic aroma of foods, which correlates with nutritional value and provides valuable insights into the nutritional composition of food products. During the bread-making process, volatile compounds are generated through both fermentation and baking. Fermentation begins when yeast is mixed into the dough, during which secondary metabolites are produced, releasing a variety of volatile compounds (Makhoul et al., 2015). Additionally, volatile compounds generated during baking result from Maillard reactions and lipid oxidation. Those derived from Maillard reactions are primarily responsible for bread’s distinctive aroma, which enhances consumer appeal. According to De Luca et al. (2021), alcohols, acids, and aldehydes are the most common volatile compounds found in fermented bread. Makhoul et al. (2015) noted that fermentation activates secondary metabolic pathways in yeast, producing volatile compounds such as propanol, 2-phenylethanol, 3-methyl-1-butanol, 2-methyl-1-propanol, ethyl acetate, ethyl hexanoate, ethyl octanoate, and ethyl benzoate. Izzreen et al. (2016) further reported that during baking, most of the produced volatile compounds belong to the pyrazine group, including 2-methylpyrazine, 2-ethylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 2,3-dimethylpyrazine, 2-ethyl-6-methylpyrazine, 2-ethyl-5-methylpyrazine, 2-ethyl-3-methylpyrazine, 2-vinylpyrazine, and 2-ethyl-3,5-dimethylpyrazine.
Several studies have explored the volatile profiles of fermented products, including traditional cereal-based foods. Ogunremi et al. (2020) identified 45 volatile compounds in Nigerian fermented cereal products, classified into organic acids, alcohols, carbonyls, and esters. Among the yeast strains evaluated, Pichia kluyveri LKC17 produced the largest proportion of carbonyl compounds, Issatchenkia orientalis OSL11 yielded the highest diversity of alcohols, and Pichia kudriavzevii OG32 was notable for its elevated ester production. Phenylethyl alcohol was found to be a dominant component produced by several strains, highlighting the role of yeast diversity in shaping aroma profiles.
Despite the richness of natural and industrial yeast sources, the potential of salak fruit (Salacca edulis Reinw.) as a novel reservoir of functional bread yeasts remains underexplored. Specifically, research on endophytic yeasts from salak and their capacity to produce aromatic volatile compounds during bread fermentation is still limited. Notably, these volatile compounds are crucial to the sensory quality of bread. Only a few studies have addressed the volatile profiles of endophytic yeasts from tropical fruits, and there has been no systematic evaluation of their leavening potential compared to commercial baker’s yeast.
Recent advances in yeast genomics have revealed significant genetic diversity between wild and domesticated strains of Saccharomyces cerevisiae, particularly in traits related to stress resistance, sugar metabolism, and aroma compound biosynthesis (Gallone et al., 2016). Endophytic yeasts, having adapted to plant microenvironments, may possess distinctive traits, such as tolerance to oxidative stress, high sugar concentrations, or antimicrobial compounds, differentiating them from laboratory or industrial strains.
Key aroma-active compounds such as phenylethyl alcohol and ethyl acetate are synthesized via the Ehrlich pathway and ester biosynthesis, respectively (Hazelwood et al., 2008). These metabolic pathways are governed by genes encoding decarboxylases, alcohol dehydrogenases, and acetyltransferases, and are influenced by yeast's metabolic flux and genetic background. Studies have demonstrated that targeted modifications of these pathways, such as overexpression of Alcohol Acetyltransferase 1 (ATF1) or deletion of Aldehyde Dehydrogenase 6 (ALD6), can significantly enhance ester production and thereby improve the aromatic profiles of fermented products (Saerens et al., 2010).
To support such biochemical insights, molecular identification of yeast species was conducted using the Internal Transcribed Spacer (ITS) region, which is widely recognized for its high resolution in species-level identification (Genç and Günay, 2020; Karimi et al., 2015). Compared to other markers, such as the D1/D2 domain of the 26S rDNA, the ITS region offers superior inter-species discrimination, making it suitable for identifying closely related yeast taxa (Schoch et al., 2012). Phylogenetic analysis based on ITS sequences was also employed to determine whether the isolates represent novel genotypes or are closely related to known strains, thereby providing insights into their evolutionary origins and genetic diversity.
This study aims to address the knowledge gap by characterizing the aroma profiles and phylogenetic identity of two endophytic yeast isolates (YIS-3 and YIS-4) derived from salak pondoh fruit. Previous research has shown that these isolates exhibit strong dough-leavening capabilities and deliver superior sensory characteristics compared to commercial baker’s yeast (Zahroh et al., 2022). This dual approach aims to establish a link between the species identity and metabolic potential of the isolates and their functional performance as alternative, indigenous bread starters.
Materials and methods
This study was conducted between January and September 2024. Yeast isolates were obtained from salak pondoh fruit (Salacca edulis Reinw.) and were selected based on their dough-leavening ability. Two yeast isolates were used in this study, designated as YIS-3 and YIS-4, and each isolate was used to produce bread. A total of four bread samples (two from each yeast strain) were prepared and analyzed.
Equipment and materials
The study employed a Laminar Air Flow (LAF; Esco, Singapore) hood, a Polymerase Chain Reaction (PCR) Thermal Cycler (Bio-Rad T100™, USA), a DNA electrophoresis apparatus (Cleaver Scientific, UK), a gel documentation system (Bio-Rad Gel Doc™ XR+, USA), and a Gas Chromatography-Mass Spectrometry (GC-MS; instrument Shimadzu QP 2010 Plus, Kyoto, Japan). Additionally, the materials included yeast isolates (YIS-3 and YIS-4) isolated from salak pondoh fruit (S. edulis Reinw.) collected in Jombang, East Java, Indonesia. These isolates were selected based on their superior bread-leavening performance demonstrated in preliminary experiments.
Culture media and reagents included Yeast Malt Broth (YMB), Yeast Malt Extract Agar (YMEA), and Yeast Peptone Glucose (YPG), all obtained from HiMedia (India); Sodium DL-lactate (Sigma-Aldrich, USA); and oligonucleotide primers for molecular identification: forward primer ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and reverse primer ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′), purchased from Integrated DNA Technologies, USA.
Bread-making process using yeast isolates YIS-3 and YIS-4
The dough preparation was carried out using a modified method adapted from Watanabe et al. (2016). The ingredients used included 50 g of wheat flour, 0.75 g of salt, 3.75 g of sugar, 4 g of butter, 2 g (4%) of yeast pellets, and 20 ml of water. All ingredients were mixed and kneaded until a smooth dough consistency was achieved. Each dough sample was placed in a mold and incubated at approximately 30 ºC for 6 h. The final step in bread making involved baking at 120 ºC for 15 min (Karki et al., 2017). After baking, the bread was sliced for organoleptic evaluation. A 3 g portion of each bread sample was collected for volatile compound analysis (Dong et al., 2018).
Volatile compound analysis of bread fermented with YIS-3 and YIS-4
Volatile compound analysis was conducted through sample extraction and GC-MS analysis. Samples were extracted based on the protocol of Dong et al. (2018). Three g of bread were placed in a 20 ml vial without solvent and tightly sealed with PTFE/silicone septa. The sample was incubated in a water bath at 60 °C for 20 min. Volatile compounds were extracted using Solid-Phase Microextraction (SPME: PK3 SPME ASSY 50/30 µm DVB/CAR/PDMS, Bellefonte, PA, USA) at 60 °C for 30 min, with the SPME fiber inserted into the vial. The fiber was desorbed for 7 min at the GC injection port set to 250 °C in splitless mode before conducting GC-MS analysis.
The volatile compound was analyzed using a GC-MS under the following conditions: oven temperature set at 35 °C, injector temperature at 250 °C, pressure at 47.6 kPa, total flow rate of 4 ml/min, column flow rate of 1 ml/min, linear velocity at 36.0 cm/s, and purge flow at 3 ml/min. The column temperature was set at 35 °C for 6 min, raised to 40 °C at a rate of 2 °C/min, further increased to 210 °C at 5 °C/min, and finally ramped to 250 °C at 10 °C/min, where the state was upheld for 5 min. For MS, conditions were as follows: m/z range of 35-500, event time of 0.3 s, interface temperature at 280 °C, and ion source temperature at 230 °C.
Organoleptic analysis of bread fermented with YIS-3 and YIS-4
The organoleptic evaluation of the bread focused on aroma and was conducted using a hedonic test method to assess consumer preference. A total of 30 untrained panelists (consumers), comprising both male and female participants aged between 20 and 35 years, were involved in the assessment. The panelists were asked to evaluate the bread's aroma based on their personal preference using a 9-point hedonic scale, which is considered more acceptable and provides greater sensitivity than the commonly used 5-point scale. The scale was defined as follows: 1=dislike extremely, 2=dislike very much, 3=dislike moderately, 4=dislike slightly, 5=neither like nor dislike, 6=like slightly, 7=like moderately, 8=like very much, and 9=like extremely. This method allows for a more nuanced measurement of liking and is widely accepted in sensory analysis. Prior to the evaluation, all panelists were given clear instructions, and the test was carried out under controlled conditions to minimize bias. The method was adapted from the procedure described by Cabello-Olmo et al. (2023), with modifications to the scale for improved accuracy in capturing consumer preferences.
Identifying yeast isolates YIS-3 and YIS-4
-DNA isolation
DNA was isolated using the direct PCR method. A direct thermal lysis method was employed to disrupt the cell wall and release genomic DNA. A single yeast colony was selected from the sub-cultured plate and transferred into a 0.2 ml microtube containing 20 μl of sterile nuclease-free water. The tube was then subjected to boiling at 95 °C for 10 min to lyse the cells and release genomic DNA. After boiling, the sample was briefly centrifuged, and 2 μl of the resulting supernatant was used as the DNA template. The PCR reaction mixture consisted of 10 µl of PCR master mix, 1 µl of forward primer (ITS1: 5’-TCC GTA GGT GAA CCT GCG G-3’), 1 µl of reverse primer (ITS4: 5’-TCC TCC GCT TAT TGA TAT GC-3’), 2 µl of DNA template, and 6 µl of nuclease-free water, yielding a final volume of 20 µl. Amplification was performed according to a modified protocol from Ben-Amar et al. (2017), involving an initial denaturation at 94 °C for 7 min, followed by 29 cycles of denaturation at 94 °C for 45 s, annealing at 56 °C for 60 s, and extension at 72 °C for 60 s, with a final extension at 72 °C for 7 min.
-Sequence analysis
The PCR products were sequenced to determine nucleotide order and composition, employing sequencing services from Limited Liability Company (LLC) Genetika Sains, Singapore. The results were analyzed through aligning process using Basic Local Alignment Search Tool (BLAST) to determine base-pair similarity percentages compared to reference sequences in the NCBI GenBank (https://www.ncbi.nlm.nih.gov/). Phylogenetic tree construction was performed using MEGA-X software. The yeast isolates’ sequences were compared with other isolates, and phylogenetic trees were reconstructed using the Neighbor-Joining method with bootstrap testing as referred to Feng et al. (2017).
Statistical analysis
Statistical analysis of the organoleptic data was carried out using IBM SPSS Statistics software version 25.0 (IBM Corp., Armonk, NY, USA). Since the sensory data did not meet the assumptions of normality and homogeneity of variances, non-parametric tests were employed. The Kruskal-Wallis test was used to identify significant differences among sample groups. When significant differences were found (p<0.05), post-hoc pairwise comparisons were performed using the Mann-Whitney U test to determine which specific groups differed from each other. The level of statistical significance was set at p<0.05.
Results and Discussion
Volatile compound analysis of bread fermented with YIS-3 and YIS-4
Based on chromatographic analysis, a total of 254 volatile compounds were identified in bread samples fermented with yeast isolate YIS-3 (Figure 1a), while 231 volatile compounds were detected in samples fermented with yeast isolate YIS-4 (Figure 1b). From each sample, the 50 volatile compounds with the highest peak quality were selected and classified into different categories based on their functional groups.
Several studies have explored the volatile profiles of fermented products, including traditional cereal-based foods. Ogunremi et al. (2020) identified 45 volatile compounds in Nigerian fermented cereal products, classified into organic acids, alcohols, carbonyls, and esters. Among the yeast strains evaluated, Pichia kluyveri LKC17 produced the largest proportion of carbonyl compounds, Issatchenkia orientalis OSL11 yielded the highest diversity of alcohols, and Pichia kudriavzevii OG32 was notable for its elevated ester production. Phenylethyl alcohol was found to be a dominant component produced by several strains, highlighting the role of yeast diversity in shaping aroma profiles.
Despite the richness of natural and industrial yeast sources, the potential of salak fruit (Salacca edulis Reinw.) as a novel reservoir of functional bread yeasts remains underexplored. Specifically, research on endophytic yeasts from salak and their capacity to produce aromatic volatile compounds during bread fermentation is still limited. Notably, these volatile compounds are crucial to the sensory quality of bread. Only a few studies have addressed the volatile profiles of endophytic yeasts from tropical fruits, and there has been no systematic evaluation of their leavening potential compared to commercial baker’s yeast.
Recent advances in yeast genomics have revealed significant genetic diversity between wild and domesticated strains of Saccharomyces cerevisiae, particularly in traits related to stress resistance, sugar metabolism, and aroma compound biosynthesis (Gallone et al., 2016). Endophytic yeasts, having adapted to plant microenvironments, may possess distinctive traits, such as tolerance to oxidative stress, high sugar concentrations, or antimicrobial compounds, differentiating them from laboratory or industrial strains.
Key aroma-active compounds such as phenylethyl alcohol and ethyl acetate are synthesized via the Ehrlich pathway and ester biosynthesis, respectively (Hazelwood et al., 2008). These metabolic pathways are governed by genes encoding decarboxylases, alcohol dehydrogenases, and acetyltransferases, and are influenced by yeast's metabolic flux and genetic background. Studies have demonstrated that targeted modifications of these pathways, such as overexpression of Alcohol Acetyltransferase 1 (ATF1) or deletion of Aldehyde Dehydrogenase 6 (ALD6), can significantly enhance ester production and thereby improve the aromatic profiles of fermented products (Saerens et al., 2010).
To support such biochemical insights, molecular identification of yeast species was conducted using the Internal Transcribed Spacer (ITS) region, which is widely recognized for its high resolution in species-level identification (Genç and Günay, 2020; Karimi et al., 2015). Compared to other markers, such as the D1/D2 domain of the 26S rDNA, the ITS region offers superior inter-species discrimination, making it suitable for identifying closely related yeast taxa (Schoch et al., 2012). Phylogenetic analysis based on ITS sequences was also employed to determine whether the isolates represent novel genotypes or are closely related to known strains, thereby providing insights into their evolutionary origins and genetic diversity.
This study aims to address the knowledge gap by characterizing the aroma profiles and phylogenetic identity of two endophytic yeast isolates (YIS-3 and YIS-4) derived from salak pondoh fruit. Previous research has shown that these isolates exhibit strong dough-leavening capabilities and deliver superior sensory characteristics compared to commercial baker’s yeast (Zahroh et al., 2022). This dual approach aims to establish a link between the species identity and metabolic potential of the isolates and their functional performance as alternative, indigenous bread starters.
Materials and methods
This study was conducted between January and September 2024. Yeast isolates were obtained from salak pondoh fruit (Salacca edulis Reinw.) and were selected based on their dough-leavening ability. Two yeast isolates were used in this study, designated as YIS-3 and YIS-4, and each isolate was used to produce bread. A total of four bread samples (two from each yeast strain) were prepared and analyzed.
Equipment and materials
The study employed a Laminar Air Flow (LAF; Esco, Singapore) hood, a Polymerase Chain Reaction (PCR) Thermal Cycler (Bio-Rad T100™, USA), a DNA electrophoresis apparatus (Cleaver Scientific, UK), a gel documentation system (Bio-Rad Gel Doc™ XR+, USA), and a Gas Chromatography-Mass Spectrometry (GC-MS; instrument Shimadzu QP 2010 Plus, Kyoto, Japan). Additionally, the materials included yeast isolates (YIS-3 and YIS-4) isolated from salak pondoh fruit (S. edulis Reinw.) collected in Jombang, East Java, Indonesia. These isolates were selected based on their superior bread-leavening performance demonstrated in preliminary experiments.
Culture media and reagents included Yeast Malt Broth (YMB), Yeast Malt Extract Agar (YMEA), and Yeast Peptone Glucose (YPG), all obtained from HiMedia (India); Sodium DL-lactate (Sigma-Aldrich, USA); and oligonucleotide primers for molecular identification: forward primer ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and reverse primer ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′), purchased from Integrated DNA Technologies, USA.
Bread-making process using yeast isolates YIS-3 and YIS-4
The dough preparation was carried out using a modified method adapted from Watanabe et al. (2016). The ingredients used included 50 g of wheat flour, 0.75 g of salt, 3.75 g of sugar, 4 g of butter, 2 g (4%) of yeast pellets, and 20 ml of water. All ingredients were mixed and kneaded until a smooth dough consistency was achieved. Each dough sample was placed in a mold and incubated at approximately 30 ºC for 6 h. The final step in bread making involved baking at 120 ºC for 15 min (Karki et al., 2017). After baking, the bread was sliced for organoleptic evaluation. A 3 g portion of each bread sample was collected for volatile compound analysis (Dong et al., 2018).
Volatile compound analysis of bread fermented with YIS-3 and YIS-4
Volatile compound analysis was conducted through sample extraction and GC-MS analysis. Samples were extracted based on the protocol of Dong et al. (2018). Three g of bread were placed in a 20 ml vial without solvent and tightly sealed with PTFE/silicone septa. The sample was incubated in a water bath at 60 °C for 20 min. Volatile compounds were extracted using Solid-Phase Microextraction (SPME: PK3 SPME ASSY 50/30 µm DVB/CAR/PDMS, Bellefonte, PA, USA) at 60 °C for 30 min, with the SPME fiber inserted into the vial. The fiber was desorbed for 7 min at the GC injection port set to 250 °C in splitless mode before conducting GC-MS analysis.
The volatile compound was analyzed using a GC-MS under the following conditions: oven temperature set at 35 °C, injector temperature at 250 °C, pressure at 47.6 kPa, total flow rate of 4 ml/min, column flow rate of 1 ml/min, linear velocity at 36.0 cm/s, and purge flow at 3 ml/min. The column temperature was set at 35 °C for 6 min, raised to 40 °C at a rate of 2 °C/min, further increased to 210 °C at 5 °C/min, and finally ramped to 250 °C at 10 °C/min, where the state was upheld for 5 min. For MS, conditions were as follows: m/z range of 35-500, event time of 0.3 s, interface temperature at 280 °C, and ion source temperature at 230 °C.
Organoleptic analysis of bread fermented with YIS-3 and YIS-4
The organoleptic evaluation of the bread focused on aroma and was conducted using a hedonic test method to assess consumer preference. A total of 30 untrained panelists (consumers), comprising both male and female participants aged between 20 and 35 years, were involved in the assessment. The panelists were asked to evaluate the bread's aroma based on their personal preference using a 9-point hedonic scale, which is considered more acceptable and provides greater sensitivity than the commonly used 5-point scale. The scale was defined as follows: 1=dislike extremely, 2=dislike very much, 3=dislike moderately, 4=dislike slightly, 5=neither like nor dislike, 6=like slightly, 7=like moderately, 8=like very much, and 9=like extremely. This method allows for a more nuanced measurement of liking and is widely accepted in sensory analysis. Prior to the evaluation, all panelists were given clear instructions, and the test was carried out under controlled conditions to minimize bias. The method was adapted from the procedure described by Cabello-Olmo et al. (2023), with modifications to the scale for improved accuracy in capturing consumer preferences.
Identifying yeast isolates YIS-3 and YIS-4
-DNA isolation
DNA was isolated using the direct PCR method. A direct thermal lysis method was employed to disrupt the cell wall and release genomic DNA. A single yeast colony was selected from the sub-cultured plate and transferred into a 0.2 ml microtube containing 20 μl of sterile nuclease-free water. The tube was then subjected to boiling at 95 °C for 10 min to lyse the cells and release genomic DNA. After boiling, the sample was briefly centrifuged, and 2 μl of the resulting supernatant was used as the DNA template. The PCR reaction mixture consisted of 10 µl of PCR master mix, 1 µl of forward primer (ITS1: 5’-TCC GTA GGT GAA CCT GCG G-3’), 1 µl of reverse primer (ITS4: 5’-TCC TCC GCT TAT TGA TAT GC-3’), 2 µl of DNA template, and 6 µl of nuclease-free water, yielding a final volume of 20 µl. Amplification was performed according to a modified protocol from Ben-Amar et al. (2017), involving an initial denaturation at 94 °C for 7 min, followed by 29 cycles of denaturation at 94 °C for 45 s, annealing at 56 °C for 60 s, and extension at 72 °C for 60 s, with a final extension at 72 °C for 7 min.
-Sequence analysis
The PCR products were sequenced to determine nucleotide order and composition, employing sequencing services from Limited Liability Company (LLC) Genetika Sains, Singapore. The results were analyzed through aligning process using Basic Local Alignment Search Tool (BLAST) to determine base-pair similarity percentages compared to reference sequences in the NCBI GenBank (https://www.ncbi.nlm.nih.gov/). Phylogenetic tree construction was performed using MEGA-X software. The yeast isolates’ sequences were compared with other isolates, and phylogenetic trees were reconstructed using the Neighbor-Joining method with bootstrap testing as referred to Feng et al. (2017).
Statistical analysis
Statistical analysis of the organoleptic data was carried out using IBM SPSS Statistics software version 25.0 (IBM Corp., Armonk, NY, USA). Since the sensory data did not meet the assumptions of normality and homogeneity of variances, non-parametric tests were employed. The Kruskal-Wallis test was used to identify significant differences among sample groups. When significant differences were found (p<0.05), post-hoc pairwise comparisons were performed using the Mann-Whitney U test to determine which specific groups differed from each other. The level of statistical significance was set at p<0.05.
Results and Discussion
Volatile compound analysis of bread fermented with YIS-3 and YIS-4
Based on chromatographic analysis, a total of 254 volatile compounds were identified in bread samples fermented with yeast isolate YIS-3 (Figure 1a), while 231 volatile compounds were detected in samples fermented with yeast isolate YIS-4 (Figure 1b). From each sample, the 50 volatile compounds with the highest peak quality were selected and classified into different categories based on their functional groups.

Figure 1: Total Ion Chromatogram (TIC) of volatile compounds detected in bread samples fermented with yeast (a): isolates YIS-3 and (b): YIS-4
As a result, the bread sample fermented with YIS-3 contained eight aldehydes, seven alcohols, one furan, eleven esters, two benzene derivatives, one alkene, six alkanes, one amine, one nitrile, one ketone, and eleven other compounds. In contrast, the bread sample fermented with YIS-4 contained eight aldehydes, six alcohols, one furan, eleven esters, five benzene derivatives, one alkene, five alkanes, one nitrile, one nitro compound, and eleven other compounds. These findings indicate that esters were the dominant group of volatile compounds in both YIS-3 and YIS-4 bread samples.
According to Mazumdar et al. (2019), esters are the primary volatile compounds in ripe salak fruit (S. edulis). This is consistent with the findings of the present study, as yeast fermentation processes typically generate a high ester profile, particularly when the substrate is rich in simple carbohydrates and lipids. However, the difference in the total number of volatile compounds between YIS-3 and YIS-4 may be attributed to variations in secondary metabolism among yeast isolates, with YIS-3 potentially producing more fatty acid-derived compounds.
The ten volatile compounds with the highest peak area percentages were identified for each sample (Table 1). Among these compounds, benzaldehyde exhibited a peak area percentage of 9.33% in the YIS-3 sample and 7.43% in the YIS-4 sample. According to De Luca et al. (2021), benzaldehyde is associated with almond, sweet, and cherry-like aromas. This compound is commonly formed through lipid oxidation, fermentation, and Maillard reactions. The higher concentration of benzaldehyde in YIS-3 suggests a greater activity of oxidative enzymes in lipid or aromatic amino acid metabolic pathways. In contrast, YIS-4 showed a higher production of nonanal, a compound more closely associated with saturated lipid oxidation, supporting the hypothesis that YIS-4 may exhibit greater activity in lipid metabolism compared to amino acid fermentation.
According to Mazumdar et al. (2019), esters are the primary volatile compounds in ripe salak fruit (S. edulis). This is consistent with the findings of the present study, as yeast fermentation processes typically generate a high ester profile, particularly when the substrate is rich in simple carbohydrates and lipids. However, the difference in the total number of volatile compounds between YIS-3 and YIS-4 may be attributed to variations in secondary metabolism among yeast isolates, with YIS-3 potentially producing more fatty acid-derived compounds.
The ten volatile compounds with the highest peak area percentages were identified for each sample (Table 1). Among these compounds, benzaldehyde exhibited a peak area percentage of 9.33% in the YIS-3 sample and 7.43% in the YIS-4 sample. According to De Luca et al. (2021), benzaldehyde is associated with almond, sweet, and cherry-like aromas. This compound is commonly formed through lipid oxidation, fermentation, and Maillard reactions. The higher concentration of benzaldehyde in YIS-3 suggests a greater activity of oxidative enzymes in lipid or aromatic amino acid metabolic pathways. In contrast, YIS-4 showed a higher production of nonanal, a compound more closely associated with saturated lipid oxidation, supporting the hypothesis that YIS-4 may exhibit greater activity in lipid metabolism compared to amino acid fermentation.
Table 1: List of the top 10 volatile compounds with the widest area percentages
| Sample | No | time retention (min) |
% area | Molecular mass |
Molecular (formula) |
Compound | Group |
| YIS-3 | 1 | 13.5 | 9.33 | 106.1219 | C7H6O | Benzaldehyde (CAS) Phenylmethanal | Aldehyde |
| 2 | 15.5 | 4.12 | 138.2069 | C9H14O | Furan, 2-pentyl- (CAS) 2- Amylfuran | Furan | |
| 3 | 15.7 | 2.94 | 296.6158 | C8H24O4Si4 | Cyclotetrasiloxane, octamethyl- (CAS) | - | |
| 4 | 16.1 | 4.88 | 144.2114 | C8H16O2 | Hexanoic acid, ethyl ester (CAS) | Ester | |
| 5 | 16.5 | 2.56 | 147.002 | C6H4Cl2 | Benzene, 1,4-dichloro- | Benzene | |
| 6 | 17.6 | 3.15 | 108.1378 | C7H8O | Benzenemethanol | Alcohol | |
| 7 | 20.9 | 13.57 | 121.1796 | C8H11N | Benzeneethanamine (CAS) | Amine | |
| 8 | 24.0 | 13.77 | 172.2646 | C10H20O2 | Octanoic acid, ethyl ester (CAS) | Ester | |
| 9 | 28.5 | 3.6 | 178.23 | C11H14O2 | Butanoic acid, phenylmethyl ester (CAS) Benzyl butanoate | Ester | |
| 10 | 29.9 | 2.78 | 200.3178 | C12H24O2 | Decanoic acid, ethyl ester (CAS) ethyl decanoate | Ester | |
| YIS-4 | 1 | 13.6 | 7.43 | 106.1219 | C7H6O | Benzaldehyde (CAS) phenylmethanal |
Aldehyde |
| 2 | 15.5 | 3.01 | 138.2069 | C9H14O | Furan, 2-pentyl- (CAS) | Furan | |
| 3 | 16.1 | 5.02 | 144.2114 | C8H16O2 | Hexanoic acid, ethyl ester (CAS) ethyl n-caproate | Ester | |
| 4 | 16.5 | 4.17 | 147.002 | C6H4Cl2 | Benzene, 1,3-dichloro- (CAS) | Benzene | |
| 5 | 17.6 | 5.16 | 108.1378 | C7H8O | Benzenemethanol (CAS) | Alcohol | |
| 6 | 20.6 | 1.99 | 142.2386 | C9H18O | Nonanal (CAS) n-nonanal | Aldehyde | |
| 7 | 20.9 | 17.08 | 149.15 | C8H7NO | O-nitrostyrene | Nitro | |
| 8 | 24.0 | 12.14 | 172.2646 | C10H20O2 | Octanoic acid, ethyl ester (CAS) | Ester | |
| 9 | 28.5 | 3.45 | 178.23 | C11H14O2 | Butanoic acid, phenylmethyl ester (CAS) benzyl butanoate | Ester | |
| 10 | 29.9 | 4.44 | 256.4241 | C16H32O2 | Tetradecanoic acid, ethyl ester (CAS) | Ester |
CAS=Chemical Abstracts Service
Furthermore, nonanal was detected in the YIS-4 bread sample at a peak area of 1.99%. Nonanal is known for its fruity aroma with rose, citrus, and orange-like notes and is mainly derived from fermentation and lipid oxidation. Both benzaldehyde and nonanal belong to the aldehyde group of volatile compounds (De Luca et al., 2021). Iranmanesh et al. (2018) noted that aldehydes can be directly formed via the Maillard reaction pathways (amadori or heyns products), particularly in thermally processed foods. Aldehydes are also major products of the autoxidation of unsaturated fatty acids via lipid oxidation mechanisms.
Another compound, 2-pentylfuran, exhibited peak areas of 4.12% in YIS-3 and 3.01% in YIS-4. This compound contributes to caramel-like, sweet, fruity, nutty, and meaty aromas (Wailzer et al., 2016). It is formed through both lipid oxidation and Maillard reactions (Izzreen et al., 2016). 2-pentylfuran belongs to the furan group, typically generated at the final stages of Maillard reactions. This finding aligns with Wailzer et al. (2016), though the concentration difference suggests that the YIS-3 strain may exhibit higher decarboxylase or peroxidase activity, accelerating furan formation from unsaturated lipid substrates.
Ethyl hexanoate, ethyl octanoate, phenylmethyl butanoate, ethyl decanoate, and ethyl tetradecanoate are classified as esters. According to Iranmanesh et al. (2018), various microorganisms, particularly yeast species, are capable of producing esters. Ester biosynthesis involves two key steps: first, triglycerides in lipids are hydrolyzed by lipase enzymes to generate Free Fatty Acids (FFAs); second, FFAs are esterified with alcohols catalyzed by esterase enzymes.
Ethyl hexanoate exhibited peak areas of 4.88% in YIS-3 and 5.02% in YIS-4. Ethyl octanoate accounted for 13.77% in YIS-3 and 12.14% in YIS-4. These compounds are known for their fatty, acidic, cheesy, and fruity aromas. Hexanoic acid is formed during fermentation and lipid oxidation (Pico et al., 2018). Phenylmethyl butanoate, which imparts cheesy and buttery aromas, contributed 3.6% in YIS-3 and 3.45% in YIS-4 (Astuti et al., 2022). Ethyl decanoate was present in YIS-3 at a peak area of 2.78%, associated with fruity and cheesy notes (Boltar et al., 2015). Ethyl tetradecanoate was observed in YIS-4 at 4.44% and is associated with cheesy, fatty, and coconut-like aromas (Astuti et al., 2022; Poveda et al., 2008).
The high ester concentrations confirm that both yeast isolates possess significant lipase and esterase activity; however, YIS-3 produced slightly higher concentrations of medium-chain esters. This may be attributed to differences in enzyme expression or the availability of precursor substrates such as ethanol.
Benzenemethanol, also known as benzyl alcohol, exhibited peak areas of 3.15% in YIS-3 and 5.16% in YIS-4. The higher benzyl alcohol concentration in YIS-4 suggests a more dominant phenylalanine metabolic pathway, consistent with the Ehrlich pathway. A similar observation was reported by Olaniran et al. (2017), who noted that specific yeast strains exhibit enhanced aromatic amino acid degradation activity. Benzyl alcohol contributes fruity, sweet, floral, and mild aromas. It is produced through yeast fermentation, specifically via the reduction of benzoic acid in the glycolytic pathway (Ardiansyah et al., 2021). This volatile compound belongs to the alcohol group. According to Olaniran et al. (2017), alcohols are synthesized via the Ehrlich pathway, wherein specific amino acids undergo transamination to form α-keto acids.
Benzene, 1,4-dichloro– was produced by YIS-3 with a peak area of 2.56%, whereas benzene, 1,3-dichloro– was produced by YIS-4 with 4.17%. Both are classified as benzene-type volatile compounds. Zhang et al. (2020) reported that benzene derivatives are produced during fermentation. Additionally, o-nitrostyrene (2-nitrostyrene), classified as a nitro compound, was detected in YIS-4 with a peak area of 17.08%. Aromatic nitro compounds such as 2-nitrostyrene are rarely reported in bread products, suggesting possible involvement of unique aromatic enzyme activity in YIS-4. Pavlovica et al. (2014) further demonstrated that the Henry reaction is influenced by pH conditions and the availability of aromatic aldehydes, which may vary between isolates. According to Pavlovica et al. (2014), 2-nitrostyrene is formed through the condensation of aromatic aldehydes with nitroalkanes via the Henry reaction, a key carbon–carbon bond-forming process in organic chemistry.
Benzeneethanamine, or phenylethylamine, accounted for 13.57% of the area in YIS-3. The higher content of phenylethylamine in YIS-3 indicates the contribution of the Ehrlich pathway in producing compounds with positive sensory characteristics. Martínez-Avila et al. (2018) showed that this compound is a critical precursor of aroma compounds such as 2-phenylethanol (2-PE) and 2-phenylethyl acetate (2-PEA). The presence of benzeneethanamine in YIS-3 suggests its potential impact on aroma quality, contributing sweet and floral notes. In contrast, o-nitrostyrene in YIS-4 may impart unique aroma characteristics such as nutty or smoky. According to Martínez-Avila et al. (2018), these compounds contribute to aroma and can be further reduced into other aromatic compounds such as 2-PE and 2-PEA. Phenylethylamine is a nitrogen-containing volatile compound classified as an amine. Jackson (2008) defined amines as organic compounds related to the ammonia group. Amine concentrations may decrease due to volatility during yeast metabolism in fermentation but can also increase through yeast autolysis.
Cyclotetrasiloxane, octamethyl-Chemical Abstracts Service (CAS), was detected in YIS-3 with a peak area of 2.94%. This finding highlights the need to evaluate potential contamination from extraction equipment, as also noted by Companioni-Damas et al. (2012). Siloxane derivatives such as cyclotetrasiloxane, octamethyl-, decamethyl cyclopentasiloxane, and dodecamethyl cyclohexasiloxane were also detected in their study, indicating that siloxane contamination may originate from polydimethylsiloxane (PDMS), the main component of divinylbenzene/ carboxen/polydimethylsiloxane (DVB/CAR/PDMS) based SPME fibers.
According to Nakamura et al. (2018), volatile compounds are formed during fermentation, baking (involving Maillard reactions), and lipid oxidation. Park and Kim (2019) classified the formation of volatile compounds into three main metabolic pathways: carbohydrate metabolism, amino acid metabolism, and fatty acid metabolism. Carbohydrate metabolism is closely linked to the production of various volatile compounds via alcohol fermentation. Amino acid metabolism, particularly through the Ehrlich pathway, is essential for the degradation of specific amino acids to yield volatile compounds. Fatty acid metabolism occurs during fermentation, wherein fats are hydrolyzed into glycerol and fatty acids by lipase enzymes. Lipid metabolic pathways encompass fatty acids and their derivatives.
Organoleptic analysis of bread fermented with YIS-3 and YIS-4
An organoleptic test was aimed to determine the sensory differences between the samples using human senses. According to the results of the Kruskal-Wallis test, for all evaluated attributes, aroma, taste, color, and texture, the p-values exceeded 0.05 (p>0.05) (Table 2). This indicates that the null hypothesis (H0) is accepted, suggesting no significant differences between the treatments (YIS-3 and YIS-4 bread) in terms of aroma, taste, color, and texture. This infers respondents perceived both bread samples as exhibiting similar sensory characteristics. The average scores for all attributes were approximately seven, which indicates a like moderately response from the respondents regarding the aroma, taste, color, and texture of both breads. This scoring was based on a scale where 1=dislike extremely, 2=dislike very much, 3=dislike moderately, 4=dislike slightly, 5=neither like nor dislike, 6=like slightly, 7=like moderately, 8=like very much, and 9=like extremely (Cabello-Olmo et al., 2023).
Table 2: Organoleptic analysis ( aroma, color, texture, and taste) of the bread samples
Another compound, 2-pentylfuran, exhibited peak areas of 4.12% in YIS-3 and 3.01% in YIS-4. This compound contributes to caramel-like, sweet, fruity, nutty, and meaty aromas (Wailzer et al., 2016). It is formed through both lipid oxidation and Maillard reactions (Izzreen et al., 2016). 2-pentylfuran belongs to the furan group, typically generated at the final stages of Maillard reactions. This finding aligns with Wailzer et al. (2016), though the concentration difference suggests that the YIS-3 strain may exhibit higher decarboxylase or peroxidase activity, accelerating furan formation from unsaturated lipid substrates.
Ethyl hexanoate, ethyl octanoate, phenylmethyl butanoate, ethyl decanoate, and ethyl tetradecanoate are classified as esters. According to Iranmanesh et al. (2018), various microorganisms, particularly yeast species, are capable of producing esters. Ester biosynthesis involves two key steps: first, triglycerides in lipids are hydrolyzed by lipase enzymes to generate Free Fatty Acids (FFAs); second, FFAs are esterified with alcohols catalyzed by esterase enzymes.
Ethyl hexanoate exhibited peak areas of 4.88% in YIS-3 and 5.02% in YIS-4. Ethyl octanoate accounted for 13.77% in YIS-3 and 12.14% in YIS-4. These compounds are known for their fatty, acidic, cheesy, and fruity aromas. Hexanoic acid is formed during fermentation and lipid oxidation (Pico et al., 2018). Phenylmethyl butanoate, which imparts cheesy and buttery aromas, contributed 3.6% in YIS-3 and 3.45% in YIS-4 (Astuti et al., 2022). Ethyl decanoate was present in YIS-3 at a peak area of 2.78%, associated with fruity and cheesy notes (Boltar et al., 2015). Ethyl tetradecanoate was observed in YIS-4 at 4.44% and is associated with cheesy, fatty, and coconut-like aromas (Astuti et al., 2022; Poveda et al., 2008).
The high ester concentrations confirm that both yeast isolates possess significant lipase and esterase activity; however, YIS-3 produced slightly higher concentrations of medium-chain esters. This may be attributed to differences in enzyme expression or the availability of precursor substrates such as ethanol.
Benzenemethanol, also known as benzyl alcohol, exhibited peak areas of 3.15% in YIS-3 and 5.16% in YIS-4. The higher benzyl alcohol concentration in YIS-4 suggests a more dominant phenylalanine metabolic pathway, consistent with the Ehrlich pathway. A similar observation was reported by Olaniran et al. (2017), who noted that specific yeast strains exhibit enhanced aromatic amino acid degradation activity. Benzyl alcohol contributes fruity, sweet, floral, and mild aromas. It is produced through yeast fermentation, specifically via the reduction of benzoic acid in the glycolytic pathway (Ardiansyah et al., 2021). This volatile compound belongs to the alcohol group. According to Olaniran et al. (2017), alcohols are synthesized via the Ehrlich pathway, wherein specific amino acids undergo transamination to form α-keto acids.
Benzene, 1,4-dichloro– was produced by YIS-3 with a peak area of 2.56%, whereas benzene, 1,3-dichloro– was produced by YIS-4 with 4.17%. Both are classified as benzene-type volatile compounds. Zhang et al. (2020) reported that benzene derivatives are produced during fermentation. Additionally, o-nitrostyrene (2-nitrostyrene), classified as a nitro compound, was detected in YIS-4 with a peak area of 17.08%. Aromatic nitro compounds such as 2-nitrostyrene are rarely reported in bread products, suggesting possible involvement of unique aromatic enzyme activity in YIS-4. Pavlovica et al. (2014) further demonstrated that the Henry reaction is influenced by pH conditions and the availability of aromatic aldehydes, which may vary between isolates. According to Pavlovica et al. (2014), 2-nitrostyrene is formed through the condensation of aromatic aldehydes with nitroalkanes via the Henry reaction, a key carbon–carbon bond-forming process in organic chemistry.
Benzeneethanamine, or phenylethylamine, accounted for 13.57% of the area in YIS-3. The higher content of phenylethylamine in YIS-3 indicates the contribution of the Ehrlich pathway in producing compounds with positive sensory characteristics. Martínez-Avila et al. (2018) showed that this compound is a critical precursor of aroma compounds such as 2-phenylethanol (2-PE) and 2-phenylethyl acetate (2-PEA). The presence of benzeneethanamine in YIS-3 suggests its potential impact on aroma quality, contributing sweet and floral notes. In contrast, o-nitrostyrene in YIS-4 may impart unique aroma characteristics such as nutty or smoky. According to Martínez-Avila et al. (2018), these compounds contribute to aroma and can be further reduced into other aromatic compounds such as 2-PE and 2-PEA. Phenylethylamine is a nitrogen-containing volatile compound classified as an amine. Jackson (2008) defined amines as organic compounds related to the ammonia group. Amine concentrations may decrease due to volatility during yeast metabolism in fermentation but can also increase through yeast autolysis.
Cyclotetrasiloxane, octamethyl-Chemical Abstracts Service (CAS), was detected in YIS-3 with a peak area of 2.94%. This finding highlights the need to evaluate potential contamination from extraction equipment, as also noted by Companioni-Damas et al. (2012). Siloxane derivatives such as cyclotetrasiloxane, octamethyl-, decamethyl cyclopentasiloxane, and dodecamethyl cyclohexasiloxane were also detected in their study, indicating that siloxane contamination may originate from polydimethylsiloxane (PDMS), the main component of divinylbenzene/ carboxen/polydimethylsiloxane (DVB/CAR/PDMS) based SPME fibers.
According to Nakamura et al. (2018), volatile compounds are formed during fermentation, baking (involving Maillard reactions), and lipid oxidation. Park and Kim (2019) classified the formation of volatile compounds into three main metabolic pathways: carbohydrate metabolism, amino acid metabolism, and fatty acid metabolism. Carbohydrate metabolism is closely linked to the production of various volatile compounds via alcohol fermentation. Amino acid metabolism, particularly through the Ehrlich pathway, is essential for the degradation of specific amino acids to yield volatile compounds. Fatty acid metabolism occurs during fermentation, wherein fats are hydrolyzed into glycerol and fatty acids by lipase enzymes. Lipid metabolic pathways encompass fatty acids and their derivatives.
Organoleptic analysis of bread fermented with YIS-3 and YIS-4
An organoleptic test was aimed to determine the sensory differences between the samples using human senses. According to the results of the Kruskal-Wallis test, for all evaluated attributes, aroma, taste, color, and texture, the p-values exceeded 0.05 (p>0.05) (Table 2). This indicates that the null hypothesis (H0) is accepted, suggesting no significant differences between the treatments (YIS-3 and YIS-4 bread) in terms of aroma, taste, color, and texture. This infers respondents perceived both bread samples as exhibiting similar sensory characteristics. The average scores for all attributes were approximately seven, which indicates a like moderately response from the respondents regarding the aroma, taste, color, and texture of both breads. This scoring was based on a scale where 1=dislike extremely, 2=dislike very much, 3=dislike moderately, 4=dislike slightly, 5=neither like nor dislike, 6=like slightly, 7=like moderately, 8=like very much, and 9=like extremely (Cabello-Olmo et al., 2023).
Table 2: Organoleptic analysis ( aroma, color, texture, and taste) of the bread samples
| Attribute | Descriptive statistics (Mean±SD) |
| Aroma Color Texture Taste |
| 7.63±0.882 a 7.60±0.764 a 7.43±0.945 a 7.18±0.854 a |
Means within a column followed by the same letter are not significantly different at p <0.05
The quality of bread is significantly influenced by the Maillard reaction and various other chemical processes that occur during baking. According to Starowicz (2021), the products of the Maillard reaction are diverse sensory-active compounds that play a critical role in determining key quality attributes such as aroma, taste, color, and texture. Among these attributes, aroma holds particular importance, as it is primarily generated through the production of secondary metabolites by yeast during fermentation. Supporting this, Pétel et al. (2017) identified approximately 300 secondary metabolites in bread, the majority of which are volatile compounds that can be categorized into several main groups, including alcohols, esters, and aldehydes.
In general, aroma is the result of a balanced composition of volatile compounds present in a specific product (Ouyang et al., 2017). These compounds are inhaled when a person smells the food, triggering a sensory response via the olfactory system. According to Agustina et al. (2021), aroma is perceived when volatile molecules enter the nasal cavity and are detected by the olfactory receptors. The resulting aroma is closely associated with the palatability and consumer acceptance of food products, which is largely influenced by the raw ingredients and processing methods. Therefore, aroma plays a crucial role in determining the overall quality and acceptance of food products, including bread.
Organoleptic analysis of bread fermented with two different yeast isolates showed no significant differences in the four evaluated sensory attributes: aroma, color, texture, and taste. Although variations in the volatile compound profiles were observed between the two bread samples, these differences were not sufficient to alter the panelists’ perception of the sensory attributes. This suggests that the variation in volatile compounds remained below the human sensory detection threshold. Even in the absence of perceptible differences in aroma, the analysis of volatile compounds remains essential for understanding the biochemical mechanisms that influence bread aroma quality. A comprehensive understanding of the relationship between volatile compounds and sensory perception is fundamental for the development of bread products with enhanced and consumer-preferred aroma and flavor characteristics.
This phenomenon can be attributed to various technological factors involved in bread-making, such as fermentation methods, fermentation temperature, baking temperature, the type and concentration of ingredients used, as well as the strain and population of yeast. The combination of these factors may lead to similar aroma profiles between samples, as reflected in the organoleptic results showing no significant difference in aroma. This finding aligns with the statement by Longin et al. (2020), who noted that both environmental and genetic factors during bread production may influence the aroma profile. Furthermore, Pétel et al. (2017) emphasized that the formation of volatile compounds is highly dependent on the complex interactions among ingredient types and concentrations, yeast activity during fermentation, and fermentation conditions such as time and temperature.
Molecular identification and phylogenetic analysis of yeast isolates YIS-3 and YIS-4
Molecular of the yeast strains YIS-3 and YIS-4 was identified using direct PCR method. Direct PCR involves direct addition of samples to the amplification reaction disregarding prior DNA extraction, purification, or quantification. This method optimizes the amount of target DNA, minimizes the risk of error and contamination from reagents, and reduces both the time and cost associated with the procedure. Amplification was performed on the ITS region, a non-coding segment within rDNA (Fajarningsih, 2016).
Results of PCR product visualization indicate that both yeast isolates successfully produced DNA bands of approximately 950 bp, as presented in Figure 2. This suggests that the PCR process was executed effectively. The target amplification size is typically less than 1,000 bp or 1 kbp, as DNA fragments exceeding this size are more susceptible to inhibitors that impair the activity of DNA polymerase, in addition, the migration time during electrophoresis is prolonged. A number of different factors influence the successful appearance of DNA bands during electrophoresis, including agarose gel concentration, DNA molecule size, DNA conformation, voltage, and DNA dyes. Optimizing these factors impose more distinct DNA bands (Moniri et al., 2020).
Samples successfully visualized, using gel documentation, were subsequently sequenced to determine the nucleotide sequence. The results were subsequently analyzed using sequence scanner software. A high-quality sequencing output is characterized by distinct, tall chromatograms with numerous blue nitrogenous base peaks, while a suboptimal sequence exhibits overlapping, shallow chromatograms with a predominance of red nitrogenous bases (Al-Shuhaib and Hashim, 2023). Following sequencing, alignment was conducted using the BLAST to assess the homology between the query sequences and those available in the NCBI database. The BLAST results for the two yeast isolates are presented in Table 3.
The quality of bread is significantly influenced by the Maillard reaction and various other chemical processes that occur during baking. According to Starowicz (2021), the products of the Maillard reaction are diverse sensory-active compounds that play a critical role in determining key quality attributes such as aroma, taste, color, and texture. Among these attributes, aroma holds particular importance, as it is primarily generated through the production of secondary metabolites by yeast during fermentation. Supporting this, Pétel et al. (2017) identified approximately 300 secondary metabolites in bread, the majority of which are volatile compounds that can be categorized into several main groups, including alcohols, esters, and aldehydes.
In general, aroma is the result of a balanced composition of volatile compounds present in a specific product (Ouyang et al., 2017). These compounds are inhaled when a person smells the food, triggering a sensory response via the olfactory system. According to Agustina et al. (2021), aroma is perceived when volatile molecules enter the nasal cavity and are detected by the olfactory receptors. The resulting aroma is closely associated with the palatability and consumer acceptance of food products, which is largely influenced by the raw ingredients and processing methods. Therefore, aroma plays a crucial role in determining the overall quality and acceptance of food products, including bread.
Organoleptic analysis of bread fermented with two different yeast isolates showed no significant differences in the four evaluated sensory attributes: aroma, color, texture, and taste. Although variations in the volatile compound profiles were observed between the two bread samples, these differences were not sufficient to alter the panelists’ perception of the sensory attributes. This suggests that the variation in volatile compounds remained below the human sensory detection threshold. Even in the absence of perceptible differences in aroma, the analysis of volatile compounds remains essential for understanding the biochemical mechanisms that influence bread aroma quality. A comprehensive understanding of the relationship between volatile compounds and sensory perception is fundamental for the development of bread products with enhanced and consumer-preferred aroma and flavor characteristics.
This phenomenon can be attributed to various technological factors involved in bread-making, such as fermentation methods, fermentation temperature, baking temperature, the type and concentration of ingredients used, as well as the strain and population of yeast. The combination of these factors may lead to similar aroma profiles between samples, as reflected in the organoleptic results showing no significant difference in aroma. This finding aligns with the statement by Longin et al. (2020), who noted that both environmental and genetic factors during bread production may influence the aroma profile. Furthermore, Pétel et al. (2017) emphasized that the formation of volatile compounds is highly dependent on the complex interactions among ingredient types and concentrations, yeast activity during fermentation, and fermentation conditions such as time and temperature.
Molecular identification and phylogenetic analysis of yeast isolates YIS-3 and YIS-4
Molecular of the yeast strains YIS-3 and YIS-4 was identified using direct PCR method. Direct PCR involves direct addition of samples to the amplification reaction disregarding prior DNA extraction, purification, or quantification. This method optimizes the amount of target DNA, minimizes the risk of error and contamination from reagents, and reduces both the time and cost associated with the procedure. Amplification was performed on the ITS region, a non-coding segment within rDNA (Fajarningsih, 2016).
Results of PCR product visualization indicate that both yeast isolates successfully produced DNA bands of approximately 950 bp, as presented in Figure 2. This suggests that the PCR process was executed effectively. The target amplification size is typically less than 1,000 bp or 1 kbp, as DNA fragments exceeding this size are more susceptible to inhibitors that impair the activity of DNA polymerase, in addition, the migration time during electrophoresis is prolonged. A number of different factors influence the successful appearance of DNA bands during electrophoresis, including agarose gel concentration, DNA molecule size, DNA conformation, voltage, and DNA dyes. Optimizing these factors impose more distinct DNA bands (Moniri et al., 2020).
Samples successfully visualized, using gel documentation, were subsequently sequenced to determine the nucleotide sequence. The results were subsequently analyzed using sequence scanner software. A high-quality sequencing output is characterized by distinct, tall chromatograms with numerous blue nitrogenous base peaks, while a suboptimal sequence exhibits overlapping, shallow chromatograms with a predominance of red nitrogenous bases (Al-Shuhaib and Hashim, 2023). Following sequencing, alignment was conducted using the BLAST to assess the homology between the query sequences and those available in the NCBI database. The BLAST results for the two yeast isolates are presented in Table 3.

Figure 2: Visualization of Polymerase Chain Reaction (PCR) results: (M)=1 kb marker; (A)=YIS-3 replicate 1; (B)=YIS-3 replicate 2; (C)=YIS-4 replicate 1; (D)=YIS-4 replicate 2
Tabel 3: Results of Basic Local Alignment Search Tool (BLAST) yeast isolates YIS-3 and YIS-4
| Isolate code | BLAST result | ||
| Species name | Identity (%) | Accession | |
| YIS-3 YIS-4 |
Saccharomyces cerevisiae strain XZFM13-1 S. cerevisiae starin HBUAS61689 |
95.88% 95.25% |
MW710189.1 OM348826.1 |
The results presented in Table 3 signify that the yeast isolate YIS-3 shares a significant similarity with S. cerevisiae strain XZFM13-1, exhibiting a percentage similarity of 95.88%. Similarly, yeast isolate YIS-4 presents 95.25% similarity with S. cerevisiae strain HBUAS61689. A higher percentage of similarity correlates with a greater degree of homology to the species in the NCBI database. According to Genç and Günay (2020), yeast species typically display sequence homology in the ITS rDNA region ranging between 99-100%. In contrast, lower homology values, particularly below 99%, suggest potential differences from known species. The lower sequence homology observed in isolates YIS-3 and YIS-4 compared to reference strains indicates that these isolates represent novel strains of S. cerevisiae. In addition, the obtained value may also indicate low sequence quality, artifacts resulting from the PCR process, or potential contamination with hybrid yeast strains or closely related species. To address this issue, raw sequencing data should be reanalyzed using software such as MOTHUR or USEARCH to identify possible chimeras or low quality sequences. Furthermore, Multi-Locus Sequence Typing (MLST), targeting conserved genes e.g., Translation Elongation Factor 1-alpha (TEF1) and RNA Polymerase II largest subunit (RPB1), is recommended to confirm the identity of the strain.
The phylogenetic relationships of the identified yeast strains are reflected through the construction of a phylogenetic tree. Phylogenetic tree potentially reveals the relationships between organisms based on shared characteristics, genetic backgrounds, and evolutionary connections. The phylogenetic tree is commonly depicted as a branching diagram, where branch lengths correspond to evolutionary distances. In this study, phylogenetic tree reconstruction was performed using the Neighbor-Joining (NJ) method in the MEGA X software, employing a 1,000-replicate Bootstrap analysis with the Kimura 2-parameter model (Figure 3). According to Davidson and Martín Del Campo (2020), the NJ method is a distance-based phylogenetic approach that calculates a tree metric derived from dissimilarities out of the biological data.
The phylogenetic relationships of the identified yeast strains are reflected through the construction of a phylogenetic tree. Phylogenetic tree potentially reveals the relationships between organisms based on shared characteristics, genetic backgrounds, and evolutionary connections. The phylogenetic tree is commonly depicted as a branching diagram, where branch lengths correspond to evolutionary distances. In this study, phylogenetic tree reconstruction was performed using the Neighbor-Joining (NJ) method in the MEGA X software, employing a 1,000-replicate Bootstrap analysis with the Kimura 2-parameter model (Figure 3). According to Davidson and Martín Del Campo (2020), the NJ method is a distance-based phylogenetic approach that calculates a tree metric derived from dissimilarities out of the biological data.

Figure 3: Reconstruction of phylogenetic tree for the yeast isolates YIS-3 and YIS-4
The results indicate that these two samples did not cluster within the same branch as the reference sequences (ingroup). This is presumably attributed to gaps (indicated by dashed lines) observed in the alignment results, due to the highly variable nature of the ITS region. Gaps represented mutations, either deletions or insertions, within the sequence. Additionally, the alignment revealed that the ITS region experienced rapid evolution, as evidenced by the multitude of nucleotide differences observed in specific regions across the sequences of various species. As such, the ITS region is commonly utilized for species-level identification, despite being applicable at finer taxonomic levels, such as strain identification (Park et al., 2020). Fajarningsih (2016) adds that the ITS region, being a highly polymorphic non-coding region, exhibits sufficient taxonomic resolution to distinguish sequences at the species level.
The phylogenetic tree reconstruction demonstrated that yeast isolate YIS-3 was closely related to the ancestor of S. cerevisiae strain XZFM13-1, while YIS-4 shared a close evolutionary relationship with both YIS-3 and the other S. cerevisiae strains represented in the phylogenetic tree. This relationship was supported by the proximity of the branches, distinctly separated from the outgroup sequences. Moreover, the relationship was evidenced by the BLAST results from NCBI. At the base of the phylogenetic tree, a scale with a value of 0.10 was depicted. According to Huynh et al. (2020), a phylogenetic tree with a scale bar of 0.01 signifies a genetic distance with one nucleotide change per 100 bp. Thus, a scale of 0.10 indicates 10 nucleotide changes per 100 bp.
The bootstrap values for the phylogenetic tree were observed between 71-77%. Hesham et al. (2014) suggest that species relationships are inferred from both the magnitude of bootstrap values and the alignment of the corresponding branches. The bootstrap value serves as an indicator of the robustness of the data model in the analysis. These values are classified into different categories: high (>85%), moderate (70-85%), weak (50-69%), or very weak (<50%). High bootstrap values imply that the branching patterns in the phylogenetic tree are reliable, whereas low bootstrap values indicate that the branches are less trustworthy (Katsura et al., 2017; Subari et al., 2021).
This study established that YIS-3 and YIS-4 were related to S. cerevisiae, albeit with different strains. Consistent with this finding, the volatile compounds contributing to the aroma of the bread produced by YIS-3 and YIS-4 were largely similar, despite the fact that each strain produced unique compounds, benzeneethanamine in bread from YIS-3 and o-nitrostyrene in bread from YIS-4. The variation in volatile compound production indicates specific characteristics of an organism. Yeast exhibits genetic variability that leads to differing capacities to produce a wide range of aroma compounds at varying concentrations (Synos et al., 2015).
Conclusion
Bread fermented with yeast isolates YIS-3 and
YIS-4 produced distinctive volatile compounds: benzeneethanamine in YIS-3 and o-nitrostyrene in YIS-4. Despite these differences in volatile profiles, organoleptic testing of aroma revealed no significant differences between the two bread samples, suggesting that the panelists perceived their aromas as relatively similar. This similarity may be attributed to the taxonomic proximity of the isolates, with YIS-3 showing 95.88% sequence similarity to S. cerevisiae strain XZFM13-1 and YIS-4 showing 95.25% similarity to strain HBUAS61689. Both YIS-3 and YIS-4 exhibit potential as sustainable alternatives to commercial yeast, suitable for use in both artisanal and industrial-scale bread production. These isolates could be developed as adaptive local yeast strains with sensory characteristics that are acceptable to consumers. However, the present study is limited by the scope of the bread formulations tested and the lack of further multigene analysis to ensure comprehensive taxonomic identification. Future studies are therefore recommended to explore large-scale production, the consistency of fermentation performance across diverse bread recipes, and the potential application of YIS-3 and YIS-4 in other fermented food products. Moreover, a more in-depth genetic analysis of aroma-related genes (e.g., ATF1, ADH2) is needed to elucidate the role of volatile metabolites in shaping the sensory characteristics.
Author contributions
N.K. designed the study, conducted yeast isolation, and preliminary characterization, and drafted the initial manuscript; J.K. performed molecular identification using PCR and conducted phylogenetic analysis; S.S. contributed to data interpretation; N.Z. analyzed volatile compounds using GC-MS and contributed to the interpretation of chemical data and manuscript preparation; U.U. developed yeast-based bread formulations, conducted dough fermentation trials, and evaluated the sensory and physical properties of the bread. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors express their sincere gratitude to all individuals and institutions who contributed to this research. Special thanks are extended to the students who assisted in sample collection and preliminary experiments. The authors also acknowledge the technical support provided by the laboratory team throughout the research process. We highly appreciate the constructive feedback from peer reviewers, which significantly improved the quality of this manuscript.
Funding
This study was funded by the Beginner Research
Grant Program (Penelitian Dasar Pemula) from
the Directorate of Research and Community
Service (DRPM), Universitas Brawijaya (Grant No.: 00146.36/UN10.A0501/B/PT.01.03.2/2024).
Ethical consideration
This study involving human participants (sensory evaluation) was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Universitas Negeri Malang (UM) (Approval No.: 03.10.1/UN32.14.2.8/LT/2024). All participants provided written informed consent prior to participation, and their confidentiality was strictly maintained.
Reference
Agustina R., Fadhil R., Mustaqimah. (2021). Organoleptic test using the hedonic and descriptive methods to determine the quality of Pliek U. IOP Conf. Series: Earth and Environmental Science. 644: 012006 [DOI: 10.1088/1755-1315/644/1/012006]
Al-Shuhaib M.B.S., Hashim H.O. (2023). Mastering DNA chromatogram analysis in Sanger sequencing for reliable clinical analysis. Journal of Genetic Engineering and Biotechnology. 21: 115. [DOI: 10.1186/s43141-023-00587-6]
Ardiansyah, Nada A., Rahmawati N.T.I., Oktriani A., David W., Astuti R.M.A., Handoko D.D., Kusbiantoro B., Budijanto S., Shirakawa H. (2021). Volatile compounds, sensory profile and phenolic compounds in fermented rice bran. Plants. 10: 1073. [DOI: 10.3390/plants10061073]
Astuti R.D., Fibri D.L.N., Handoko D.D., David W., Budijanto S., Shirakawa H., Ardiansyah. (2022). The volatile compounds and aroma description in various Rhizopus oligosporus solid-state fermented and nonfermented rice bran. Fermentation. 8: 120. [DOI: 10.3390/fermentation8030120]
Ben-Amar A., Oueslati S., Mliki A. (2017). Universal direct PCR amplification system: a time- and cost-effective tool for high-throughput applications. 3 Biotech. 7: 246. [DOI: 10.1007/s13205-017-0890-7]
Boltar I., Čanžek Majhenič A., Jarni K., Jug T., Bavcon Kralj M. (2015). Volatile compounds in Nanos cheese: their formation during ripening and sesonal variation. Journal of Food Science and Technology. 52: 608-623. [DOI: 10.1007/s13197-014-1565-6]
Cabello-Olmo M., Krishnan P.G., Araña M., Oneca M., Díaz J.V., Barajas M., Rovai M. (2023). Development, analysis, and sensory evaluation of improved bread fortified with a plant-based fermented food product. Foods. 12: 2817. [DOI: 10.3390/ foods12152817]
Companioni-Damas E.Y., Santos F.J., Galceran M.T. (2012). Analysis of linear and cyclic methylsiloxanes in water by headspace-solid phase microextraction and gas chromatography-mass spectrometry. Talanta. 89: 63-69. [DOI: 10.1016/j.talanta. 2011.11.058]
Davidson R., Martín Del Campo A. (2020). Combinatorial and computational investigations of neighbor-joining bias. Frontiers in Genetics. 11: 584785. [DOI: 10.3389/fgene.2020.584785]
De Luca L., Aiello A., Pizzolongo F., Blaiotta G., Aponte M., Romano R. (2021). Volatile organic compounds in breads prepared with different sourdoughs. Applied Sciences. 11: 1330. [DOI: 10.3390/app11031330]
Dong X., Hu X., Sun L., Zhang H., Wu L., Wang B. (2018). Volatile compounds of wheat flour and steamed bread as affected by wheat storage time. SM Analytical and Bioanalytical Techniques. 3: 1015. [DOI: 10.36876/smabt.1015]
Fajarningsih N.D. (2016). Internal transcribed spacer (ITS) as Dna barcoding to identify fungal species: a review. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology. 11: 37. [DOI: 10.15578/squalen.v11i2.213]
Feng B., Lin Y., Zhou L., Guo Y., Friedman R., Xia R., Hu F., Liu C., Tang J. (2017). Reconstructing yeasts phylogenies and ancestors from whole genome data. Scientific Reports. 7: 15209. [DOI: 10.1038/s41598-017-15484-5]
Gallone B., Steensels J., Prahl T., Soriaga L., Saels V., Herrera-Malaver B., Merlevede A., Roncoroni M., Voordeckers K., Miraglia L., Teiling C., Steffy B., et al. (2016). Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell. 166: 1397-1410. [DOI: 10.1016/j.cell.2016.08.020]
Genç T.T., Günay M. (2020). Internal transcribed spacer (ITS) sequence-based identification of yeast biota on pomegranate surface and determination of extracellular enzyme profile. Nusantara Bioscience. 12: 59-67. [DOI: 10.13057/ nusbiosci/ n120111]
Hazelwood L.A., Daran J.-M., Van Maris A.J.A., Pronk J.T., Dickinson J.R. (2008). The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Applied and Environmental Microbiology. 74: 2259-2266. [DOI: 10.1128/AEM.02625-07]
Hesham A.E.-L., Wambui V., Henry Ogola J.O., Maina J.M. (2014). Phylogenetic analysis of isolated biofuel yeasts based on 5.8S-ITS rDNA and D1/D2 26S rDNA sequences. Journal of Genetic Engineering and Biotechnology. 12: 37-43. [DOI: 10.1016/ j.jgeb.2014.01.001]
Huynh B.-T., Passet V., Rakotondrasoa A., Diallo T., Kerleguer A., Hennart M., De Lauzanne A., Herindrainy P., Seck A., Bercion R., Borand L., De La Gandara M.P., et al. (2020): Klebsiella pneumoniae carriage in low-income countries: antimicrobial resistance, genomic diversity and risk factors. 11: 1287-1299. Gut Microbes. [DOI: 10.1080/19490976.2020.1748257]
Iranmanesh M., Ezzatpanah H., Akbari-Adergani B., Karimi Torshizi M.A. (2018). SPME/GC-MS characterization of volatile compounds of Iranian traditional dried Kashk. International Journal of Food Properties. 21: 1067-1079. [DOI: 10.1080/10942912.2018.1466323]
Izzreen M.N.N.Q., Hansen S.S., Petersen M.A. (2016). Volatile compounds in whole meal bread crust: the effects of yeast level and fermentation temperature. Food Chemistry. 210: 566-576. [DOI: 10.1016/j.foodchem.2016.04.110]
Jackson R.S. (2008). Wine science: principles and applications. 3rd Edition. Academic Press, London.
Karimi L., Mirhendi H., Khodadadi H., Mohammadi R. (2015). Molecular identification of uncommon clinical yeast species in Iran. Current Medical Mycology. 1: 1-6. [DOI: 10.18869/ acadpub.cmm.1.2.1]
Karki T.B., Timilsina P.M., Yadav A., Pandey G.R., Joshi Y., Bhujel S., Adhikari R., Neupane K. (2017). Selection and characterization of potential baker's yeast from indigenous resources of Nepal. Biotechnology Research International. 2017. [DOI: 10.1155/2017/1925820]
Katsura Y., Stanley Jr C.E., Kumar S., Nei M. (2017). The reliability and stability of an inferred phylogenetic tree from empirical data. Molecular Biology and Evolution. 34: 718-723. [DOI: 10.1093/molbev/msw272]
Longin F., Beck H., Gütler H., Heilig W., Kleinert M., Rapp M., Philipp N., Erban A., Brilhaus D., Mettler-Altmann T., Stich B. (2020). Aroma and quality of breads baked from old and modern wheat varieties and their prediction from genomic and flour-based metabolite profiles. Food Research International. 129: 108748. [DOI: 10.1016/j.foodres.2019.108748].
Makhoul S., Romano A., Capozzi V., Spano G., Aprea E., Cappellin L., Benozzi E., Scampicchio M., Märk T.D., Gasperi F., El-Nakat H., Guzzo J., et al. (2015). Volatile compound production during the bread-making process: effect of flour, yeast and their interaction. Food and Bioprocess Technology. 8: 1925-1937. [DOI: 10.1007/s11947-015-1549-1]
Martínez-Avila O., Sánchez A., Font X., Barrena R. (2018). Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: current state and perspectives. Applied Microbiology and Biotechnology. 102: 9991-10004. [DOI: 10.1007/s00253-018-9384-8]
Mazumdar P., Pratama H., Lau S.E., Teo C.H., Harikrishna J.A. (2019). Biology, phytochemical profile and prospects for snake fruit: an antioxidant-rich fruit of South East Asia. Trends in Food Science and Technology. 91: 147-158. [DOI: 10.1016/j.tifs. 2019.06.017]
Moniri A., Miglietta L., Malpartida-Cardenas K., Pennisi I., Cacho-Soblechero M., Moser N., Holmes A., Georgiou P., Rodriguez-Manzano J. (2020). Amplification curve analysis: data-driven multiplexing using real-time digital PCR. Analytical chemistry. 92: 13134-13143. [DOI: 10.1021/acs.analchem.0c02253]
Nakamura T., Tomita S., Saito K. (2018). Metabolite profiling in dough during fermentation. Food Science and Technology Research. 24: 509-517. [DOI: 10.3136/fstr.24.509]. [Japanese with English abdtract]
Ogunremi O.R., Agrawal R., Sanni A. (2020). Production and characterization of volatile compounds and phytase from potentially probiotic yeasts isolated from traditional fermented cereal foods in Nigeria. Journal of Genetic Engineering and Biotechnology. 18: 16. [DOI: 10.1186/s43141-020-00031-z]
Olaniran A.O., Hiralal L., Mokoena M.P., Pillay B. (2017). Flavour-active volatile compounds in beer: production, regulation and control. Journal of the Institute of Brewing. 123: 13-23. [DOI: 10.1002/jib.389]
Ouyang X., Yuan G., Ren J., Wang L., Wang M., Li Y., Zhang B., Zhu B. (2017). Aromatic compounds and organoleptic features of fermented wolfberry wine: effects of maceration time. International Journal of Food Properties. 20: 2234-2248. [DOI: 10.1080/10942912.2016.1233435]
Park H., Park J.H., Jeon H.H., Woo D.U., Lee Y., Kang Y.J. (2020). Characterization of the complete chloroplast genome sequence of Wolffia globosa (Lemnoideae) and its phylogenetic relationships to other Araceae family. Mitochondrial DNA Part B. 5: 1905-1907. [DOI: 10.1080/23802359.2020.1754948]
Park M.K., Kim Y.-S. (2019). Distinctive formation of volatile compounds in fermented rice inoculated by different molds, yeasts, and lactic acid bacteria. Molecules. 24: 2123. [DOI: 10.3390/molecules24112123]
Pavlovica S., Gaidule A., Zicmanis A. (2014). Synthesis of β-nitrostyrene in highly hydrophilic ionic liquid media. Latvian Journal of Chemistry. 52: 49-53. [DOI: 10.2478/ljc-2013-0005]
Pétel C., Onno B., Prost C. (2017). Sourdough volatile compounds and their contribution to bread: a review. Trends in Food Science and Technology. 59: 105-123. [DOI: 10.1016/j.tifs.2016.10.015]
Pico J., Antolín B., Román L., Gómez M., Bernal J. (2018). Analysis of volatile compounds in gluten-free bread crusts with an optimised and validated SPME-GC/QTOF methodology. Food Research International. 106: 686-695. [DOI: 10.1016/ j.foodres.2018.01.048]
Poveda J.M., Sánchez-Palomo E., Pérez-Coello M.S., Cabezas L. (2008). Volatile composition, olfactometry profile and sensory evaluation of semi-hard Spanish goat cheeses. Dairy Science and Technology. 88: 355-367. [DOI: 10.1051/dst:2007021]
Saerens S.M.G., Delvaux F.R., Verstrepen K.J., Thevelein J.M. (2010). Production and biological function of volatile esters in Saccharomyces cerevisiae. Microbial Biotechnology. 3: 165-177. [DOI: 10.1111/j.1751-7915.2009.00106.x]
Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Fungal Barcoding Consortium. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences. 109: 6241-6246. [DOI: 10.1073/pnas.1117018109]
Starowicz M. (2021). Analysis of volatiles in food products. Separations. 8: 157. [DOI: 10.3390/separations8090157]
Subari A., Razak A., Sumarmin R. (2021). Phylogenetic analysis of Rasbora spp. based on the mitochondrial DNA COI gene in Harapan forest. Jurnal Biologi Tropis. 21: 89-94. [DOI: 10.29303/jbt.v21i1.2351]
Synos K., Reynolds A.G., Bowen A.J. (2015). Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT - Food Science and Technology. 64: 227-235. [DOI: 10.1016/j.lwt. 2015.05.044]
Wailzer B., Kocker J., Wolschann P., Buchbauer G. (2016). Structural features for furan-derived fruity and meaty aroma impressions. Natural Product Communications. 11: 1475-1479. [DOI: 10.1177/1934578X1601101014]
Watanabe M., Uchida N., Fujita K., Yoshino T., Sakaguchi T. (2016). Bread and effervescent beverage productions with local microbes for the local revitalization. International Journal on Advanced Science, Engineering and Information Technology. 6: 381-384. [DOI: 10.18517/ijaseit.6.3.841]
Zahroh N., Utami U., Kusmiyati N. (2022). Effect of nitrogen source on growth endophytic yeast from Salacca edulis reinw. and bread quality analysis. Jurnal Biodjati. 7: 95-108. [DOI: 10.15575/biodjati.v7i1.16854]
Zhang L., Mi S., Liu R.-B., Sang Y.-X., Wang X.-H. (2020). Evaluation of volatile compounds during the fermentation process of yogurts by Streptococcus thermophilus based on odor activity value and heat map analysis. International Journal of Analytical Chemistry. 2020: 3242854. [DOI: 10.1155/2020/ 3242854]
The phylogenetic tree reconstruction demonstrated that yeast isolate YIS-3 was closely related to the ancestor of S. cerevisiae strain XZFM13-1, while YIS-4 shared a close evolutionary relationship with both YIS-3 and the other S. cerevisiae strains represented in the phylogenetic tree. This relationship was supported by the proximity of the branches, distinctly separated from the outgroup sequences. Moreover, the relationship was evidenced by the BLAST results from NCBI. At the base of the phylogenetic tree, a scale with a value of 0.10 was depicted. According to Huynh et al. (2020), a phylogenetic tree with a scale bar of 0.01 signifies a genetic distance with one nucleotide change per 100 bp. Thus, a scale of 0.10 indicates 10 nucleotide changes per 100 bp.
The bootstrap values for the phylogenetic tree were observed between 71-77%. Hesham et al. (2014) suggest that species relationships are inferred from both the magnitude of bootstrap values and the alignment of the corresponding branches. The bootstrap value serves as an indicator of the robustness of the data model in the analysis. These values are classified into different categories: high (>85%), moderate (70-85%), weak (50-69%), or very weak (<50%). High bootstrap values imply that the branching patterns in the phylogenetic tree are reliable, whereas low bootstrap values indicate that the branches are less trustworthy (Katsura et al., 2017; Subari et al., 2021).
This study established that YIS-3 and YIS-4 were related to S. cerevisiae, albeit with different strains. Consistent with this finding, the volatile compounds contributing to the aroma of the bread produced by YIS-3 and YIS-4 were largely similar, despite the fact that each strain produced unique compounds, benzeneethanamine in bread from YIS-3 and o-nitrostyrene in bread from YIS-4. The variation in volatile compound production indicates specific characteristics of an organism. Yeast exhibits genetic variability that leads to differing capacities to produce a wide range of aroma compounds at varying concentrations (Synos et al., 2015).
Conclusion
Bread fermented with yeast isolates YIS-3 and
YIS-4 produced distinctive volatile compounds: benzeneethanamine in YIS-3 and o-nitrostyrene in YIS-4. Despite these differences in volatile profiles, organoleptic testing of aroma revealed no significant differences between the two bread samples, suggesting that the panelists perceived their aromas as relatively similar. This similarity may be attributed to the taxonomic proximity of the isolates, with YIS-3 showing 95.88% sequence similarity to S. cerevisiae strain XZFM13-1 and YIS-4 showing 95.25% similarity to strain HBUAS61689. Both YIS-3 and YIS-4 exhibit potential as sustainable alternatives to commercial yeast, suitable for use in both artisanal and industrial-scale bread production. These isolates could be developed as adaptive local yeast strains with sensory characteristics that are acceptable to consumers. However, the present study is limited by the scope of the bread formulations tested and the lack of further multigene analysis to ensure comprehensive taxonomic identification. Future studies are therefore recommended to explore large-scale production, the consistency of fermentation performance across diverse bread recipes, and the potential application of YIS-3 and YIS-4 in other fermented food products. Moreover, a more in-depth genetic analysis of aroma-related genes (e.g., ATF1, ADH2) is needed to elucidate the role of volatile metabolites in shaping the sensory characteristics.
Author contributions
N.K. designed the study, conducted yeast isolation, and preliminary characterization, and drafted the initial manuscript; J.K. performed molecular identification using PCR and conducted phylogenetic analysis; S.S. contributed to data interpretation; N.Z. analyzed volatile compounds using GC-MS and contributed to the interpretation of chemical data and manuscript preparation; U.U. developed yeast-based bread formulations, conducted dough fermentation trials, and evaluated the sensory and physical properties of the bread. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors express their sincere gratitude to all individuals and institutions who contributed to this research. Special thanks are extended to the students who assisted in sample collection and preliminary experiments. The authors also acknowledge the technical support provided by the laboratory team throughout the research process. We highly appreciate the constructive feedback from peer reviewers, which significantly improved the quality of this manuscript.
Funding
This study was funded by the Beginner Research
Grant Program (Penelitian Dasar Pemula) from
the Directorate of Research and Community
Service (DRPM), Universitas Brawijaya (Grant No.: 00146.36/UN10.A0501/B/PT.01.03.2/2024).
Ethical consideration
This study involving human participants (sensory evaluation) was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Universitas Negeri Malang (UM) (Approval No.: 03.10.1/UN32.14.2.8/LT/2024). All participants provided written informed consent prior to participation, and their confidentiality was strictly maintained.
Reference
Agustina R., Fadhil R., Mustaqimah. (2021). Organoleptic test using the hedonic and descriptive methods to determine the quality of Pliek U. IOP Conf. Series: Earth and Environmental Science. 644: 012006 [DOI: 10.1088/1755-1315/644/1/012006]
Al-Shuhaib M.B.S., Hashim H.O. (2023). Mastering DNA chromatogram analysis in Sanger sequencing for reliable clinical analysis. Journal of Genetic Engineering and Biotechnology. 21: 115. [DOI: 10.1186/s43141-023-00587-6]
Ardiansyah, Nada A., Rahmawati N.T.I., Oktriani A., David W., Astuti R.M.A., Handoko D.D., Kusbiantoro B., Budijanto S., Shirakawa H. (2021). Volatile compounds, sensory profile and phenolic compounds in fermented rice bran. Plants. 10: 1073. [DOI: 10.3390/plants10061073]
Astuti R.D., Fibri D.L.N., Handoko D.D., David W., Budijanto S., Shirakawa H., Ardiansyah. (2022). The volatile compounds and aroma description in various Rhizopus oligosporus solid-state fermented and nonfermented rice bran. Fermentation. 8: 120. [DOI: 10.3390/fermentation8030120]
Ben-Amar A., Oueslati S., Mliki A. (2017). Universal direct PCR amplification system: a time- and cost-effective tool for high-throughput applications. 3 Biotech. 7: 246. [DOI: 10.1007/s13205-017-0890-7]
Boltar I., Čanžek Majhenič A., Jarni K., Jug T., Bavcon Kralj M. (2015). Volatile compounds in Nanos cheese: their formation during ripening and sesonal variation. Journal of Food Science and Technology. 52: 608-623. [DOI: 10.1007/s13197-014-1565-6]
Cabello-Olmo M., Krishnan P.G., Araña M., Oneca M., Díaz J.V., Barajas M., Rovai M. (2023). Development, analysis, and sensory evaluation of improved bread fortified with a plant-based fermented food product. Foods. 12: 2817. [DOI: 10.3390/ foods12152817]
Companioni-Damas E.Y., Santos F.J., Galceran M.T. (2012). Analysis of linear and cyclic methylsiloxanes in water by headspace-solid phase microextraction and gas chromatography-mass spectrometry. Talanta. 89: 63-69. [DOI: 10.1016/j.talanta. 2011.11.058]
Davidson R., Martín Del Campo A. (2020). Combinatorial and computational investigations of neighbor-joining bias. Frontiers in Genetics. 11: 584785. [DOI: 10.3389/fgene.2020.584785]
De Luca L., Aiello A., Pizzolongo F., Blaiotta G., Aponte M., Romano R. (2021). Volatile organic compounds in breads prepared with different sourdoughs. Applied Sciences. 11: 1330. [DOI: 10.3390/app11031330]
Dong X., Hu X., Sun L., Zhang H., Wu L., Wang B. (2018). Volatile compounds of wheat flour and steamed bread as affected by wheat storage time. SM Analytical and Bioanalytical Techniques. 3: 1015. [DOI: 10.36876/smabt.1015]
Fajarningsih N.D. (2016). Internal transcribed spacer (ITS) as Dna barcoding to identify fungal species: a review. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology. 11: 37. [DOI: 10.15578/squalen.v11i2.213]
Feng B., Lin Y., Zhou L., Guo Y., Friedman R., Xia R., Hu F., Liu C., Tang J. (2017). Reconstructing yeasts phylogenies and ancestors from whole genome data. Scientific Reports. 7: 15209. [DOI: 10.1038/s41598-017-15484-5]
Gallone B., Steensels J., Prahl T., Soriaga L., Saels V., Herrera-Malaver B., Merlevede A., Roncoroni M., Voordeckers K., Miraglia L., Teiling C., Steffy B., et al. (2016). Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell. 166: 1397-1410. [DOI: 10.1016/j.cell.2016.08.020]
Genç T.T., Günay M. (2020). Internal transcribed spacer (ITS) sequence-based identification of yeast biota on pomegranate surface and determination of extracellular enzyme profile. Nusantara Bioscience. 12: 59-67. [DOI: 10.13057/ nusbiosci/ n120111]
Hazelwood L.A., Daran J.-M., Van Maris A.J.A., Pronk J.T., Dickinson J.R. (2008). The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Applied and Environmental Microbiology. 74: 2259-2266. [DOI: 10.1128/AEM.02625-07]
Hesham A.E.-L., Wambui V., Henry Ogola J.O., Maina J.M. (2014). Phylogenetic analysis of isolated biofuel yeasts based on 5.8S-ITS rDNA and D1/D2 26S rDNA sequences. Journal of Genetic Engineering and Biotechnology. 12: 37-43. [DOI: 10.1016/ j.jgeb.2014.01.001]
Huynh B.-T., Passet V., Rakotondrasoa A., Diallo T., Kerleguer A., Hennart M., De Lauzanne A., Herindrainy P., Seck A., Bercion R., Borand L., De La Gandara M.P., et al. (2020): Klebsiella pneumoniae carriage in low-income countries: antimicrobial resistance, genomic diversity and risk factors. 11: 1287-1299. Gut Microbes. [DOI: 10.1080/19490976.2020.1748257]
Iranmanesh M., Ezzatpanah H., Akbari-Adergani B., Karimi Torshizi M.A. (2018). SPME/GC-MS characterization of volatile compounds of Iranian traditional dried Kashk. International Journal of Food Properties. 21: 1067-1079. [DOI: 10.1080/10942912.2018.1466323]
Izzreen M.N.N.Q., Hansen S.S., Petersen M.A. (2016). Volatile compounds in whole meal bread crust: the effects of yeast level and fermentation temperature. Food Chemistry. 210: 566-576. [DOI: 10.1016/j.foodchem.2016.04.110]
Jackson R.S. (2008). Wine science: principles and applications. 3rd Edition. Academic Press, London.
Karimi L., Mirhendi H., Khodadadi H., Mohammadi R. (2015). Molecular identification of uncommon clinical yeast species in Iran. Current Medical Mycology. 1: 1-6. [DOI: 10.18869/ acadpub.cmm.1.2.1]
Karki T.B., Timilsina P.M., Yadav A., Pandey G.R., Joshi Y., Bhujel S., Adhikari R., Neupane K. (2017). Selection and characterization of potential baker's yeast from indigenous resources of Nepal. Biotechnology Research International. 2017. [DOI: 10.1155/2017/1925820]
Katsura Y., Stanley Jr C.E., Kumar S., Nei M. (2017). The reliability and stability of an inferred phylogenetic tree from empirical data. Molecular Biology and Evolution. 34: 718-723. [DOI: 10.1093/molbev/msw272]
Longin F., Beck H., Gütler H., Heilig W., Kleinert M., Rapp M., Philipp N., Erban A., Brilhaus D., Mettler-Altmann T., Stich B. (2020). Aroma and quality of breads baked from old and modern wheat varieties and their prediction from genomic and flour-based metabolite profiles. Food Research International. 129: 108748. [DOI: 10.1016/j.foodres.2019.108748].
Makhoul S., Romano A., Capozzi V., Spano G., Aprea E., Cappellin L., Benozzi E., Scampicchio M., Märk T.D., Gasperi F., El-Nakat H., Guzzo J., et al. (2015). Volatile compound production during the bread-making process: effect of flour, yeast and their interaction. Food and Bioprocess Technology. 8: 1925-1937. [DOI: 10.1007/s11947-015-1549-1]
Martínez-Avila O., Sánchez A., Font X., Barrena R. (2018). Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: current state and perspectives. Applied Microbiology and Biotechnology. 102: 9991-10004. [DOI: 10.1007/s00253-018-9384-8]
Mazumdar P., Pratama H., Lau S.E., Teo C.H., Harikrishna J.A. (2019). Biology, phytochemical profile and prospects for snake fruit: an antioxidant-rich fruit of South East Asia. Trends in Food Science and Technology. 91: 147-158. [DOI: 10.1016/j.tifs. 2019.06.017]
Moniri A., Miglietta L., Malpartida-Cardenas K., Pennisi I., Cacho-Soblechero M., Moser N., Holmes A., Georgiou P., Rodriguez-Manzano J. (2020). Amplification curve analysis: data-driven multiplexing using real-time digital PCR. Analytical chemistry. 92: 13134-13143. [DOI: 10.1021/acs.analchem.0c02253]
Nakamura T., Tomita S., Saito K. (2018). Metabolite profiling in dough during fermentation. Food Science and Technology Research. 24: 509-517. [DOI: 10.3136/fstr.24.509]. [Japanese with English abdtract]
Ogunremi O.R., Agrawal R., Sanni A. (2020). Production and characterization of volatile compounds and phytase from potentially probiotic yeasts isolated from traditional fermented cereal foods in Nigeria. Journal of Genetic Engineering and Biotechnology. 18: 16. [DOI: 10.1186/s43141-020-00031-z]
Olaniran A.O., Hiralal L., Mokoena M.P., Pillay B. (2017). Flavour-active volatile compounds in beer: production, regulation and control. Journal of the Institute of Brewing. 123: 13-23. [DOI: 10.1002/jib.389]
Ouyang X., Yuan G., Ren J., Wang L., Wang M., Li Y., Zhang B., Zhu B. (2017). Aromatic compounds and organoleptic features of fermented wolfberry wine: effects of maceration time. International Journal of Food Properties. 20: 2234-2248. [DOI: 10.1080/10942912.2016.1233435]
Park H., Park J.H., Jeon H.H., Woo D.U., Lee Y., Kang Y.J. (2020). Characterization of the complete chloroplast genome sequence of Wolffia globosa (Lemnoideae) and its phylogenetic relationships to other Araceae family. Mitochondrial DNA Part B. 5: 1905-1907. [DOI: 10.1080/23802359.2020.1754948]
Park M.K., Kim Y.-S. (2019). Distinctive formation of volatile compounds in fermented rice inoculated by different molds, yeasts, and lactic acid bacteria. Molecules. 24: 2123. [DOI: 10.3390/molecules24112123]
Pavlovica S., Gaidule A., Zicmanis A. (2014). Synthesis of β-nitrostyrene in highly hydrophilic ionic liquid media. Latvian Journal of Chemistry. 52: 49-53. [DOI: 10.2478/ljc-2013-0005]
Pétel C., Onno B., Prost C. (2017). Sourdough volatile compounds and their contribution to bread: a review. Trends in Food Science and Technology. 59: 105-123. [DOI: 10.1016/j.tifs.2016.10.015]
Pico J., Antolín B., Román L., Gómez M., Bernal J. (2018). Analysis of volatile compounds in gluten-free bread crusts with an optimised and validated SPME-GC/QTOF methodology. Food Research International. 106: 686-695. [DOI: 10.1016/ j.foodres.2018.01.048]
Poveda J.M., Sánchez-Palomo E., Pérez-Coello M.S., Cabezas L. (2008). Volatile composition, olfactometry profile and sensory evaluation of semi-hard Spanish goat cheeses. Dairy Science and Technology. 88: 355-367. [DOI: 10.1051/dst:2007021]
Saerens S.M.G., Delvaux F.R., Verstrepen K.J., Thevelein J.M. (2010). Production and biological function of volatile esters in Saccharomyces cerevisiae. Microbial Biotechnology. 3: 165-177. [DOI: 10.1111/j.1751-7915.2009.00106.x]
Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Fungal Barcoding Consortium. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences. 109: 6241-6246. [DOI: 10.1073/pnas.1117018109]
Starowicz M. (2021). Analysis of volatiles in food products. Separations. 8: 157. [DOI: 10.3390/separations8090157]
Subari A., Razak A., Sumarmin R. (2021). Phylogenetic analysis of Rasbora spp. based on the mitochondrial DNA COI gene in Harapan forest. Jurnal Biologi Tropis. 21: 89-94. [DOI: 10.29303/jbt.v21i1.2351]
Synos K., Reynolds A.G., Bowen A.J. (2015). Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT - Food Science and Technology. 64: 227-235. [DOI: 10.1016/j.lwt. 2015.05.044]
Wailzer B., Kocker J., Wolschann P., Buchbauer G. (2016). Structural features for furan-derived fruity and meaty aroma impressions. Natural Product Communications. 11: 1475-1479. [DOI: 10.1177/1934578X1601101014]
Watanabe M., Uchida N., Fujita K., Yoshino T., Sakaguchi T. (2016). Bread and effervescent beverage productions with local microbes for the local revitalization. International Journal on Advanced Science, Engineering and Information Technology. 6: 381-384. [DOI: 10.18517/ijaseit.6.3.841]
Zahroh N., Utami U., Kusmiyati N. (2022). Effect of nitrogen source on growth endophytic yeast from Salacca edulis reinw. and bread quality analysis. Jurnal Biodjati. 7: 95-108. [DOI: 10.15575/biodjati.v7i1.16854]
Zhang L., Mi S., Liu R.-B., Sang Y.-X., Wang X.-H. (2020). Evaluation of volatile compounds during the fermentation process of yogurts by Streptococcus thermophilus based on odor activity value and heat map analysis. International Journal of Analytical Chemistry. 2020: 3242854. [DOI: 10.1155/2020/ 3242854]
*Corresponding author (N. Kusmiyati)
* E-mail: nurkusmiyati@ub.ac.id
ORCID ID: https://orcid.org/0000-0003-3368-5723
* E-mail: nurkusmiyati@ub.ac.id
ORCID ID: https://orcid.org/0000-0003-3368-5723
Type of Study: Original article |
Subject:
Special
Received: 24/12/04 | Accepted: 25/08/25 | Published: 25/09/30
Received: 24/12/04 | Accepted: 25/08/25 | Published: 25/09/30
References
1. Agustina R., Fadhil R., Mustaqimah. (2021). Organoleptic test using the hedonic and descriptive methods to determine the quality of Pliek U. IOP Conf. Series: Earth and Environmental Science. 644: 012006 [DOI: 10.1088/1755-1315/644/1/012006] [DOI:10.1088/1755-1315/644/1/012013]
2. Al-Shuhaib M.B.S., Hashim H.O. (2023). Mastering DNA chromatogram analysis in Sanger sequencing for reliable clinical analysis. Journal of Genetic Engineering and Biotechnology. 21: 115. [DOI: 10.1186/s43141-023-00587-6] [DOI:10.1186/s43141-023-00587-6] [PMID] [PMCID]
3. Ardiansyah, Nada A., Rahmawati N.T.I., Oktriani A., David W., Astuti R.M.A., Handoko D.D., Kusbiantoro B., Budijanto S., Shirakawa H. (2021). Volatile compounds, sensory profile and phenolic compounds in fermented rice bran. Plants. 10: 1073. [DOI: 10.3390/plants10061073] [DOI:10.3390/plants10061073] [PMID] [PMCID]
4. Astuti R.D., Fibri D.L.N., Handoko D.D., David W., Budijanto S., Shirakawa H., Ardiansyah. (2022). The volatile compounds and aroma description in various Rhizopus oligosporus solid-state fermented and nonfermented rice bran. Fermentation. 8: 120. [DOI: 10.3390/fermentation8030120] [DOI:10.3390/fermentation8030120]
5. Ben-Amar A., Oueslati S., Mliki A. (2017). Universal direct PCR amplification system: a time- and cost-effective tool for high-throughput applications. 3 Biotech. 7: 246. [DOI: 10.1007/s13205-017-0890-7] [DOI:10.1007/s13205-017-0890-7] [PMID] [PMCID]
6. Boltar I., Čanžek Majhenič A., Jarni K., Jug T., Bavcon Kralj M. (2015). Volatile compounds in Nanos cheese: their formation during ripening and sesonal variation. Journal of Food Science and Technology. 52: 608-623. [DOI: 10.1007/s13197-014-1565-6] [DOI:10.1007/s13197-014-1565-6]
7. Cabello-Olmo M., Krishnan P.G., Araña M., Oneca M., Díaz J.V., Barajas M., Rovai M. (2023). Development, analysis, and sensory evaluation of improved bread fortified with a plant-based fermented food product. Foods. 12: 2817. [DOI: 10.3390/ foods12152817] [DOI:10.3390/foods12152817] [PMID] [PMCID]
8. Companioni-Damas E.Y., Santos F.J., Galceran M.T. (2012). Analysis of linear and cyclic methylsiloxanes in water by headspace-solid phase microextraction and gas chromatography-mass spectrometry. Talanta. 89: 63-69. [DOI: 10.1016/j.talanta. 2011.11.058] [DOI:10.1016/j.talanta.2011.11.058] [PMID]
9. Davidson R., Martín Del Campo A. (2020). Combinatorial and computational investigations of neighbor-joining bias. Frontiers in Genetics. 11: 584785. [DOI: 10.3389/fgene.2020.584785] [DOI:10.3389/fgene.2020.584785] [PMID] [PMCID]
10. De Luca L., Aiello A., Pizzolongo F., Blaiotta G., Aponte M., Romano R. (2021). Volatile organic compounds in breads prepared with different sourdoughs. Applied Sciences. 11: 1330. [DOI: 10.3390/app11031330] [DOI:10.3390/app11031330]
11. Dong X., Hu X., Sun L., Zhang H., Wu L., Wang B. (2018). Volatile compounds of wheat flour and steamed bread as affected by wheat storage time. SM Analytical and Bioanalytical Techniques. 3: 1015. [DOI: 10.36876/smabt.1015] [DOI:10.36876/smabt.1015]
12. Fajarningsih N.D. (2016). Internal transcribed spacer (ITS) as Dna barcoding to identify fungal species: a review. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology. 11: 37. [DOI: 10.15578/squalen.v11i2.213] [DOI:10.15578/squalen.v11i2.213]
13. Feng B., Lin Y., Zhou L., Guo Y., Friedman R., Xia R., Hu F., Liu C., Tang J. (2017). Reconstructing yeasts phylogenies and ancestors from whole genome data. Scientific Reports. 7: 15209. [DOI: 10.1038/s41598-017-15484-5] [DOI:10.1038/s41598-017-15484-5] [PMID] [PMCID]
14. Gallone B., Steensels J., Prahl T., Soriaga L., Saels V., Herrera-Malaver B., Merlevede A., Roncoroni M., Voordeckers K., Miraglia L., Teiling C., Steffy B., et al. (2016). Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell. 166: 1397-1410. [DOI: 10.1016/j.cell.2016.08.020] [DOI:10.1016/j.cell.2016.08.020] [PMID] [PMCID]
15. Genç T.T., Günay M. (2020). Internal transcribed spacer (ITS) sequence-based identification of yeast biota on pomegranate surface and determination of extracellular enzyme profile. Nusantara Bioscience. 12: 59-67. [DOI: 10.13057/ nusbiosci/ n120111] [DOI:10.13057/nusbiosci/n120111]
16. Hazelwood L.A., Daran J.-M., Van Maris A.J.A., Pronk J.T., Dickinson J.R. (2008). The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Applied and Environmental Microbiology. 74: 2259-2266. [DOI: 10.1128/AEM.02625-07] [DOI:10.1128/AEM.02625-07] [PMID] [PMCID]
17. Hesham A.E.-L., Wambui V., Henry Ogola J.O., Maina J.M. (2014). Phylogenetic analysis of isolated biofuel yeasts based on 5.8S-ITS rDNA and D1/D2 26S rDNA sequences. Journal of Genetic Engineering and Biotechnology. 12: 37-43. [DOI: 10.1016/ j.jgeb.2014.01.001] [DOI:10.1016/j.jgeb.2014.01.001]
18. Huynh B.-T., Passet V., Rakotondrasoa A., Diallo T., Kerleguer A., Hennart M., De Lauzanne A., Herindrainy P., Seck A., Bercion R., Borand L., De La Gandara M.P., et al. (2020): Klebsiella pneumoniae carriage in low-income countries: antimicrobial resistance, genomic diversity and risk factors. 11: 1287-1299. Gut Microbes. [DOI: 10.1080/19490976.2020.1748257] [DOI:10.1080/19490976.2020.1748257] [PMID] [PMCID]
19. Iranmanesh M., Ezzatpanah H., Akbari-Adergani B., Karimi Torshizi M.A. (2018). SPME/GC-MS characterization of volatile compounds of Iranian traditional dried Kashk. International Journal of Food Properties. 21: 1067-1079. [DOI: 10.1080/10942912.2018.1466323] [DOI:10.1080/10942912.2018.1466323]
20. Izzreen M.N.N.Q., Hansen S.S., Petersen M.A. (2016). Volatile compounds in whole meal bread crust: the effects of yeast level and fermentation temperature. Food Chemistry. 210: 566-576. [DOI: 10.1016/j.foodchem.2016.04.110] [DOI:10.1016/j.foodchem.2016.04.110] [PMID]
21. Jackson R.S. (2008). Wine science: principles and applications. 3rd Edition. Academic Press, London.
22. Karimi L., Mirhendi H., Khodadadi H., Mohammadi R. (2015). Molecular identification of uncommon clinical yeast species in Iran. Current Medical Mycology. 1: 1-6. [DOI: 10.18869/ acadpub.cmm.1.2.1] [DOI:10.18869/acadpub.cmm.1.2.1] [PMID] [PMCID]
23. Karki T.B., Timilsina P.M., Yadav A., Pandey G.R., Joshi Y., Bhujel S., Adhikari R., Neupane K. (2017). Selection and characterization of potential baker's yeast from indigenous resources of Nepal. Biotechnology Research International. 2017. [DOI: 10.1155/2017/1925820] [DOI:10.1155/2017/1925820] [PMID] [PMCID]
24. Katsura Y., Stanley Jr C.E., Kumar S., Nei M. (2017). The reliability and stability of an inferred phylogenetic tree from empirical data. Molecular Biology and Evolution. 34: 718-723. [DOI: 10.1093/molbev/msw272] [DOI:10.1093/molbev/msw272] [PMID] [PMCID]
25. Longin F., Beck H., Gütler H., Heilig W., Kleinert M., Rapp M., Philipp N., Erban A., Brilhaus D., Mettler-Altmann T., Stich B. (2020). Aroma and quality of breads baked from old and modern wheat varieties and their prediction from genomic and flour-based metabolite profiles. Food Research International. 129: 108748. [DOI: 10.1016/j.foodres.2019.108748]. [DOI:10.1016/j.foodres.2019.108748] [PMID]
26. Makhoul S., Romano A., Capozzi V., Spano G., Aprea E., Cappellin L., Benozzi E., Scampicchio M., Märk T.D., Gasperi F., El-Nakat H., Guzzo J., et al. (2015). Volatile compound production during the bread-making process: effect of flour, yeast and their interaction. Food and Bioprocess Technology. 8: 1925-1937. [DOI: 10.1007/s11947-015-1549-1] [DOI:10.1007/s11947-015-1549-1]
27. Martínez-Avila O., Sánchez A., Font X., Barrena R. (2018). Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: current state and perspectives. Applied Microbiology and Biotechnology. 102: 9991-10004. [DOI: 10.1007/s00253-018-9384-8] [DOI:10.1007/s00253-018-9384-8] [PMID]
28. Mazumdar P., Pratama H., Lau S.E., Teo C.H., Harikrishna J.A. (2019). Biology, phytochemical profile and prospects for snake fruit: an antioxidant-rich fruit of South East Asia. Trends in Food Science and Technology. 91: 147-158. [DOI: 10.1016/j.tifs. 2019.06.017] [DOI:10.1016/j.tifs.2019.06.017]
29. Moniri A., Miglietta L., Malpartida-Cardenas K., Pennisi I., Cacho-Soblechero M., Moser N., Holmes A., Georgiou P., Rodriguez-Manzano J. (2020). Amplification curve analysis: data-driven multiplexing using real-time digital PCR. Analytical chemistry. 92: 13134-13143. [DOI: 10.1021/acs.analchem.0c02253] [DOI:10.1021/acs.analchem.0c02253] [PMID]
30. Nakamura T., Tomita S., Saito K. (2018). Metabolite profiling in dough during fermentation. Food Science and Technology Research. 24: 509-517. [DOI: 10.3136/fstr.24.509]. [Japanese with English abdtract] [DOI:10.3136/fstr.24.509]
31. Ogunremi O.R., Agrawal R., Sanni A. (2020). Production and characterization of volatile compounds and phytase from potentially probiotic yeasts isolated from traditional fermented cereal foods in Nigeria. Journal of Genetic Engineering and Biotechnology. 18: 16. [DOI: 10.1186/s43141-020-00031-z] [DOI:10.1186/s43141-020-00031-z] [PMID] [PMCID]
32. Olaniran A.O., Hiralal L., Mokoena M.P., Pillay B. (2017). Flavour-active volatile compounds in beer: production, regulation and control. Journal of the Institute of Brewing. 123: 13-23. [DOI: 10.1002/jib.389] [DOI:10.1002/jib.389]
33. Ouyang X., Yuan G., Ren J., Wang L., Wang M., Li Y., Zhang B., Zhu B. (2017). Aromatic compounds and organoleptic features of fermented wolfberry wine: effects of maceration time. International Journal of Food Properties. 20: 2234-2248. [DOI: 10.1080/10942912.2016.1233435] [DOI:10.1080/10942912.2016.1233435]
34. Park H., Park J.H., Jeon H.H., Woo D.U., Lee Y., Kang Y.J. (2020). Characterization of the complete chloroplast genome sequence of Wolffia globosa (Lemnoideae) and its phylogenetic relationships to other Araceae family. Mitochondrial DNA Part B. 5: 1905-1907. [DOI: 10.1080/23802359.2020.1754948] [DOI:10.1080/23802359.2020.1754948]
35. Park M.K., Kim Y.-S. (2019). Distinctive formation of volatile compounds in fermented rice inoculated by different molds, yeasts, and lactic acid bacteria. Molecules. 24: 2123. [DOI: 10.3390/molecules24112123] [DOI:10.3390/molecules24112123] [PMID] [PMCID]
36. Pavlovica S., Gaidule A., Zicmanis A. (2014). Synthesis of β-nitrostyrene in highly hydrophilic ionic liquid media. Latvian Journal of Chemistry. 52: 49-53. [DOI: 10.2478/ljc-2013-0005] [DOI:10.2478/ljc-2013-0005]
37. Pétel C., Onno B., Prost C. (2017). Sourdough volatile compounds and their contribution to bread: a review. Trends in Food Science and Technology. 59: 105-123. [DOI: 10.1016/j.tifs.2016.10.015] [DOI:10.1016/j.tifs.2016.10.015]
38. Pico J., Antolín B., Román L., Gómez M., Bernal J. (2018). Analysis of volatile compounds in gluten-free bread crusts with an optimised and validated SPME-GC/QTOF methodology. Food Research International. 106: 686-695. [DOI: 10.1016/ j.foodres.2018.01.048] [DOI:10.1016/j.foodres.2018.01.048] [PMID]
39. Poveda J.M., Sánchez-Palomo E., Pérez-Coello M.S., Cabezas L. (2008). Volatile composition, olfactometry profile and sensory evaluation of semi-hard Spanish goat cheeses. Dairy Science and Technology. 88: 355-367. [DOI: 10.1051/dst:2007021] [DOI:10.1051/dst:2007021]
40. Saerens S.M.G., Delvaux F.R., Verstrepen K.J., Thevelein J.M. (2010). Production and biological function of volatile esters in Saccharomyces cerevisiae. Microbial Biotechnology. 3: 165-177. [DOI: 10.1111/j.1751-7915.2009.00106.x] [DOI:10.1111/j.1751-7915.2009.00106.x] [PMID] [PMCID]
41. Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Fungal Barcoding Consortium. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences. 109: 6241-6246. [DOI: 10.1073/pnas.1117018109] [DOI:10.1073/pnas.1117018109] [PMID] [PMCID]
42. Starowicz M. (2021). Analysis of volatiles in food products. Separations. 8: 157. [DOI: 10.3390/separations8090157] [DOI:10.3390/separations8090157]
43. Subari A., Razak A., Sumarmin R. (2021). Phylogenetic analysis of Rasbora spp. based on the mitochondrial DNA COI gene in Harapan forest. Jurnal Biologi Tropis. 21: 89-94. [DOI: 10.29303/jbt.v21i1.2351] [DOI:10.29303/jbt.v21i1.2351]
44. Synos K., Reynolds A.G., Bowen A.J. (2015). Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT - Food Science and Technology. 64: 227-235. [DOI: 10.1016/j.lwt. 2015.05.044] [DOI:10.1016/j.lwt.2015.05.044]
45. Wailzer B., Kocker J., Wolschann P., Buchbauer G. (2016). Structural features for furan-derived fruity and meaty aroma impressions. Natural Product Communications. 11: 1475-1479. [DOI: 10.1177/1934578X1601101014] [DOI:10.1177/1934578X1601101014]
46. Watanabe M., Uchida N., Fujita K., Yoshino T., Sakaguchi T. (2016). Bread and effervescent beverage productions with local microbes for the local revitalization. International Journal on Advanced Science, Engineering and Information Technology. 6: 381-384. [DOI: 10.18517/ijaseit.6.3.841] [DOI:10.18517/ijaseit.6.3.841]
47. Zahroh N., Utami U., Kusmiyati N. (2022). Effect of nitrogen source on growth endophytic yeast from Salacca edulis reinw. and bread quality analysis. Jurnal Biodjati. 7: 95-108. [DOI: 10.15575/biodjati.v7i1.16854] [DOI:10.15575/biodjati.v7i1.16854]
48. Zhang L., Mi S., Liu R.-B., Sang Y.-X., Wang X.-H. (2020). Evaluation of volatile compounds during the fermentation process of yogurts by Streptococcus thermophilus based on odor activity value and heat map analysis. International Journal of Analytical Chemistry. 2020: 3242854. [DOI: 10.1155/2020/ 3242854] [DOI:10.1155/2020/3242854] [PMID] [PMCID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







